Abstract
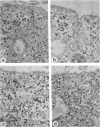
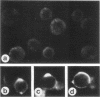
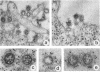
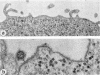
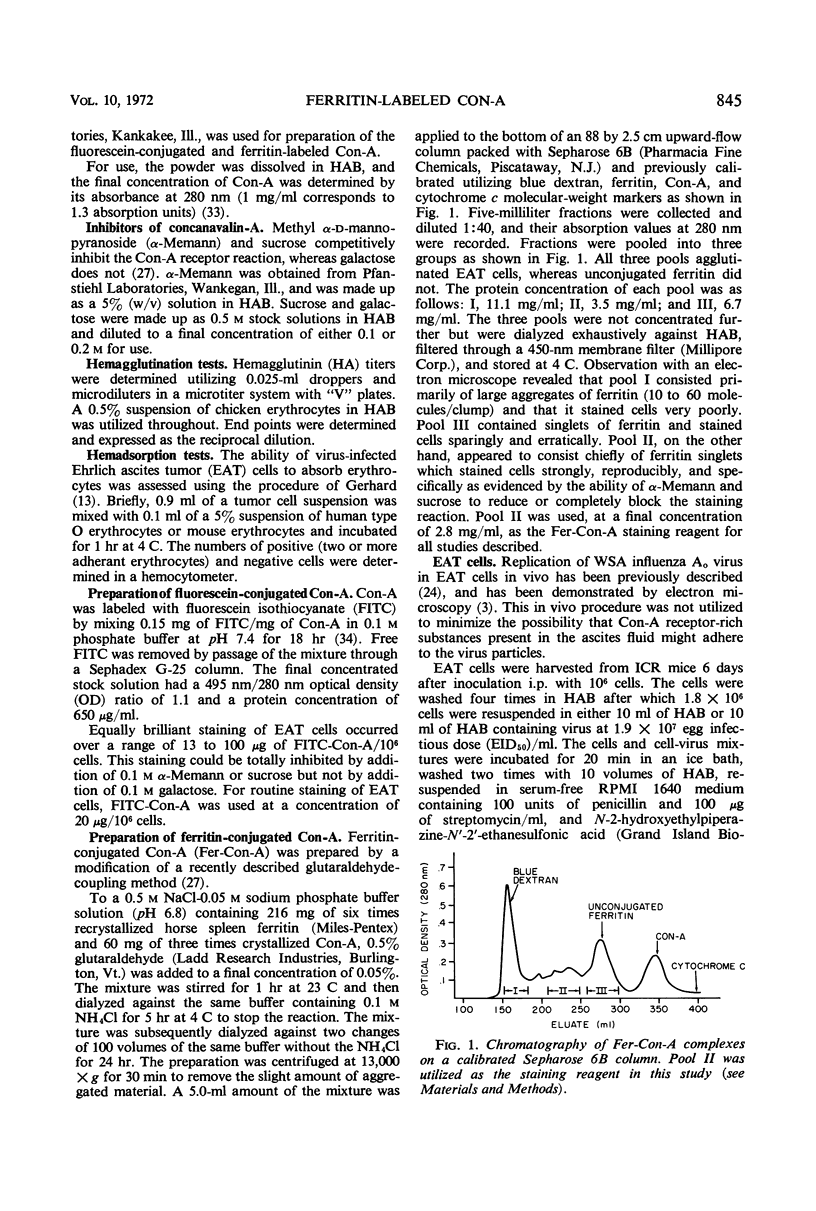
Concanavalin A (Con-A) was linked to ferritin with glutaraldehyde and chromatographed on Sepharose 6B to separate unconjugated Con-A and ferritin from covalently cross-linked molecules. Ehrlich ascites tumor cells were infected with WSA influenza virus, stained at intervals with the ferritin-labeled Con-A and examined by electron microscopy. The surfaces of most mature viruses were specifically stained, providing direct evidence that influenza viruses maturing in this cell type have exposed Con-A receptor sites. The ferritin cores of the staining reagent were found at an average distance of 21.3 nm from the virus membrane and 10.8 nm from the uninfected cell membrane. This finding was interpreted to mean that the population of Con-A receptor sites on influenza virus particles is located at an average distance from the virus membrane twice that of the population of Con-A receptor sites found on uninfected cells. The structural elements of viral membranes can provide a reliable means for evaluating electron microscopy staining reagents, thereby enhancing their usefulness as probes for the study of membrane relationships.
Full text
PDF

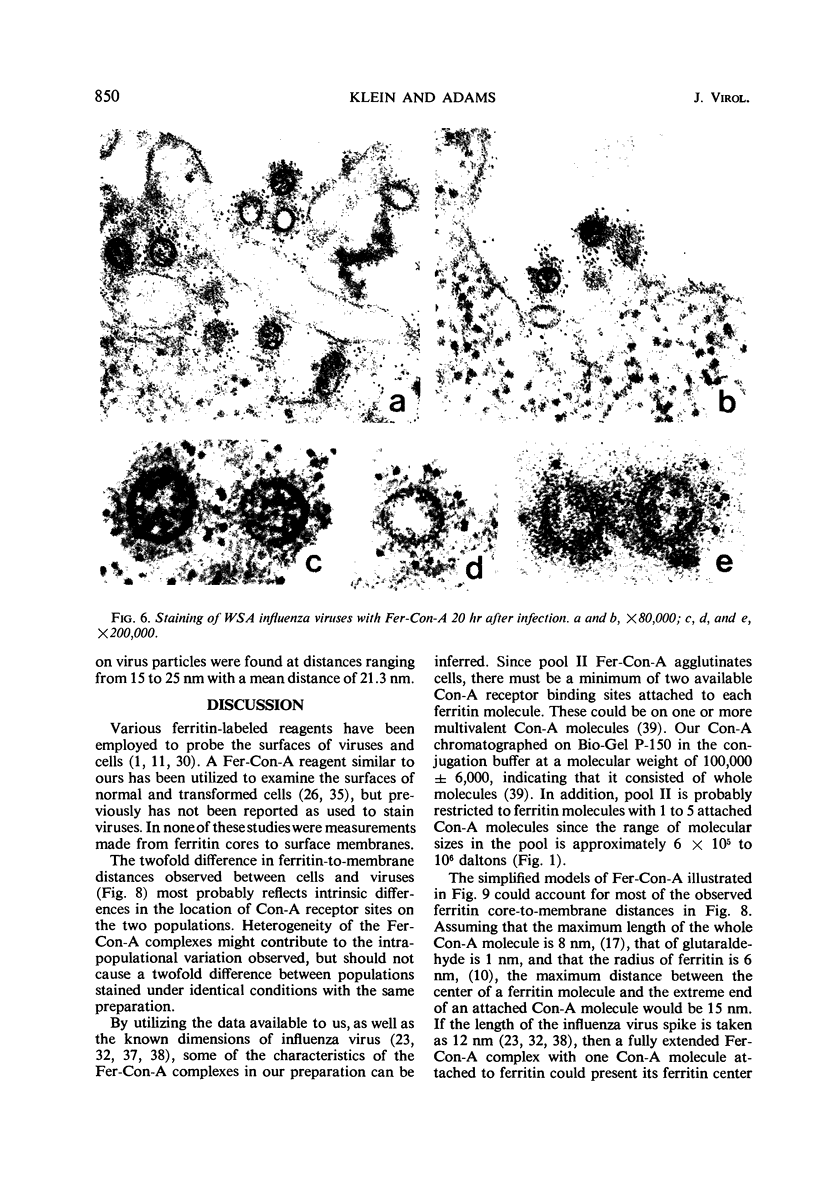
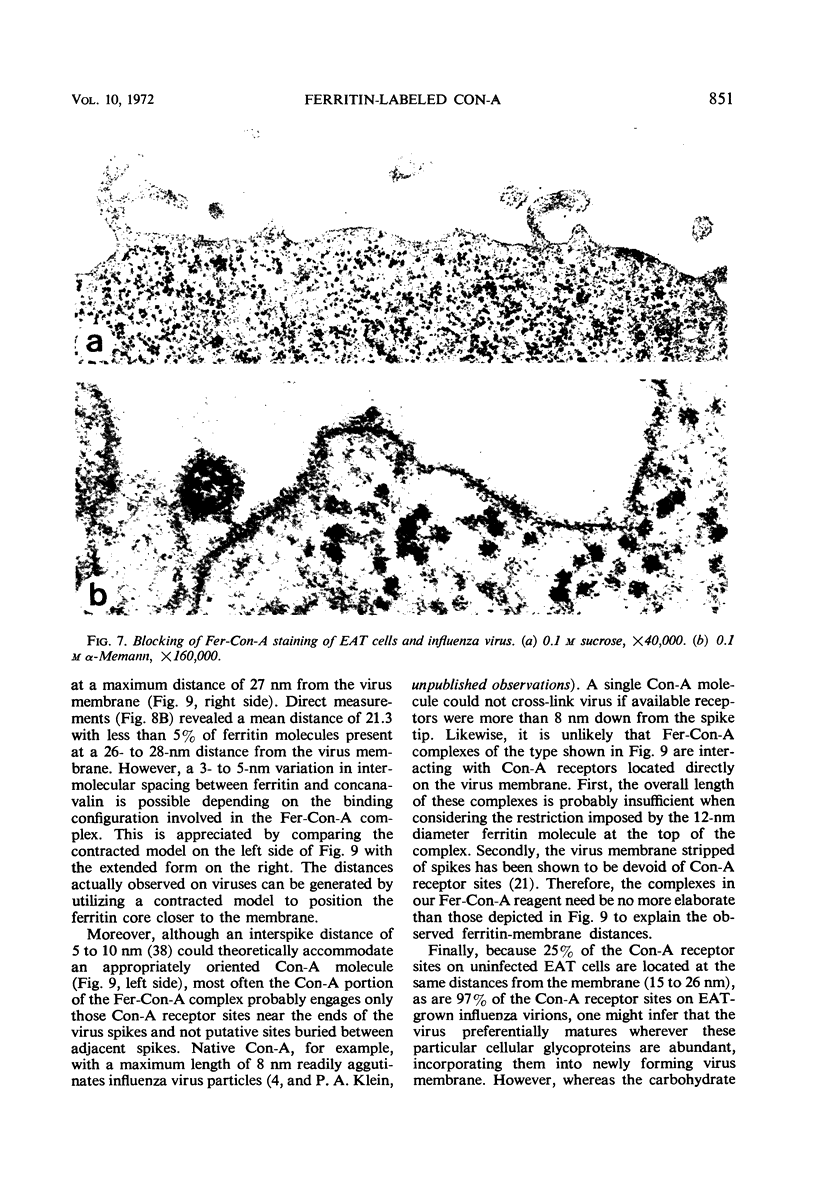
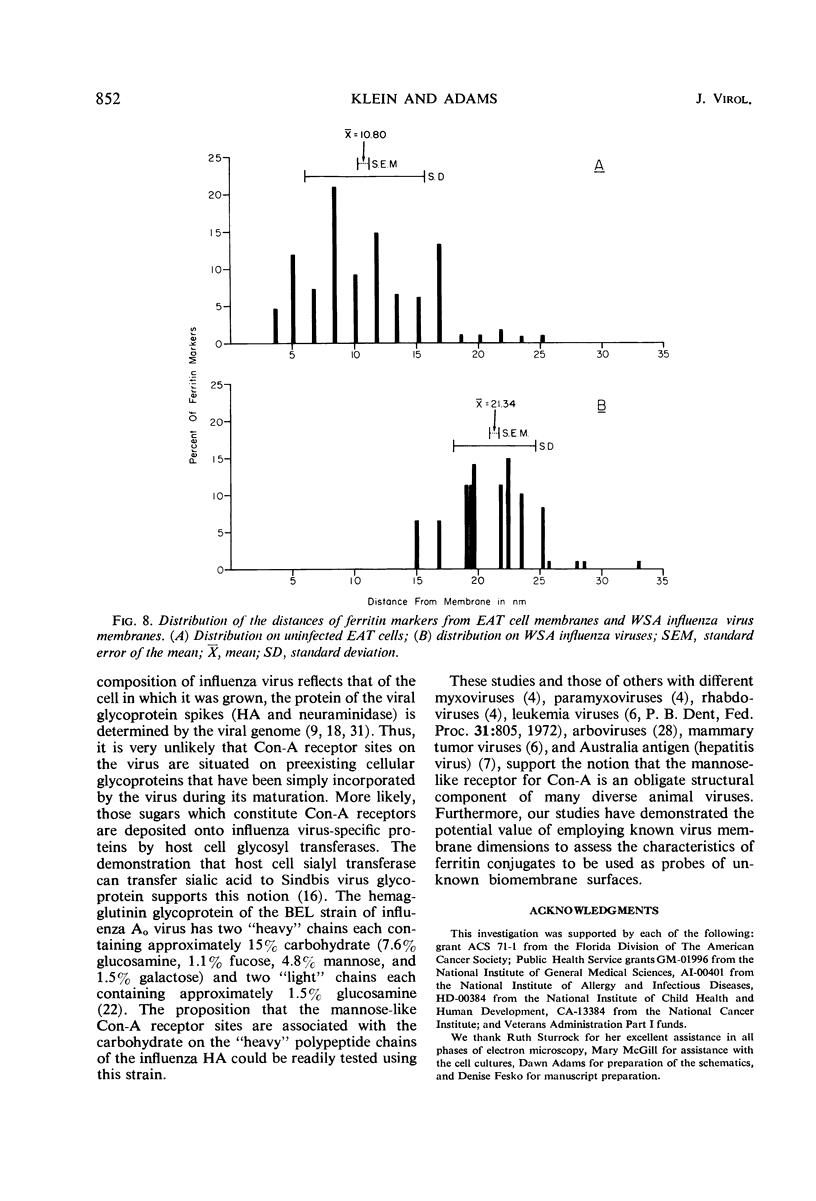


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Takahashi T. Viral and cellular surface antigens of murine leukemias and myelomas. Serological analysis by immunoelectron microscopy. J Exp Med. 1972 Mar 1;135(3):443–457. doi: 10.1084/jem.135.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt-Jovin D. J., Berg P. Quantitative binding of 125 I-concanavalin A to normal and transformed cells. J Virol. 1971 Nov;8(5):716–721. doi: 10.1128/jvi.8.5.716-721.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becht H., Rott R., Klenk H. D. Effect of Concanavalin A on cells infected with enveloped RNA viruses. J Gen Virol. 1972 Jan;14(1):1–8. doi: 10.1099/0022-1317-14-1-1. [DOI] [PubMed] [Google Scholar]
- Bächi T., Gerhard W., Lindenmann J., Mühlethaler K. Morphogenesis of influenza A virus in Ehrlich ascites tumor cells as revealed by thin-sectioning and freeze-etching. J Virol. 1969 Nov;4(5):769–776. doi: 10.1128/jvi.4.5.769-776.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat J., Hageman P. C. Binding of Concanavalin A to the envelope of two murine RNA tumour viruses. J Gen Virol. 1972 Jan;14(1):103–106. doi: 10.1099/0022-1317-14-1-103. [DOI] [PubMed] [Google Scholar]
- Cawley L. P. Reaction between concanavalin A and the Australia antigen. Am J Clin Pathol. 1972 Feb;57(2):253–253. doi: 10.1093/ajcp/57.2.253. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Livingston D. C. Binding of 3 H-concanavalin A by normal and transformed cells. Nat New Biol. 1971 Aug 4;232(31):155–156. doi: 10.1038/newbio232155a0. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Crichton R. R. Ferritin: structure, synthesis and function. N Engl J Med. 1971 Jun 24;284(25):1413–1422. doi: 10.1056/NEJM197106242842506. [DOI] [PubMed] [Google Scholar]
- Feller U., Dougherty R. M., Di Stefano H. S. Comparative morphology of avian and murine leukemia viruses. J Natl Cancer Inst. 1971 Dec;47(6):1289–1298. [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., MERRICK J. M. PROTEIN-CARBOHYDRATE INTERACTION. I. THE INTERACTION OF POLYSACCHARIDES WITH CONCANAVALIN A. Biochim Biophys Acta. 1965 Jan 4;97:68–76. doi: 10.1016/0304-4165(65)90270-9. [DOI] [PubMed] [Google Scholar]
- Gerhard W. Die Vermehrung eines tumoradaptierten Stammes von Influenza A 0 -Virus in Suspensionen von Ehrlich-Aszites-Tumorzellen in vitro. Pathol Microbiol (Basel) 1971;37(2):132–156. [PubMed] [Google Scholar]
- Goldstein I. J., So L. L. Protein-carbonhydrate interaction. 3. Agar gel-diffusion studies on the interaction of Concanavalin A, a lectin isolated from jack bean, with polysaccharides. Arch Biochem Biophys. 1965 Aug;111(2):407–414. doi: 10.1016/0003-9861(65)90203-1. [DOI] [PubMed] [Google Scholar]
- Grimes W. J., Burge B. W. Modification of Sindbis virus glycoprotein by host-specified glycosyl transferases. J Virol. 1971 Mar;7(3):309–313. doi: 10.1128/jvi.7.3.309-313.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman K. D., Wood M. K., Schiffer M., Edmundson A. B., Ainsworth C. F. Structure of concanavalin A at 4.25-ångström resolution. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1393–1397. doi: 10.1073/pnas.68.7.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Influenza virus effects on cell membrane proteins. Science. 1970 Jan 9;167(3915):202–205. doi: 10.1126/science.167.3915.202. [DOI] [PubMed] [Google Scholar]
- Inbar M., Ben-Bassat H., Sachs L. Membrane changes associated with malignancy. Nat New Biol. 1972 Mar 1;236(61):3–passim. doi: 10.1038/newbio236003a0. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Becht H. On the structure of the influenza virus envelope. Virology. 1972 Mar;47(3):579–591. doi: 10.1016/0042-6822(72)90547-8. [DOI] [PubMed] [Google Scholar]
- Laver W. G. Separation of two polypeptide chains from the hemagglutinin subunit of influenza virus. Virology. 1971 Jul;45(1):275–288. doi: 10.1016/0042-6822(71)90134-6. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- Lindenmann J., Klein P. A. Viral oncolysis: increased immunogenicity of host cell antigen associated with influenza virus. J Exp Med. 1967 Jul 1;126(1):93–108. doi: 10.1084/jem.126.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. G., Temin H. M. Lack of correlation between conversion of RNA tumour viruses and increased agglutinability of cells by concanavalin A and wheat germ agglutinin. Nature. 1971 May 14;231(5298):117–118. doi: 10.1038/231117a0. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Singer S. J. Ferritin-conjugated plant agglutinins as specific saccharide stains for electron microscopy: application to saccharides bound to cell membranes. Proc Natl Acad Sci U S A. 1971 May;68(5):942–945. doi: 10.1073/pnas.68.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Ellwood D. C., Appleyard G., Stanley J. L. Agglutination of an arbovirus by concanavalin A. Nat New Biol. 1971 Sep 8;233(36):50–51. doi: 10.1038/newbio233050a0. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Sambrook J. Binding of radioactively labelled concanavalin A and wheat germ agglutinin to normal and virus-transformed cells. Nat New Biol. 1971 Aug 4;232(31):156–160. doi: 10.1038/newbio232156a0. [DOI] [PubMed] [Google Scholar]
- Parsons D. F., Subjeck J. R. The morphology of the polysaccharide coat of mammalian cells. Biochim Biophys Acta. 1972 Feb 14;265(1):85–113. doi: 10.1016/0304-4157(72)90020-2. [DOI] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. I. The polypeptides of the virion. Virology. 1970 Dec;42(4):890–904. doi: 10.1016/0042-6822(70)90338-7. [DOI] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. II. A model based on the morphology and composition of subviral particles. Virology. 1972 Jan;47(1):181–196. doi: 10.1016/0042-6822(72)90251-6. [DOI] [PubMed] [Google Scholar]
- Shoham J., Inbar M., Sachs L. Differential toxicity on normal and transformed cells in vitro and inhibition of tumour development in vivo by concanavalin A. Nature. 1970 Sep 19;227(5264):1244–1246. doi: 10.1038/2271244a0. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C. The pattern of binding of fluorescein-labeled concanavalin A to the motile lymphocyte. J Reticuloendothel Soc. 1970 Nov;8(5):458–464. [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Tevethia S. S., Lowry S., Rawls W. E., Melnick J. L., McMillan V. Detection of early cell surface changes in herpes simplex virus infected cells by agglutination with concanavalin A. J Gen Virol. 1972 Apr;15(1):93–97. doi: 10.1099/0022-1317-15-1-93. [DOI] [PubMed] [Google Scholar]
- Tiffany J. M., Blough H. A. Estimation of the number of surface projections on myxo- and paramyxoviruses. Virology. 1970 Jun;41(2):392–394. doi: 10.1016/0042-6822(70)90096-6. [DOI] [PubMed] [Google Scholar]
- Tiffany J. M., Blough H. A. Models of structure of the envelope of influenza virus. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1105–1112. doi: 10.1073/pnas.65.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L., Cunningham B. A., Edelman G. M. Unusual fragments in the subunit structure of concanavalin A. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1130–1134. doi: 10.1073/pnas.68.6.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Tevethia S. S. Expression of concanavalin A binding sites in rabbit kidney cells infected with vaccinia virus. Virology. 1971 Jul;45(1):313–316. doi: 10.1016/0042-6822(71)90140-1. [DOI] [PubMed] [Google Scholar]