Abstract
Background
Numerous studies have examined the elution characteristics and the effects of antibiotics from bone cement. this study seeks to determine the effect that surface area and volume have on the elution characteristics and bioavailability of tobramycin and vancomycin when mixed in polymethylmethacralate (PMMA) bone cement in various combinations. It also investigates the mechanical properties of antibiotic-impregnated bone cement and its relationship to surface area and volume.
Methods
Three antibiotic-bone cement combinations were used, and these consisted of PMMA mixed with tobramycin and vancomycin or tobramycin alone. Four groups of specimens (different surface area and volume) were made. the elution characteristics of the different specimens were examined using the minimum inhibitory concentration (MIc) method at different time intervals. the bacteria used during testing were methicillin-sensitive staphylococcus aureus (MssA). the ultimate compressive strength (Ucs) of the specimens was also determined at various time intervals.
Results
the bactericidal activity of a tobramycin/vancomycin combination against MssA was not significantly greater than tobramycin alone. tobramycin was more effective than vancomycin against MssA (average: 168%, p<0.05). the inhibitory capabilities of tobramycin and vancomycin individually were not found to be additive. combination 2 (1.0g tobramycin/1.0g vancomycin) had a higher antibiotic elution mass and rate for all sample sizes compared to the other two combinations (average: 170%, p<0.05). surface area and volume did not have a significant effect on the elution rate of the antibiotics. the Ucs of all samples tested was greater than 70MPa at all three testing intervals.
Discussion
Mixing tobramycin and vancomycin did not have a synergistic effect against the bacteria as expected. Increasing the concentration of antibiotics in bone cement increases both elution mass and elution rate over time. Although the Ucs of the antibiotic-impregnated bone cement was affected by antibiotic elution and sample geometry, all testing results fell within previously accepted standards.
Clinical Relevance
This study advanced our overall understanding of the elution characteristics and biomechanics of PMMA bone cement impregnated with tobramycin and vancomycin.
Keywords: antibiotic, bone-cement, elution, surface, volume
Introduction
Local delivery of antibiotics using antibiotic depots has become a major factor in the management of deep musculoskeletal infections1, 2. The use of antibiotic-impregnated bone cement to treat musculoskeletal infection has been reported in the literature for more than three decades1, 3 and 4. The elution of antibiotics from polymethylmethacrylate (PMMA) beads has been studied extensively both in vitro and in vivo 5-10. The elution characteristics of antibiotic-impregnated bone cement can be affected by certain factors including the type of cement used11, preparation methods12, surface characteristics13, porosity of the cement6, and the amount and/or type of antibiotics used10, 14-17. Despite all of this data, the current literature still leaves to question the effect that surface area and volume have on the overall performance of antibiotic-loaded cement.
Minimum inhibitory concentration (MIC) is the lowest concentration of an antimicrobial substance that will inhibit the visible growth of a microorganism after overnight incubation. MICs are important in diagnostic laboratories to confirm resistance of microorganisms to an antimicrobial agent and also to monitor the activity of new antimicrobial agents18. MIC is generally regarded as the most basic laboratory measurement of the activity, or strength, of an antimicrobial substance against an organism19.
The two most common antibiotics mixed with bone cement are tobramycin and vancomycin. Tobramycin sulfate is an aminoglycoside antibiotic used to treat various types of bacterial infections, particularly those caused by gram-negative organisms. Tobramycin is preferred over gentamicin for Pseudomonas aeruginosa pneumonia due to better lung penetration and bactericidal activity. Vancomycin is a glycopeptide antibiotic used in the treatment of gram-positive organisms and is best known for its effectiveness against methicillin-resistant Staphyllococcus aureus (MRSA).
Several questions, however, still remain. First, will both tobramycin and vancomycin mix well with PMMA bone cement? Second, will combining tobramycin and vancomycin increase their overall bioactivity against microorganisms? Third, what kind of elution characteristics will these antibiotics have once the cement has cured? Fourth, do the physical dimensions of the cement have an effect on the elution characteristics of the antibiotics? Finally, how will the elution of the antibiotics alter the mechanical properties of the PMMA bone cement? To our knowledge, no one has previously addressed all of these issues. This study seeks to evaluate the elution characteristics of antibiotic-impregnated PMMA bone cement with varying surface areas and volumes. Additionally, it also examines the mechanical properties of the bone cement over time as the antibiotics are eluted.
The specific objectives for this study were two-fold: 1) investigate the elution characteristics and the mechanical properties of PMMA bone cement impregnated either with tobramycin and vancomycin, or with tobramycin only; and 2) evaluate the relationship surface area and volume have with the elution characteristics and mechanical properties of antibiotic-impregnated bone cement. The null hypotheses for this study was: 1) the antibiotic profile of the PMMA bone cement will be favorable, 2) its mechanical properties will not significantly change as the antibiotics are eluted, and 3) the surface area and volume of the cement will affect both the antibiotic elution and its mechanical performance.
Materials and Methods
Antibiotic-Impregnated PMMA Specimen Preparation
Three different antibiotic-impregnated bone cement mixtures were used based on the clinical practice at our institution. Combination 1 was a pre-mixed Simplex P bone cement with tobramycin, which contains 1.0g (2.5 wt.%) of tobramycin in 40g of polymer powder (Stryker Howmedica Osteonics, Mahwah, NJ). The next two antibiotic-impregnated bone cement mixtures used finely powdered tobramycin (X-Gen Pharmaceuticals, Inc., northport, NY) and vancomycin (Hospira, Inc., Lake Forest, IL) mixed with 40g of Simplex P bone cement powder (Stryker Howmedica Osteonics, Mahwah, NJ). Combination 2 contained 1.0g (2.5 wt.%) of tobramycin and 1.0g (2.5 wt.%) of vancomycin in 40g of polymer powder. Combination 3 contained 0.5g (1.25 wt.%) of tobramycin and 0.5g (1.25 wt.%) of vancomycin in 40g of polymer powder. A total antibiotics concentration of 5% by mass is considered to be the gold standard20, 21.
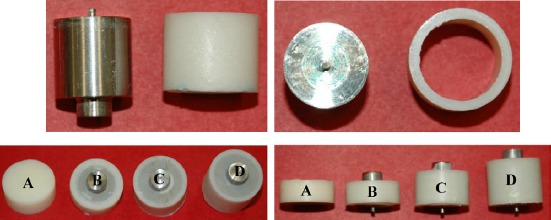
Four groups (A, B, C, D) of eighteen specimens each were made from each of the three antibiotic-impregnated bone cement combinations. Figure 1 and Table 1 show the design and dimensions, respectively, for the four groups of specimens. The samples for each group had a unique volume and surface area that were carefully predetermined (Table 1). Group A specimens consisted of a solid disk designed to serve as a baseline for later comparison. The samples in groups B, C, and D were made up of cylinders each with a particular inner diameter, outer diameter and thickness. The central core of the specimens consisted of a professionally machined stainless steel rod that mimicked a prosthetic implant. This rod was left in place for all of the samples through each arm of the testing. Figure 2 shows images of actual samples.
Figure 1. Dimension drawing of the antibiotic-impregnated PMMA cement specimens *Note: oD - outer Diameter; ID - Inner Diameter.
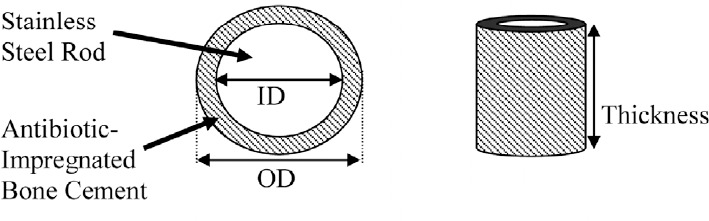
Table 1.
Physical dimension of antibiotic-impregnated PMMA cement
| Group | Od (cm) |
ID (cm) |
Thickness (cm) |
Volume (cm3) |
% Change volume | Surface Area (cm2) |
% change Surface area |
|---|---|---|---|---|---|---|---|
| A (Baseline) | 2.54 | 0.00 | 1.00 | 5.07 | 0 | 18.11 | 0 |
| B | 2.54 | 1.75 | 1.00 | 2.66 | -47 | 13.30 | -27 |
| C | 2.54 | 2.06 | 1.54 | 2.67 | -47 | 15.76 | -3 |
| D | 2.54 | 2.06 | 2.07 | 3.59 | +29 | 19.99 | 10 |
Note: OD - Outer Diameter; ID - Inner Diameter
Figure 2. Testing specimens (antibiotic-impregnated PMMA cement).
The cement was prepared using a manual mixer. Centrifuging and vacuum techniques were not used in this study. The cement was transferred to the appropriate polyethylene molds and allowed to cure for 24 hours. After 24 hours, each specimen was inspected for any voids or powder clots, and any flawed specimens were excluded. All of the approved specimens were fully immersed separately in sterile phosphate buffered saline (PBS) inside plastic containers with lids. The volume of the saline was measured for each group of specimens. The containers were then placed on a swirl shaker inside an incubator (95% humidity, 37°C, 6% CO2).
Elution Kinetics Testing
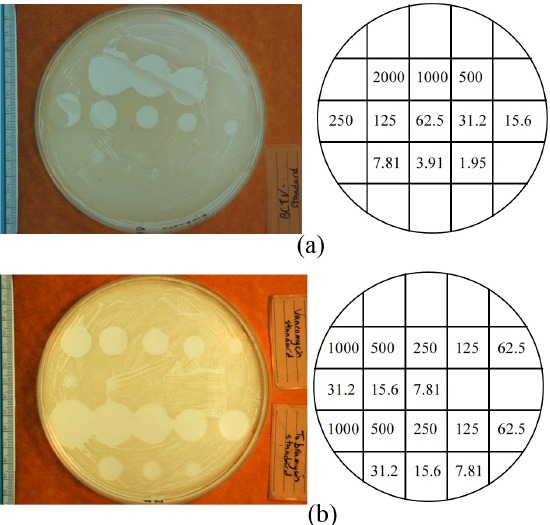
The measurement of the bioactivity of tobramycin, vancomycin and a combination of the two antibiotics was assessed using the MIC method. For this study standard Petri dishes containing Mueller-Hinton agar were plated with methicillin-sensitive Staphylococcus aureus ATCC 25923 (MSSA, NCTC 12981). Four dishes were used for each type of antibiotic and the combination of the two. A series of 8 two-fold dilutions ranging from 2000mg/mL to 7.81mg/mL was prepared, and 10mL of each dilution was placed in individual wells of the Petri dish (Figure 3). After overnight incubation of the plates at 37°C, the wells were examined for presence or absence of growth. Digital pictures were taken of the dishes and used to measure the diameter of the zone inhibition. The area of the inhibitory zone was then calculated. These dishes served as a standard data set to determine antibiotic concentrations during the elution kinetics testing.
Figure 3. Representative digital pictures of the minimum inhibitory concentration (MIC) with methicillin-sensitive Staphylococcus aureus ATCC 25923 for each type of antibiotic used. (a) 1.0g tobramycin and 1.0g vancomycin combination, (b) vancomycin (top) and tobramycin (bottom) individually.
To test the antibiotic elution characteristics, sequential samples were assayed for the MIC similar to the antibiotic activity tests. Only 6 of the 18 specimens from each group were used for this portion of the investigation. Sample intervals representative of 1, 3, 5, and 7 days were tested for their antibiotic elution characteristics. For each sample interval, the solution was removed and stored in another container at 4°C until analyzed. Then the appropriate amount of fresh PBS was placed back into the plastic container with the specimen. A series of three-fold dilutions (1:1, 1:3, and 1:9) was prepared, and 10mL of each dilution was placed in individual wells of the Petri dish. Digital pictures were taken of the dishes and used to measure the diameter of the zone inhibition. The antibiotic concentration for each solution was extrapolated from the diameter of the zone of inhibition using the computer growth equation constructed from the standard curves.
Mechanical Testing
In terms of mechanical performance, all testing was performed with strict adherence to the American Society for Testing and Materials (ASTM) F451-99a standards - Standard Specification for Acrylic Bone Cement (ASTM Standard, 2006). The dimensions for each cylindrical-shaped specimen were measured using a digital caliper and recorded prior to each mechanical test. Surface area and volume for the antibiotic-impregnated PMMA samples were determined. For each antibiotic combination, six specimens from each group were tested at intervals of 0, 5, and 7 days resulting in eighteen specimens per group. All the specimens were tested in compression using an MTS Bionix servohydraulic materials testing system (MTS Model 858, Eden Prairie, MN). The specimens were loaded from -50N to complete structural failure at the rate of 20mm/min. Load and deflection data were measured and collected every 0.1 seconds by the MTS system. The ultimate compressive strength (UCS) was then determined. The mean and standard deviation of the groups were calculated for each type of antibiotic-impregnated bone cement. All mechanical tests were performed in air at environmental temperature.
Statistical Analysis
Data retrieved for bioactivity, antibiotic elution rate, and ultimate compressive strength were analyzed using one-way analysis of variance (ANOVA) of SPSS software (Version 16.0; SPSS, Chicago, IL) with p<0.05 denoting significance. Post hoc tests were conducted using the Least Significant Difference (LSD) multiple comparisons test method. These analyses were used to determine the statistical relevance of the difference in bioactivity of the antibiotics, mechanical properties and elution characteristics in each group.
Results
Elution Kinetics Testing
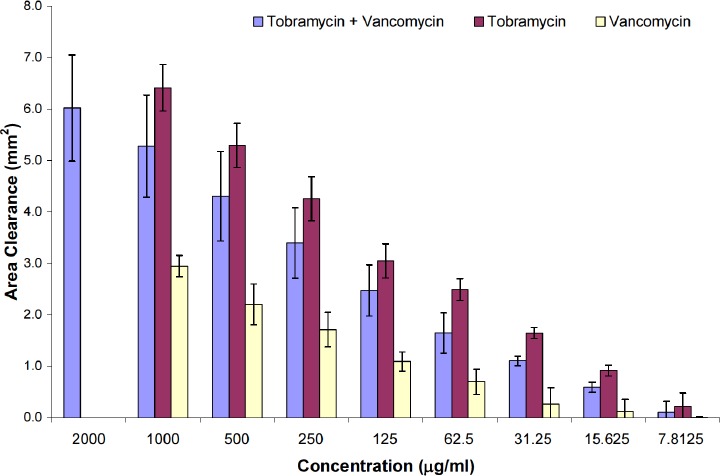
The mean inhibitory zones for vancomycin, tobramycin, and a combination of the two antibiotics against the S. aureus used in this study are shown in Figure 4. The graph illustrates the activity of the antibiotics at different concentrations. Overall, the activity of the tobramycin and vancomycin combination was not significantly (p>0.05) higher than the activity of tobramycin alone. However, tobramycin alone was significantly more efficacious than vancomycin (average: 168%, range: 117%-257%; p<0.05). Consequently, the inhibitory capabilities of tobramycin and vancomycin individually, were not found to be additive.
Figure 4. Antibiotic activity using MIC for tobramycin, vancomycin and combination of both tobramycin and vancomycin (mean ± standard deviation).
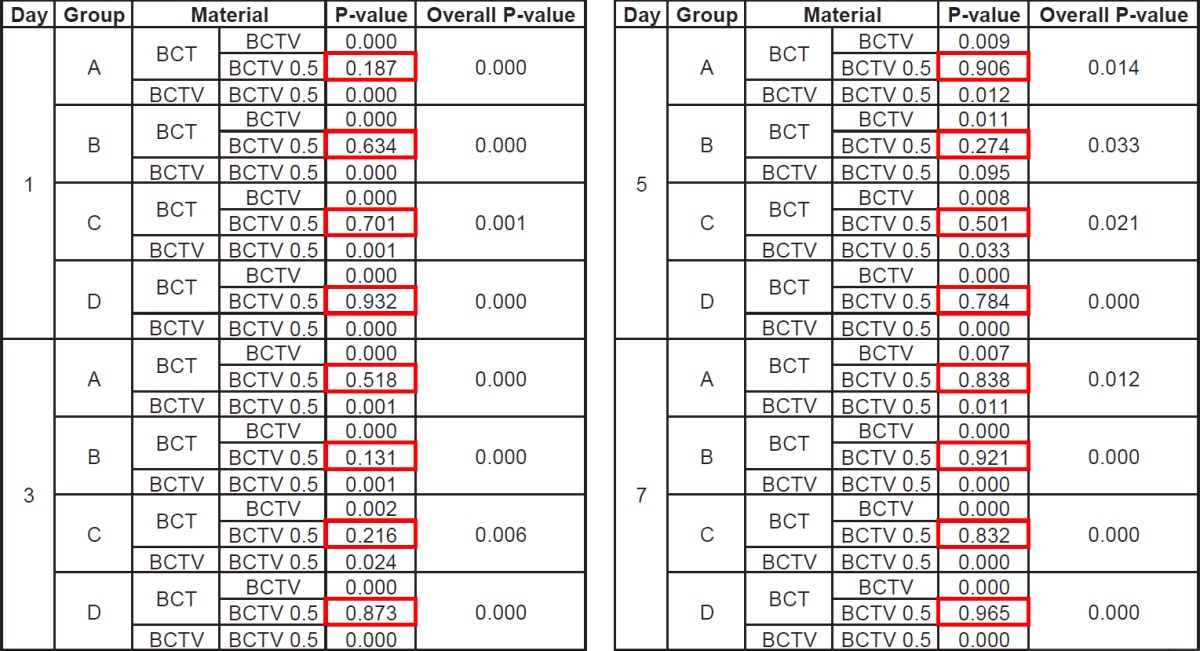
After seven days in the 37°C buffered solution, the antibiotic-impregnated PMMA specimens did not show any evidence of gross deterioration. Figure 5 shows the total concentration of antibiotics released at four separate intervals during this time period. Table 2 lists the amount of antibiotics released and the rate of their release for each of the bone cement combinations and their four sample groups. The specimens from Combination 2 (1.0g tobramycin/1.0g vancomycin) had a higher antibiotic elution rate for all sample sizes when compared to Combination 1 and Combination 3 (average: 170%, range: 66% – 236%, Table 2). This difference was statistically significant (Table 3). The specimens from Combination 3 (0.5g tobramycin/0.5g vancomycin) had a higher antibiotic elution rate (average: 52.6%, range: 28.7% – 69.5%, Table 2) than those from Combination 1 (1.0g tobramycin), however this was not statistically significant (Table 3).
Figure 5. Total concentration of antibiotics released (mean ± 95% Confidence Interval) from all PMMA specimens used in the study over time.
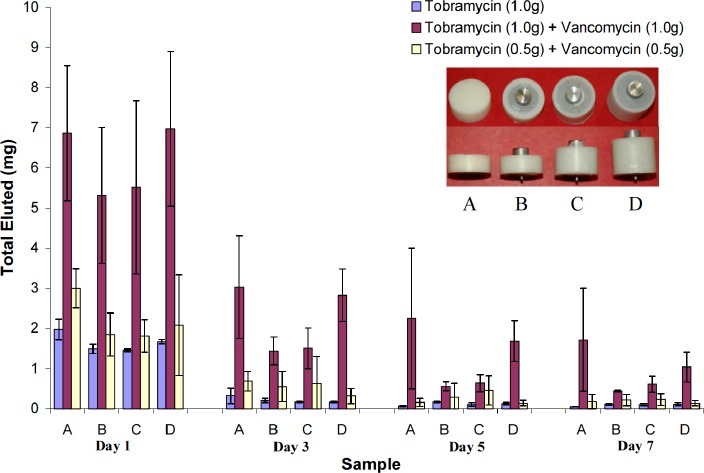
Table 2.
Mean overall Antibiotics Released and Release Rate Over Total 7-Days Period
| Group | Od (cm) | ID (cm) | Designed Thickness (cm) | Volume (cm3) | Surface area (cm2) | Total amount of antibiotics released (mg) | Initial antibiotics Release rate (mg/day) (Day 1 – Day 3) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BCT | BCTV | BCTV 0.5 | BCT | BCTV | BCTV 0.5 | ||||||
| A | 2.54 | 0.00 | 1.00 | 5.07 | 18.11 | 2.43 | 13.86 | 4.03 | 0.82 | 1.92 | 1.15 |
| B | 2.54 | 1.75 | 1.00 | 2.66 | 13.30 | 1.98 | 7.77 | 2.91 | 0.64 | 1.94 | 0.65 |
| C | 2.54 | 2.06 | 1.54 | 2.67 | 15.76 | 1.85 | 8.27 | 3.13 | 0.64 | 2.00 | 0.60 |
| D | 2.54 | 2.06 | 2.07 | 3.59 | 19.99 | 2.08 | 12.52 | 2.68 | 0.75 | 2.07 | 0.88 |
Note: BCT represents PMMA impregnated with 1.0g tobramycin;
BCTV represents PMMA impregnated with 1.0g tobramycin and 1.0g vancomycin;
BCTV 0.5 represents PMMA impregnated with 0.5g tobramycin and 0.5g vancomycin.
Table 3.
Statistical Analysis of Effect of Different Antibiotics Combination on Antibiotic Release (one-way ANOVA, SPSS)
Note: BCT represents PMMA impregnated with 1.0g tobramycin;
BCTV represents PMMA impregnated with 1.0g tobramycin and 1.0g vancomycin;
BCTV 0.5 represents PMMA impregnated with 0.5g tobramycin and 0.5g vancomycin.
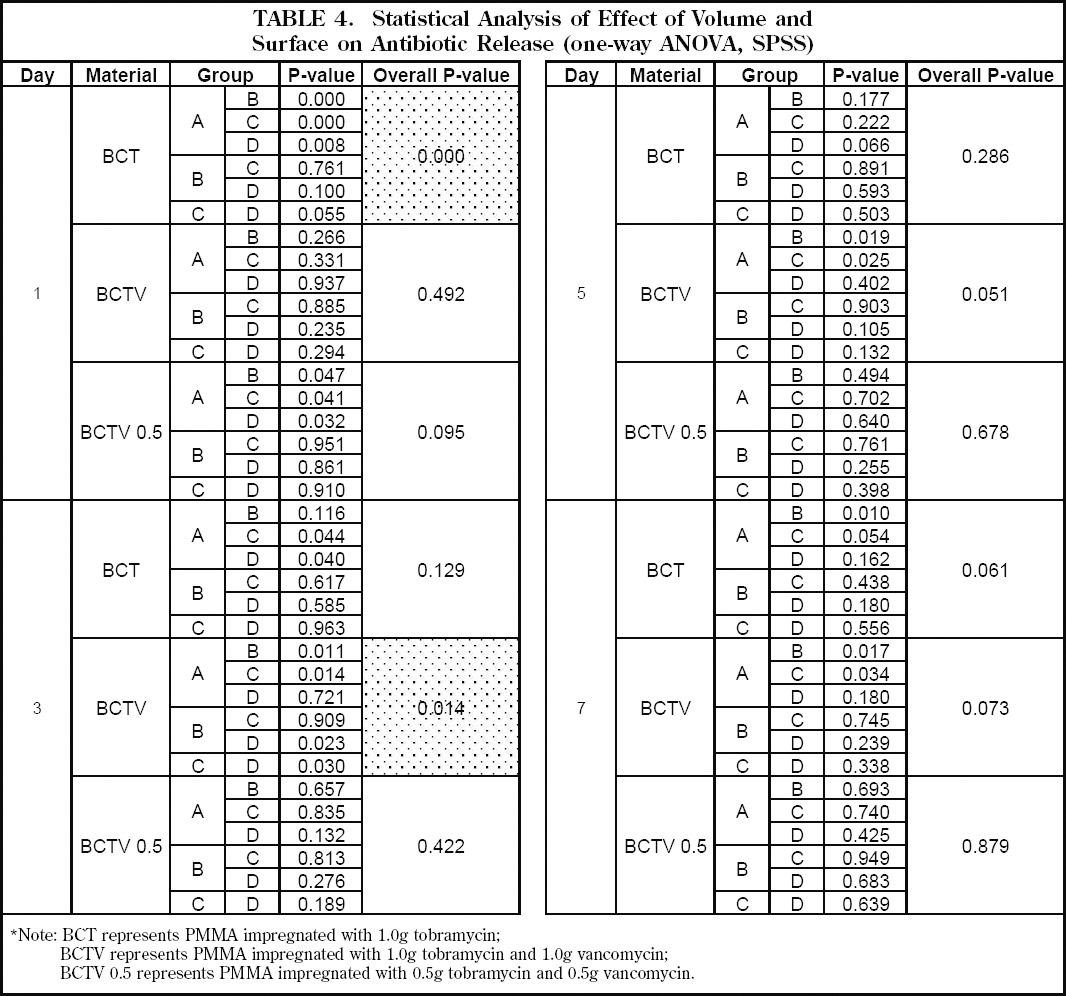
On day one there was a large amount of total antibiotics released for all samples followed by an exponential decay (Figure 5). For those specimens impregnated with both tobramycin and vancomycin, there was no significant difference (p>0.05) in the amount of antibiotics eluted when comparing groups with the same antibiotic combination but different surface areas and volumes (Table 4). However, the larger volume samples did tend to elute a higher amount of antibiotics for each of the combinations. On day three, samples containing either the low-dose combination of tobramycin and vancomycin or tobramycin alone eluted less than 0.7mg of antibiotics, thereby making it difficult to determine the total elution mass of antibiotics as a function of surface area and volume. For the specimens containing the high-dose combination of the two antibiotics, the amount of antibiotics eluted was high enough to show there was no difference based on surface area when holding volume constant (Group B vs C) (Figure 5, Table 4).
Table 4.
Statistical Analysis of Effect of Volume and Surface on Antibiotic Release (one-way ANOVA, SPSS)
|
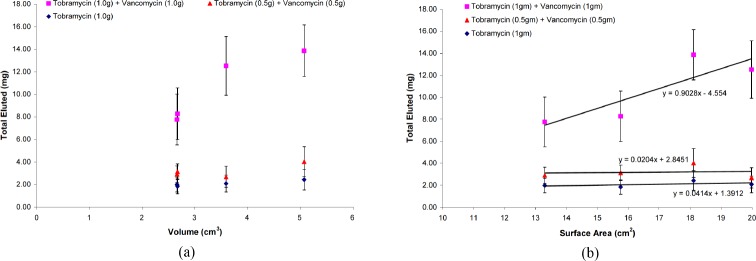
The total elution mass of antibiotics as a function of volume could not be determined due to the limited number of data points collected and the variable performance pattern of the different antibiotic combinations. No consistent relationship was identified (Figure 6a). Figure 6. As for the total elution mass of antibiotics as a function of surface area, a linear relationship was identified between increasing surface area and increasing amount of antibiotics released. However, this was dependent upon the initial concentration as well as the combination of antibiotics, with the specimens containing the high-dose combination of vancomycin and tobramycin exhibiting this relationship (Figure 6b).
Figure 6. Average mass of antibiotics eluted as a function of: (a) specimen volume and (b) specimen surface area. Error bars represent the uncertainties of the total eluted mass at the 95% confidence interval.
Mechanical Testing
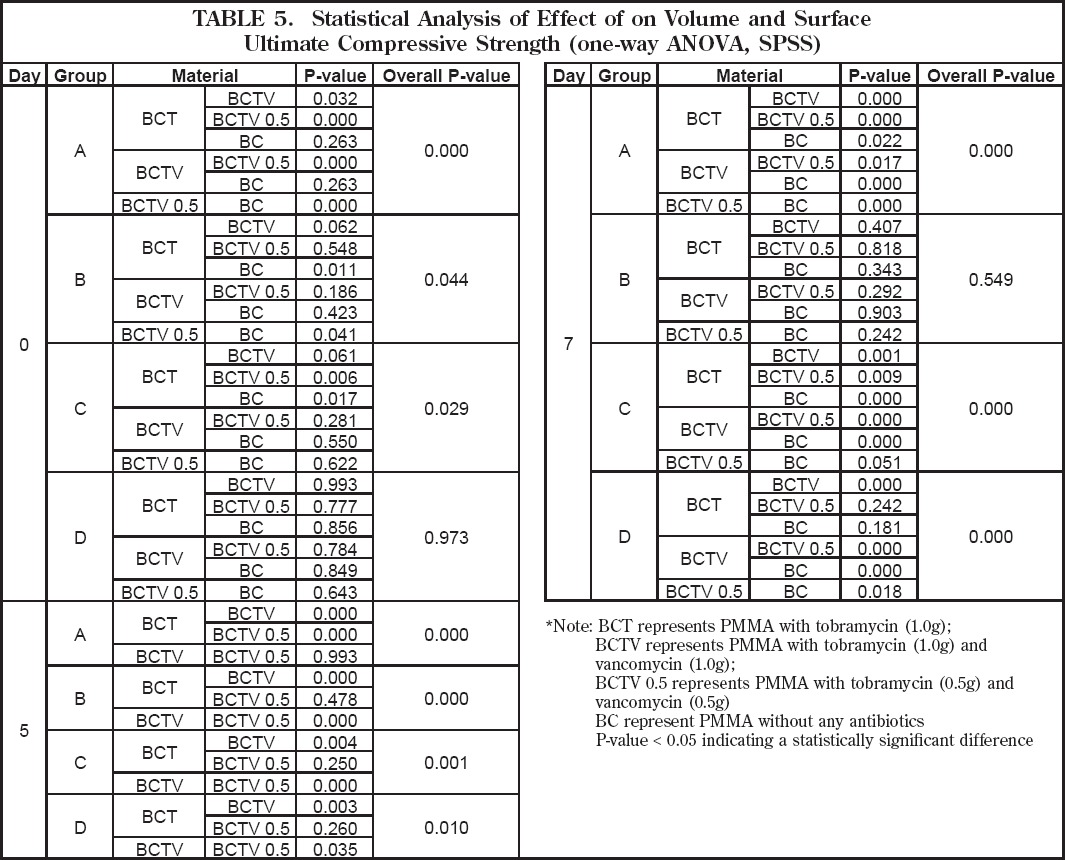
Ultimate compressive strength (UCS) testing was carried out, and six samples from each group were tested at each interval day for a total of 18 test samples. Results are shown in Figure 7. The UCS of all specimens tested during the seven days of incubation in the 37oC buffer was above the ASTM F541 and ISO 5833 minimum of 70MPa. At Day 0, the mean UCS for Group A was found to be significantly different (p<0.05) between the antibiotic-impregnated specimens. The same held true for Groups B and C. When antibiotic-free specimens in Group A were compared to antibiotic-impregnated specimens no significant difference was detected, except for those specimens containing a low-dose tobramycin/ vancomycin combination (Table 5). For Group B the mean UCS for antibiotic-free specimens was found to be significantly different from specimens with a high-dose tobramycin/vancomycin combination (Table 5). For Group C a significant difference in UCS was found between antibiotic-free specimens and those impregnated with tobramycin alone. For Group D no significant difference in mean UCS was found when comparing all specimen groups (Table 5).
Figure 7. Mechanical properties from all PMMA specimens used in the study over time. Error bars represent the uncertainties of the compression strength at the 95% confidence interva.
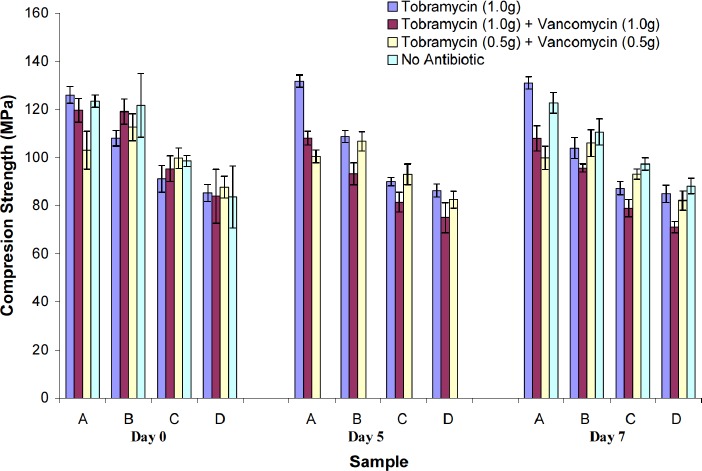
Table 5.
Statistical Analysis of Effect of Volume and Surface on Antibiotic Release (one-way ANOVA, SPSS)
|
At day 7, a significant difference (p<0.05) was detected in the mean UCS for Group A, and when comparing antibiotic-impregnated specimens to antibiotic-free specimens (Table 5). For Group B no significant difference was detected between specimens. For Group C the mean UCS was found to be significantly different (p<0.05) when comparing antibiotic-free specimens to antibiotic-impregnated specimens, except for those specimens with a low-dose tobramycin/vancomycin combination (Table 5). For Group D the mean UCS was also significantly different when comparing antibiotic-free specimens to antibiotic-impregnated specimens except for those with tobramycin alone.
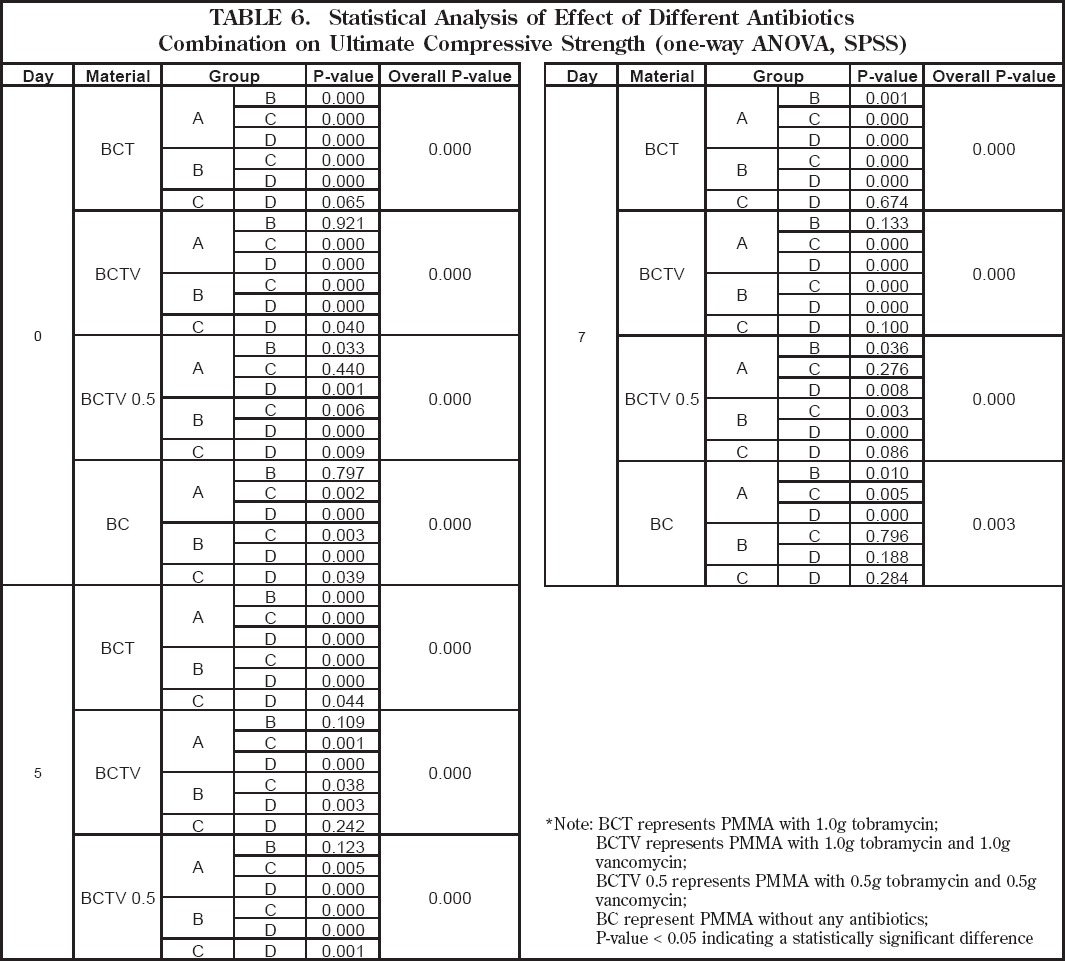
When comparing UCS among different specimens in relationship to their geometry (i.e. surface area and volume) statistically significant differences (p<0.05) were detected (Table 6). At Day 0 there was a significant difference in UCS between groups without antibiotics and those with antibiotics except when comparing Groups A and B. At Day 7 the mean UCS of the original PMMA specimens without antibiotics was found to be significantly different (p<0.05). When looking at specimens with antibiotics, the mean UCS of group A was significantly different when compared to all other groups.
Table 6.
Statistical Analysis of Effect of Volume and Surface on Antibiotic Release (one-way ANOVA, SPSS)
|
The UCS of the specimens containing tobramycin alone (BCT) was significantly different (p<0.05), except between Groups C and D for day 0 and day 7 (Table 6). A significant difference in UCS was also found between samples impregnated with a high-dose tobramycin/vancomycin combination (p<0.05), except between Groups A and B (Days 0, 5 and 7) and Groups C and D (Days 5 and 7). Those specimens with a low-dose tobramycin/ vancomycin combination had significantly different measurements of UCS, except between Groups A and C (Days 0 and 7), Groups A and B (Day 5) and Groups C and D (Day 7) (Table 6).
When comparing the UCS of all specimens between day 0 and day 7, a significant difference (p<0.05) was detected in the groups with a high-dose tobramycin/ vancomycin combination, and in Group C with specimens containing a low-dose tobramycin/vancomycin combination (Table 7).
Table 7.
Ultimate compressive strength (Ucs) comparison between Day 0 and Day 7 for different sample sizes
| UCS Comparison in Percent (Day 0 vs Day 7) | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | ||
| BCT | -4.0 | 3.8 | 4.3 | 0.5 | 0.06 | 0.64 | 0.30 | 0.95 | |
| BCTV | 9.7 | 19.7 | 17.3 | 15.3 | 0.01 | 0.02 | 0.00 | 0.04 | |
| BCTV | 0.5 | 3.1 | 5.8 | 6.7 | 6.3 | 0.87 | 0.71 | 0.02 | 0.13 |
| BC | 0.6 | 9.1 | 1.3 | -5.5 | 0.97 | 0.13 | 0.49 | 0.52 | |
Note: BCT represents PMMA with 1.0g tobramycin;
BCTV represents PMMA with 1.0g tobramycin and 1.0g vancomycin;
BCTV 0.5 represents PMMA with 0.5g tobramycin and 0.5g vancomycin;
BC represent PMMA without any antibiotics;
P-value < 0.05 indicating a statistically significant difference
Discussion
Antibiotic-impregnated PMMA is used routinely in treatment of infected total joint arthroplasties and occasionally in the management of chronic osteomyelitis1, 3, 4, 22-27. Penner et al9 demonstrated that combining tobramycin and vancomycin in bone cement may have clinical advantages by increasing the antimicrobial spectrum and providing a synergistic effect. Their study used the fluorescence polarization immunoassay method to measure the concentrations of the eluted antibiotics, but had no way of showing whether the antibiotics were biologically active against bacteria. In 2001 González Della Valle et al28 demonstrated that the presence of tobramycin has a synergistic-like effect on the bactericidal activity of vancomycin, and their study used 3 patients who underwent total hip reimplantation with cement after chronic infection caused by methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis.
Our current study found that tobramycin released from PMMA is more bioactive than vancomycin against methicillin-sensitive Staphylococcus aureus (MSSA). Another important observation was that mixing tobramycin and vancomycin with PMMA bone cement does not have a synergistic affect against MSSA. This is the first report that we know of in the literature to demonstrate this finding. The non-additive, non-synergistic properties of this antibiotic combination may be due to less elution of vancomycin secondary to its greater molecular weight. Klekamp et al 8 stated that the molecular weight of vancomycin is 1,468 atomic mass units and the molecular weight of tobramycin is almost one-third (485 atomic mass units). This factor may also explain the discrepancy in bioactivity of vancomycin and tobramycin when obtaining the standard data set using for MIC testing. The increased mass of vancomycin may inhibit its dissipation across the Petri dish in liquid form.
Penner et al9 used 1.0g of vancomycin and 2.4g of tobramycin added to each 40g pack of bone cement powder to study in vitro elution characteristics, and they concluded that the elution rate of tobramycin was increased by 68% and that of vancomycin by 103% simply by combining these two antibiotics in the same batch of cement. Klekamp et al8 also studied the elution characteristics of tobramycin and vancomycin combined with Simplex or Palacos cement, using enzyme-linked immunosorbent assay, and they observed that the elution of tobramycin was compromised by the presence of vancomycin. Greeme et al29 also reported that the tobramycin elutes at higher levels and for longer periods than vancomycin. However, their results only compare the elution of tobramycin and vancomycin individually from Palacos and Simplex cement. Our results agree with those of both Klekamp et al8 and Penner et al9 that the combining tobramycin and vancomycin in PMMA bone cement had an additive effect on the in vitro elution rate.
Based on previous studies6, 8, 10, 17, there is no doubt that elution of antibiotics is a surface phenomenon related to pores and cracks within the bone cement matrix created by the antibiotics themselves. A majority of impregnated antibiotics remain entrapped within the cement core, therefore, it is no surprise that elution is improved with increasing surface area. Our results demonstrate that mixing vancomycin with the tobramycin in bone cement helps to increase the elution rate.
Kelm et al14 noted the effective antibiotic elution in vivo may be different from that observed in vitro . And according to Masri et al30, it is apparent that clinical application of these in vitro results is not straightforward. Work suggests that high antibiotic doses and maximal elution efficiency are necessary to maintain tissue antibiotic levels above the breakpoint sensitivity limit for long-term therapy. Our results support the use of two antibiotics in situations in which the absolute maximal amount of antibiotic elution is desired.
Additionally, our current study disproves the null hypothesis that the mechanical properties of PMMA bone cement will not significantly change as the antibiotics are eluted. The percentage drop in UCS between Day 0 and Day 7 was much higher for specimens made with larger amounts of antibiotics. However, the loading results of all groups and specimens with different combination doses of antibiotics after the seven days of incubation in the 37°C buffer still had the mean UCS above the ASTM F54131 and ISO 5833 minimum of 70MPa.
The UCS of the specimens was affected by two factors: 1) increased time within the solution, and 2) the thickness of the specimens. The time-related change in mechanical properties has been reported previously. Pelletier et al32 showed that after 4 weeks the PMMA bone cement was stronger than then the PMMA at 24 hours, which concluded that the PMMA specimen has time-dependent properties. These may explain why our mechanical test results after Day 7 the UCS of the high dosage antibiotic-loaded PMMA bone cement still within the ASTM and the ISO standards. The other factor affecting the UCS of the specimen was the thickness of the specimens. Paradoxically, even though there is an increase both in volume and surface area, the thicker the specimens the more likely the specimen will failure.
Areas of future interest include testing of other mechanical properties of PMMA bone cement, such as diametral tensile strength and fatigue properties. Also, further research is needed to investigate the biological activity of these antibiotics against other strains of bacteria such as Staphylococcus epidermidis (BK 2), methicillin-resistant Staphylococcus aureus (MRSA) and Staphylococcus capitis (ED2D), all bacterial species that frequently cause orthopaedic implant infections33.
Acknowledgments
The authors wish to thank Teresa L. Jones, MPH, MT (ASCP) for her assistant, revision and critical comments on the papers. The authors also wish to thank Tom Aldag, Shin Mah, Dimuthu Tilakaratne, and Rita Rai from National Aviation Institute of Aviation for their technical help and for the use of the MTS system. No benefits of any form have been received directly or indirectly to the subject of this article.
References
- 1.Duncan CP, Masri BA. Antibiotic depots. J Bone Joint Surg Br. 1993;75(3):349–50. doi: 10.1302/0301-620X.75B3.8496197. PMID:8496197. May. [DOI] [PubMed] [Google Scholar]
- 2.Duncan CP, Masri BA. The role of antibiotic-loaded cement in the treatment of an infection after a hip replacement. Instr Course Lect. 1995;44:305–13. PMID:7797868. [PubMed] [Google Scholar]
- 3.Calhoun JH, Mader JT. Antibiotic beads in the management of surgical infections. Am J Surg. 1989;157(4):443–9. doi: 10.1016/0002-9610(89)90597-7. Apr. PMID:2648886. [DOI] [PubMed] [Google Scholar]
- 4.Henry SL, Seligson D, Mangino P, Popham GJ. Antibiotic-impregnated beads. Part I: Bead implantation versus systemic therapy. Orthop Rev. 1991;20(3):242–7. PMID:2023787 Mar; [PubMed] [Google Scholar]
- 5.Adams K, Couch L, Cierny, g, calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethyl-methacrylate beads. Clin Orthop Relat Res. 1992;(278):244–52. PMID:1563160 May; [PubMed] [Google Scholar]
- 6.Baker AS, Greenham LW. Release of gentamicin from acrylic bone cement. Elution and diffusion studies. J Bone Joint Surg Am. 1988;70(10):1551–7. PMID:3198680 Dec; [PubMed] [Google Scholar]
- 7.Holtom PD, Warren CA, Greene NW, Bravos PD, Ressler RL, Shepherd L, Mc Pherson EJ. Patzakis MJ. Relation of surface area to in vitro elution characteristics of vancomycin-impregnated polymethylmethacrylate spacers. Am J Orthop. 1998;27(3):207–10. PMID: 9544362 Mar; [PubMed] [Google Scholar]
- 8.Klekamp J, Dawson JM, Haas DW, Deboer D, Christie M. The use of vancomycin and tobramycin in acrylic bone cement: biomechanical effects and elution kinetics for use in joint arthroplasty. J Arthro-plasty. 1999;14(3):339–46. doi: 10.1016/s0883-5403(99)90061-x. PMID: 10220189 Apr. [DOI] [PubMed] [Google Scholar]
- 9.Penner MJ, Duncan CP, Masri BA. The in vitro elution characteristics of antibiotic-loaded CMW and Palacos-R bone cements. J Arthroplasty. 1999;14(2):209–14. doi: 10.1016/s0883-5403(99)90128-6. PMID:10065729 Feb. [DOI] [PubMed] [Google Scholar]
- 10.Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11(8):939–44. doi: 10.1016/s0883-5403(96)80135-5. PMID:8986572 Dec. [DOI] [PubMed] [Google Scholar]
- 11.Marks KE, Nelson CL, Lautenschlager E P. Antibiotic-impregnated acrylic bone cement. J Bone Joint Surg Am. 1976;58(3):358–64. PMID:770477 Apr. [PubMed] [Google Scholar]
- 12.Askew MJ, Kufel MF, Fleissner PR, Jr, Gradisar IA, Jr, Salstrom SJ, Tan JS. Effect of vacuum mixing on the mechanical properties of antibiotic-impregnated polymethylmethacrylate bone cement. J Biomed Mater Res. 1990;24(5):573–80. doi: 10.1002/jbm.820240504. PMID: 2324127 May. [DOI] [PubMed] [Google Scholar]
- 13.Masri BA, Duncan CP, Beauchamp CP, Paris NJ, Arntorp J. Effect of varying surface patterns on antibiotic elution from antibiotic-loaded bone cement. J Arthroplasty. 1995;10(4):453–9. doi: 10.1016/s0883-5403(05)80145-7. PMID: 8523003 Aug. [DOI] [PubMed] [Google Scholar]
- 14.Kelm J, Regitz T, Schmitt E, Jung W, Anagnostakos K. In vivo and in vitro studies of antibiotic release from and bacterial growth inhibition by antibiotic-impregnated polymethylmethacrylate hip spacers. Antimicrob Agents Chemother. 2006;50(1):332–5. doi: 10.1128/AAC.50.1.332-335.2006. PMID:16377705 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res. 2005;23(1):27–33. doi: 10.1016/j.orthres.2004.03.003. PMID:15607871 Jan. [DOI] [PubMed] [Google Scholar]
- 16.Picknell B, Mizen L, Sutherland R. Antibacterial activity of antibiotics in acrylic bone cement. J Bone Joint Surg Br. 1977;59(3):302–7. doi: 10.1302/0301-620X.59B3.408356. PMID:408356 Aug. [DOI] [PubMed] [Google Scholar]
- 17.Kuechle DK, Landon GC, Musher DM, Noble PC. Elution of vancomycin daptomycin, and amika-cin from acrylic bone cement. Clin Orthop Relat Res. 1991;(264):302–8. PMID: 1705191 Mar. [PubMed] [Google Scholar]
- 18.Andrews JM. Determination of minimum inhibitor y concentrations. J Antimicrob Chemother. 2001 doi: 10.1093/jac/48.suppl_1.5. Jul;48 Suppl 1:5-16 Erratum in: J Antimicrob Chemother 2002 Jun; 49(6)1049. PMID:11420333. [DOI] [PubMed] [Google Scholar]
- 19.Turnidge JD, Ferraro MJ, Jorgensen JH. Susceptibility Test Methods: General Considerations. In: Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH, editors. Manual of Clinical Microbiology. 8th Ed. Washington: American Society of Clinical Microbiology; 2003. p. 1103. ISBN 1-55581-255-4. [Google Scholar]
- 20.He Y, Trotignon JP, Loty B, Tcharkhtchi A, Verdu J. Effect of antibiotics on the properties of poly(methylmethacrylate)-based bone cement. J Biomed Mater Res. 2002;63(6):800–6. doi: 10.1002/jbm.10405. PMID: 12418027. [DOI] [PubMed] [Google Scholar]
- 21.Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am. 2006;88(11):2487–500. doi: 10.2106/JBJS.E.01126. PMID: 17079409 Nov. [DOI] [PubMed] [Google Scholar]
- 22.Buchholz HW, Elson RA, Heinert K. Antibioticloaded acrylic cement: current concepts. Clin Orthop Relat Res. 1984:96–108. PMID: 6386264 Nov;(190) [PubMed] [Google Scholar]
- 23.Trippel SB. Antibioticimpregnated cement in total joint arthroplasty. J Bone Joint Surg Am. 1986;68(8):1297–302. PMID:3095327 Oct. [PubMed] [Google Scholar]
- 24.Calhoun JH, Henry SL, Anger DM, Cobos JA, Mader JT. The treatment of infected nonunions with gentamicin-polymethylmethacrylate antibiotic beads. Clin Orthop Relat Res. 1993:23–7. PMID: 8403654 Oct;(295) [PubMed] [Google Scholar]
- 25.Dash AK, Suryanarayanan R. An implantable dosage form for the treatment of bone infections. Pharm Res. 1992;9(8):993–1002. doi: 10.1023/a:1015842108772. Aug. PMID:1409389. [DOI] [PubMed] [Google Scholar]
- 26.Evans RP, Nelson CL. Gentamicin-impregnated polymethylmethacrylate beads compared with systemic antibiotic therapy in the treatment of chronic osteomyelitis. Clin Orthop Relat Res. 1993:37–42. PMID:8403668 Oct;(295) [PubMed] [Google Scholar]
- 27.Nelson CL, Evans RP, Blaha JD, Calhoun J, Henry SL, Patzakis MJ. A comparison of genta-micin-impregnated polymethylmethacrylate bead implantation to conventional parenteral antibiotic therapy in infected total hip and knee arthroplasty. Clin Orthop Relat Res. 1993:96–101. PMID: 8403676 Oct;(295) [PubMed] [Google Scholar]
- 28.González Della VALLE A, Bostrom M, Brause B, Harney C, Salvati EA. Effective bactericidal activity of tobramycin and vancomycin eluted from acrylic bone cement. Acta Orthop Scand. 2001;72(3):237–40. doi: 10.1080/00016470152846547. PMID: 11480597 Jun. [DOI] [PubMed] [Google Scholar]
- 29.Greene N, Holtom PD, Warren CA, Ressler RL, Shepherd L, Mcpherson EJ, Patzakis MJ. In vitro elution of tobramycin and vancomycin poly-methylmethacrylate beads and spacers from Simplex and Palacos. Am J Orthop. 1998;27(3):201–5. PMID:9544361 Mar. [PubMed] [Google Scholar]
- 30.Masri BA, Duncan CP, Beauchamp CP. Long term elution of antibiotics from bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplasty. 1998;13(3):331–8. doi: 10.1016/s0883-5403(98)90179-6. PMID:9590645 Apr. [DOI] [PubMed] [Google Scholar]
- 31.ASTM . American Society for Testing and Materials Specifcation F451-99a, Standard Specifcation for Acrylic bone Cement Annual Book of Astm Standards. Vol. 13.01. West Conshohocken, PA: 2006. pp. 104–11. [Google Scholar]
- 32.Pelletier MH, Malisano L, Smitham PJ, Okamoto K, Walsh WR. The Compressive Properties of Bone Cements Containing Large Doses of Antibiotics. J Arthroplasty. 2009;24(3):454–60. doi: 10.1016/j.arth.2007.10.023. Epub 2008 Apr 3. PMID:18534462 Apr. [DOI] [PubMed] [Google Scholar]
- 33.Tunney MM, Patrick S, Gorman SP, Nixon JR, Anderson N, Davis RI, Hanna D, Ramage G. Improved detection of infection in hip replacements. A currently underestimated problem. J Bone Joint Surg Br. 1998;80(4):568–72. doi: 10.1302/0301-620x.80b4.8473. PMID:9699813 Jul. [DOI] [PubMed] [Google Scholar]


