Abstract
Chest CT scans are often used to monitor patients after excision of a sarcoma. Although sensitive, CT scans are more expensive than chest radiographs and are associated with possible health risks from a higher radiation dose. We hypothesized that a program based upon limited CT scans in lower-grade sarcoma could be efficacious and less expensive. We retrospectively assigned patients to a high-risk or low-risk hypothetical protocol. Eighty-three low- or intermediate-grade soft tissue sarcomas met our inclusion criteria. Eight patients had pulmonary metastasis. A protocol based on selective CT scans for high-risk patients would have identified seven out of eight lesions. The incremental cost-effectiveness ratio for routine CT scans was $731,400. A program based upon selective CT scans for higher-risk patients is accurate, spares unnecessary radiation to many patients, and is less expensive.
Introduction
The identification of pulmonary metastatic disease is important, as its presence or absence affects treatment and prognosis.1-14 The two primary imaging options are chest roentgenograms and computed tomography (CT). Radiographs are quick, relatively inexpensive and accessible, and they minimize radiation exposure.15 CT scans expose the patient to higher doses of radiation and are more expensive.16-20 However, the detail and information provided in a CT scan is superior to a radiograph.15, 21, 22 The preferred method of pulmonary surveillance in soft tissue sarcoma remains controversial.23-31
Elimination of unnecessary CT scans would be beneficial to patient safety and cost savings. Recent reports in the literature have raised concerns about a causative effect with excessive radiation exposure from CT scans and subsequent development of malignancy.16, 19 In this tumultuous era of health care reform, there is continuing pressure to eliminate superfluous diagnostic studies and interventions. It is an appropriate time to make a real effort in determining the most effective form and frequency of monitoring post-resection sarcoma patients.
Our hypothesis was that CT scans are over utilized in low-risk patients. We questioned whether CT scans could be used selectively while retaining overall efficacy. We determined risk factors for pulmonary metastasis to aid in the hypothetical implementation of selective CT scans. Any reduction in CT scans would be expected to result in a cost benefit.
Materials and Methods
We identified 139 low- and intermediate-grade soft tissue sarcomas resected for cure and monitored postoperatively at our institution from March 1994 to April 2008. Patient charts, including clinical notes, operative notes, pathology reports, and radiology reports were reviewed for completeness of documentation. All included patients required an official pathology report from our institution stating the diagnosis, grade, and margin. When a grading system was reported, grades 1-2/3 and 1-2/4 were allowed into the study. Two years of radiographic follow-up were required. We excluded patients with high-grade sarcoma, metastatic disease at diagnosis, absent pathology reports, retroperitoneal location, and primary bone sarcoma. Patients with local recurrence or distant metastasis after their initial surgery were included regardless of length of follow-up. This study was approved by our Institutional Review Board.
The algorithm for pulmonary surveillance currently used at our institution for low- and intermediate-grade lesions is a CT scan at baseline and every four months for years 1 and 2, every six months for years 3 through 5, and a chest radiograph annually after five years. The primary outcome measures were evidence of local recurrence, distant lung metastasis, distant non-lung metastasis, or no evidence of disease. All local recurrences were confirmed histologically. Lung and non-lung metastases were either confirmed histologically or had progressed with serial imaging studies consistent with metastatic disease.
We compared patients with pulmonary metastases to those without, to determine corresponding risk factors. Chi-square or Fisher's exact test were used for statistical analysis for nominal variables depending on the group size. Continuous variables were analyzed using t tests. Logistic regression was performed in an attempt to elucidate further associations between independent variables.
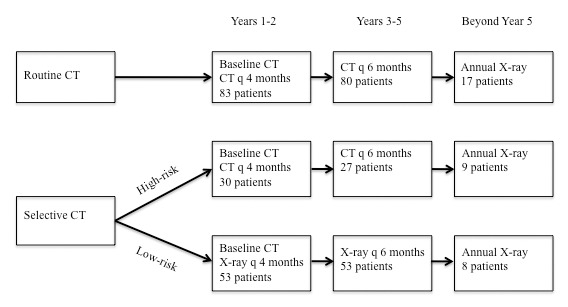
Factors from our outcome analysis that had p values < 0.05 were considered characteristics that placed patients at high risk for developing pulmonary metastasis. We then retrospectively “assigned” our cohort into a low-risk, chest radiograph-based protocol, or a highrisk, routine chest CT-based protocol (Fig 1). We then re-analyzed the patients with pulmonary metastasis to see if their lesions would have been accurately identified using hypothetical selective CT scans. A low-risk protocol based upon chest radiographs was selected for comparison simply because it is the most reasonable alternative to chest CT scans. 24, 26, 31 We did not attempt to compare the diagnostic ability of chest CT scans and chest radiographs.
Figure 1. Flowchart detailing the allocation of patients in the routine and selective CT protocols.
We performed a cost analysis by using the Incremental Cost-Effectiveness Ratio (ICER). This tool has previously been implemented for comparisons such as this.32, 33 It is calculated by dividing the difference in the costs of the test per patient by the gain in diagnostic yield. This value represents the spending required to identify each additional lesion in the higher-yield protocol. Our institution's current hospital and professional fees for a chest CT without contrast is $1,571. The cost for a PA/lateral chest radiograph is $191. We also performed a sensitivity analysis to look at the change in ICER given two separate assumptions. The first assumption is that the cost of a chest CT is half as much as it is currently; the second, that the yield of the selective CT protocol is only half as much as with routine CT scans for all patients.
Results
We initially found 139 low- and intermediate-grade soft tissue sarcomas that met our inclusion criteria. Twentytwo patients had dermatofibrosarcoma protuberans (DFSP) and 30 had well-differentiated liposarcoma. None of these had pulmonary metastases. The documented pulmonary metastatic rates of these tumors are so low4, 34, 35 that we did not think it appropriate to include them in our analysis. Four patients died of non-oncologic processes at less than two years, leaving a cohort of 83 patients for analysis.
There were 47 females and 36 males. The median age was 52.1 years (range 11-86 years). The median radiographic follow-up was 4.4 years (range 0.6-14.9 years) with a minimum of two years in patients without local recurrence or distant metastasis. There were 46 low-grade and 37 intermediate-grade lesions. The histologic diagnoses consisted of liposarcoma (28), fibrosarcoma (27), malignant fibrous histiocytoma (8), leiomyosarcoma (8), spindle cell sarcoma (3), hemangiopericytoma (3), hemangioendothelioma (2), malignant giant cell tumor of soft parts (2), and malignant peripheral nerve sheath tumor (2). Both of the hemangioendotheliomas were isolated lesions. For purposes of analysis, “lipomatous and fibrous tumors” included any histologic diagnosis of liposarcoma, fibrosarcoma, malignant fibrous histiocytoma, and their variants.
In our cohort of 83 patients, we noted local recurrence, distant metastases, and/or nodal spread in 20 patients. Twelve patients had low-grade tumors and eight had intermediate-grade. Eight patients had pulmonary metastases (9.6%) (Table 1). The median time to pulmonary metastasis was 13.2 months (Table 2). All of the pulmonary metastases were discovered during routine surveillance and none of the patients were symptomatic.
Table 1.
Oncologic recurrence and spread by histology
| Histology | Total number | Local recurrence | Pulmonary metastasis | Non-lung distance metastasis | Nodal metastasis | Any recurrence or metastasis |
|---|---|---|---|---|---|---|
| Liposarcoma | ||||||
| Myxoid | 21 | 0 | 1 | 4 | 0 | 4 |
| Non myxoid | 7 | 0 | 0 | 0 | 0 | 0 |
| MFH | 8 | 1 | 0 | 0 | 0 | 1 |
| Fibrosarcoma | ||||||
| Myxofibrosarcoma | 14 | 2 | 1 | 1 | 0 | 3 |
| Fibromyxoid sarcoma | 8 | 1 | 0 | 0 | 0 | 1 |
| DFSP transformation | 2 | 1 | 0 | 0 | 0 | 1 |
| Myxoinflammatory fibroblastic sarcoma | 2 | 0 | 0 | 0 | 0 | 0 |
| Myofibrosarcoma | 1 | 0 | 0 | 0 | 0 | 0 |
| Leiomyosarcoma | 8 | 2 | 0 | 0 | 0 | 2 |
| Spindle cell sarcoma | 3 | 2 | 1 | 0 | 1 | 2 |
| Hemangiopericytoma | 3 | 1 | 2 | 0 | 2 | 3 |
| Hemangioendothelioma | 2 | 0 | 0 | 0 | 0 | 0 |
| Malignant giant cell tumor of soft parts | 2 | 1 | 2 | 0 | 0 | 2 |
| MPNST | 2 | 1 | 1 | 0 | 0 | 1 |
| All | 83 | 12 | 8 | 5 | 3 | 20 |
MFH – malignant fibrous histiocytoma, DFSP – dermatofibrosarcoma protuberans, MPNST – malignant peripheral nerve sheath tumor
Table 2.
Details of patients with pulmonary metastasis
| Patient Diagnosis | Other disease1 | Time to metastasis (mo)2 |
|---|---|---|
| 1 Spindle cell sarcoma | Yes | 7.0 |
| 2 Myxoid liposarcoma | Yes | 9.3 |
| 3 Myxofibrosarcoma | Yes | 20.0 |
| 4 Malignant giant cell tumor of soft parts | No | 3.4 |
| 5 Hemangiopericytoma | Yes | 11.2 |
| 6 Malignant giant cell tumor of soft parts | Yes | 56.4 |
| 7 MPNST | Yes | 73.6 |
| 8 Hemangiopericytoma | No | 15.2 |
1Local recurrence or non-lung metastasis
2Measured from date of primary tumor resection MPNST – malignant peripheral nerve sheath tumor
We analyzed various tumor and patient characteristics in an attempt to quantify risk factors for pulmonary metastasis in order to determine when a selective CT scan should be performed (Table 3). Notable differences were found with regard to the presence of local recurrence (p = 0.013), non-lung metastasis (p = 0.002), and histology (p = 0.002). Patients with these clinical attributes were considered at high risk for metastases and placed into the selective CT limb of our hypothetical analysis. Although patients with local recurrence or distant metastasis would initially be started on the low-risk protocol, clinically, and then switched over when disease progression was recognized, for ease of comparison we assigned them to the hypothetical high-risk algorithm at the time of definitive resection.
Table 3.
Patient and tumor characteristics for pulmonary metastasis
| Variable | Pulmonary metastasis | No pulmonary metastasis | P value |
|---|---|---|---|
| Age | |||
| Median (range) | 51.6 (39-86) | 52.1 (11-80) | 0.375 |
| Mean (std dev) | 57.7 (16.0) | 50.1 (17.9) | |
| Sex | |||
| Male | 5 | 31 | 0.285 |
| Female | 3 | 44 | |
| Grade | |||
| Low | 6 | 40 | 0.289 |
| Intermediate | 2 | 35 | |
| Size | |||
| >5 cm | 6 | 27 | 0.134 |
| <5 cm | 2 | 36 | |
| Depth | |||
| Deep | 7 | 55 | 0.673 |
| Superficial | 1 | 20 | |
| Location | |||
| Axial | 1 | 3 | 0.565 |
| Lower extremity | 5 | 52 | |
| Upper extremity | 2 | 20 | |
| Proximal limb/trunk | 4 | 53 | 0.251 |
| Distal limb | 4 | 22 | |
| Margins | |||
| Intralesional | 1 | 5 | 0.732 |
| Marginal | 2 | 27 | |
| Wide | 5 | 43 | |
| Outside excision | |||
| Yes | 2 | 32 | 0.462 |
| No | 6 | 43 | |
| Procedure | |||
| Primary excision | 6 | 42 | 0.458 |
| Tumor bed re-excision | 2 | 33 | |
| Recurrence on | |||
| presentation | |||
| Yes | 2 | 7 | 0.207 |
| No | 6 | 68 | |
| Radiation | |||
| Yes | 7 | 38 | 0.065 |
| No | 1 | 37 | |
| Local recurrence | |||
| Yes | 4 | 8 | 0.013 |
| No | 4 | 67 | |
| Non-lung metastasis | |||
| Yes | 4 | 4 | 0.002 |
| No | 4 | 71 | |
| Histology | |||
| Lipomatous /Fibrous | 2 | 61 | 0.002 |
| Other | 6 | 14 |
Our current algorithm of routine CT scans identified eight patients with pulmonary metastasis. Our hypothetical selective CT protocol would have identified all but one of these lesions. Seven of these patients would have been labeled high-risk, six for unusual histology and one for a nodal metastasis prior to pulmonary dissemination. In the remaining patient (patient 3), selective CT scans would not have been implemented. In reality, a surveillance CT scan showed diffuse pulmonary and abdominal metastases without a concurrent radiograph, and success or failure of our algorithm in this patient is unclear.
Using institution-specific administrative claims data from 2009, the cost per patient of the CT-based protocol was $15,289, compared to the selective CT protocol of $6,477. The yield of metastatic detection of the CT-based protocol was 9.6% (8/83), compared to 8.4% for selective CTs (7/83). This results in an incremental cost-effectiveness ratio of $731,400. This is the cost to identify an additional case of pulmonary metastasis using a routine, rather than a selective, CT protocol. The sensitivity analysis investigates how the ICER changes, given different assumptions (Table 4).
Table 4.
ICER calculation and sensitivity analysis
| Assumption | Cost per patient ($) | Yield | ICER ($) | |
| Current data | Routine CT Selective CT | 16,860 8,048 | 0.096 0.084 | 731,400 |
| CT half as expensive | Routine CT Selective CT | 9,298 5,502 | 0.096 0.084 | 315,085 |
| Selective CT yield decreased | Routine CT Selective CT | 16,860 8,047 | 0.096 0.048 | 182,850 |
Discussion
Although recommendations pertaining to pulmonary surveillance after sarcoma excision exist, no uniform regimen is agreed upon. In higher-grade sarcoma, where tumor progression can be quick and relentless, close monitoring is warranted. This is not necessarily the case in low-grade lesions. We retrospectively separated our cohort of patients with lower-grade sarcoma into a low-risk or high-risk protocol in an attempt to minimize the number of CT scans. Our goals were to compare the diagnostic yield of selective and routine CT scans, and to calculate the incremental cost-effectiveness ratio. This would provide greater insight into the implications of limiting the number of CT scans for pulmonary surveillance.
We found an overall rate of pulmonary metastasis of 9.6%. There have been several investigations documenting the incidence of metastatic disease in soft tissue sarcoma with rates of 22-40%.1, 3, 7, 9, 11-13, 31 When low-grade (grade 1) sarcomas are observed individually, the reported rates are 6-9%.1-3 Pulmonary metastases represent 55-85% of all distant metastases1, 36; in our study, eight of the 11 patients with distant disease had pulmonary metastases. We chose a minimum radiographic followup of two years. Previous studies have documented that 75-80% of distant metastasis occur within this time.10, 11
Several authors have advocated minimal surveillance in low-grade sarcoma. Whooley et al concluded that chest radiographs were sufficiently effective for pulmonary surveillance and questioned whether early detection with CT results in any survival benefit.24, 31 Kane et al felt that chest x-rays annually were sufficient for the monitoring of low-risk lesions.26 Lord et al actually questioned the necessity of any radiographic follow-up for low-grade tumors.28 The concern with an all-radiograph based protocol is the potential to miss relevant lesions that would be detected by CT scan.
A counter argument is that CT scans are overly sensitive. Reportedly, 80% to more than 90% of non-calcified nodules on screening studies are felt to be benign.17, 21 At least, these findings warrant follow-up examinations - at most, an invasive procedure. Even in lesions that are felt to be worrisome enough to mandate biopsy or resection, 18-55% are found to be false positives.18 In patients with sarcoma, Rissing et al reported that only 28% of those with indeterminate nodules on CT had eventual evidence of metastatic disease, and that patients with lesions <5 mm had equivalent survival to those with completely normal scans.29 Although worrisome nodules in sarcoma patients should be addressed with suspicion, there is still a possibility of subjecting someone to the inherent risks of pulmonary resection or biopsy for an otherwise benign entity.
The consequences of radiation exposure from screening CT scans are becoming significant concerns in the literature and media. A generally accepted, although still debatable, means to assess low-dose radiation risk is the linear non-threshold model,20 in which the risk of radiation-induced malignancy increases incrementally with dose exposure.16 Brenner and Hall estimated that as many as 1.5-2.0% of all cancers in the United States may be caused by radiation exposure from CT scans.19 The risks are cumulative and more pronounced in children.16, 19 The radiation dose from a chest CT scan is estimated to be nearly 100 times greater than a standard posterioranterior and lateral chest radiograph.19 Although the risk of undetected cancer spread is greater than the risk of radiation-induced malignancy in these patients, it is sound practice to minimize unnecessary radiation when possible.
Monetary considerations should never be the sole reason to determine appropriate diagnostic or therapeutic intervention. However, as health care is continually becoming a more limited resource, it is responsible to ensure that we are at least being cost-conscious in our decision-making. Using current pricing, we estimated that it costs over $700,000 to find each additional case of pulmonary metastatic disease with our current routine CT protocol rather than one which utilizes selective CT scans for high-risk patients.
Weaknesses of this study include the heterogeneity in diagnosis and treatment inherent in most studies analyzing outcomes in rare conditions. The grading of tumors is a subjective art, and it is possible that the histologic appearance was initially misinterpreted in the clinically aggressive lesions. However, all slides were reviewed at the time of recurrence or metastasis and the retrospective grading remained consistent even with knowledge of the subsequent clinical course.
The small sample size limits the conclusions we can draw. The number of subjects was somewhat restricted by the nature of our institution as a large referral center requiring a substantial amount of travel for patients in many cases. As some patients elected to be monitored closer to their residence after surgery, our investigation was limited to those who agreed to be monitored under the guidance of our institution. We are not attempting to propose a protocol for generalized acceptance, but simply suggesting that the number of diagnostic CT scans in lower-grade sarcoma may potentially be decreased with few adverse consequences. Again, the lack of definitive conclusions is primarily attributable to the rarity of the condition and cannot be easily overcome in a single institutional study. As there is a paucity of literature on this subject, our hope is that our preliminary data will evoke thought and act as an impetus for continuing efforts to address this important issue with further investigations.
The goal of pulmonary surveillance is to detect lesions in a timely manner so that an intervention that changes the natural history of the disease may be implemented. A secondary goal is to comment on disease status and prognosis. An ideal algorithm would be able to predictably detect metastases, yet minimize the number of studies needed to accomplish these objectives. This is a complex topic which will require well-designed multi-institutional prospective studies to adequately address it. Larger studies will also help to better define criteria for designating high-risk patients. We found that implementation of selective CT scans could reduce unnecessary radiation exposure, accurately detect pulmonary lesions, and decrease the cost of monitoring lower-grade sarcoma.
Acknowledgments
The authors wish to thank Wei Hou, PhD for assistance with the statistical analysis.
Conflict of Interest Statement
We declare that we have no conflicts of interest relevant to this investigation. Dr. Gibbs is a consultant for Zimmer.
References
- Alkis N, Muallaoglu S, Kocer M, et al. Primary adult soft tissue sarcomas: analysis of 294 patients. Med Oncol. Mar 2011;28(1):391–396. doi: 10.1007/s12032-010-9450-2. [DOI] [PubMed] [Google Scholar]
- Coindre JM, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. May 15 2001;91(10):1914–1926. doi: 10.1002/1097-0142(20010515)91:10<1914::aid-cncr1214>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. Mar 1996;14(3):869–877. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- Gaynor JJ, Tan CC, Casper ES, et al. Refinement of clinicopathologic staging for localized soft tissue sarcoma of the extremity: a study of 423 adults. J Clin Oncol. Aug 1992;10(8):1317–1329. doi: 10.1200/JCO.1992.10.8.1317. [DOI] [PubMed] [Google Scholar]
- Goodlad JR, Fletcher CD, Smith MA. Surgical resection of primary soft-tissue sarcoma. Incidence of residual tumour in 95 patients needing re-excision after local resection. J Bone Joint Surg Br. Jul 1996;78(4):658–661. [PubMed] [Google Scholar]
- Noria S, Davis A, Kandel R, et al. Residual disease following unplanned excision of soft-tissue sarcoma of an extremity. J Bone Joint Surg Am. May 1996;78(5):650–655. doi: 10.2106/00004623-199605000-00003. [DOI] [PubMed] [Google Scholar]
- Gronchi A, Casali PG, Mariani L, et al. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: a series of patients treated at a single institution. J Clin Oncol. Jan 1 2005;23(1):96–104. doi: 10.1200/JCO.2005.04.160. [DOI] [PubMed] [Google Scholar]
- Liu CY, Yen CC, Chen WM, et al. Soft Tissue Sarcoma of Extremities: The Prognostic Significance of Adequate Surgical Margins in Primary Operation and Reoperation After Recurrence. Ann Surg Oncol. Aug 2010;17(8):2102–2111. doi: 10.1245/s10434-010-0997-0. [DOI] [PubMed] [Google Scholar]
- Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. May 1996;14(5):1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- Shiu MH, Castro EB, Hajdu SI, Fortner JG. Surgical treatment of 297 soft tissue sarcomas of the lower extremity. Ann Surg. Nov 1975;182(5):597–602. doi: 10.1097/00000658-197511000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songur N, Dinc M, Ozdilekcan C, Eke S, Ok U, Oz M. Analysis of lung metastases in patients with primary extremity sarcoma. Sarcoma. 2003;7(2):63–67. doi: 10.1080/13577140310001607284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trovik CS, Bauer HC, Alvegard TA, et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer. Apr 2000;36(6):710–716. doi: 10.1016/s0959-8049(99)00287-7. [DOI] [PubMed] [Google Scholar]
- Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: improved knowledge with unchanged survival over time. J Clin Oncol. Jul 15 2003;21(14):2719–2725. doi: 10.1200/JCO.2003.02.026. [DOI] [PubMed] [Google Scholar]
- Zornig C, Peiper M, Schroder S. Re-excision of soft tissue sarcoma after inadequate initial operation. Br J Surg. Feb 1995;82(2):278–279. doi: 10.1002/bjs.1800820247. [DOI] [PubMed] [Google Scholar]
- Christie-Large M, James SL, Tiessen L, Davies AM, Grimer RJ. Imaging strategy for detecting lung metastases at presentation in patients with soft tissue sarcomas. Eur J Cancer. Sep 2008;44(13):1841–1845. doi: 10.1016/j.ejca.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Huppmann MV, Johnson WB, Javitt MC. Radiation risks from exposure to chest computed tomography. Semin Ultrasound CT MR. Feb 2010;31(1):14–28. doi: 10.1053/j.sult.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Black C, de Verteuil R, Walker S, et al. Population screening for lung cancer using computed tomography, is there evidence of clinical effectiveness? A systematic review of the literature. Thorax. Feb 2007;62(2):131–138. doi: 10.1136/thx.2006.064659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett JR, Midthun DE. Commentary: CT screening for lung cancer--caveat emptor. Oncologist. Apr 2008;13(4):439–444. doi: 10.1634/theoncologist.2008-0027. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. Nov 29 2007;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- Preston RJ. Update on linear non-threshold doseresponse model and implications for diagnostic radiology procedures. Health Phys. Nov 2008;95(5):541–546. doi: 10.1097/01.HP.0000326332.80829.63. [DOI] [PubMed] [Google Scholar]
- Chalmers N, Best JJ. The significance of pulmonary nodules detected by CT but not by chest radiography in tumour staging. Clin Radiol. Dec 1991;44(6):410–412. doi: 10.1016/s0009-9260(05)80661-0. [DOI] [PubMed] [Google Scholar]
- Chang AE, Schaner EG, Conkle DM, Flye MW, Doppman JL, Rosenberg SA. Evaluation of computed tomography in the detection of pulmonary metastases: a prospective study. Cancer. Mar 1979;43(3):913–916. doi: 10.1002/1097-0142(197903)43:3<913::aid-cncr2820430319>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Casali PG, Jost L, Sleijfer S, Verweij J, Blay JY. Soft tissue sarcomas: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. May 2008;19 Suppl 2(ii):89–93. doi: 10.1093/annonc/mdn101. [DOI] [PubMed] [Google Scholar]
- Whooley BP, Mooney MM, Gibbs JF, Kraybill WG. Effective follow-up strategies in soft tissue sarcoma. Semin Surg Oncol. Jul-Aug 1999;17(1):83–87. doi: 10.1002/(sici)1098-2388(199907/08)17:1<83::aid-ssu11>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Cool P, Grimer R, Rees R. Surveillance in patients with sarcoma of the extremities. Eur J Surg Oncol. Nov 2005;31(9):1020–1024. doi: 10.1016/j.ejso.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Kane JM 3rd. Surveillance strategies for patients following surgical resection of soft tissue sarcomas. Curr Opin Oncol. Jul 2004;16(4):328–332. doi: 10.1097/01.cco.0000127879.62254.d3. [DOI] [PubMed] [Google Scholar]
- James SL, Davies AM. Post-operative imaging of soft tissue sarcomas. Cancer Imaging. Feb 27 2008;8:8–18. doi: 10.1102/1470-7330.2008.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord HK, Salter DM, MacDougall RH, Kerr GR. Is routine chest radiography a useful test in the follow up of all adult patients with soft tissue sarcoma? Br J Radiol. Oct 2006;79(946):799–800. doi: 10.1259/bjr/69175634. [DOI] [PubMed] [Google Scholar]
- Rissing S, Rougraff BT, Davis K. Indeterminate pulmonary nodules in patients with sarcoma affect survival. Clin Orthop Relat Res. Jun 2007;459:118–121. doi: 10.1097/BLO.0b013e31805d8606. [DOI] [PubMed] [Google Scholar]
- Sakata K, Johnson FE, Beitler AL, Kraybill WG, Virgo KS. Extremity soft tissue sarcoma patient follow-up: tumor grade and size affect surveillance strategies after potentially curative surgery. Int J Oncol. Jun 2003;22(6):1335–1343. [PubMed] [Google Scholar]
- Whooley BP, Gibbs JF, Mooney MM, McGrath BE, Kraybill WG. Primary extremity sarcoma: what is the appropriate follow-up? Ann Surg Oncol. Jan-Feb 2000;7(1):9–14. doi: 10.1007/s10434-000-0009-x. [DOI] [PubMed] [Google Scholar]
- Fleming JB, Cantor SB, Varma DG, et al. Utility of chest computed tomography for staging in patients with T1 extremity soft tissue sarcomas. Cancer. Aug 15 2001;92(4):863–868. doi: 10.1002/1097-0142(20010815)92:4<863::aid-cncr1394>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Porter GA, Cantor SB, Ahmad SA, et al. Costeffectiveness of staging computed tomography of the chest in patients with T2 soft tissue sarcomas. Cancer. Jan 1 2002;94(1):197–204. doi: 10.1002/cncr.10184. [DOI] [PubMed] [Google Scholar]
- Lemm D, Mugge LO, Mentzel T, Hoffken K. Current treatment options in dermatofibrosarcoma protuberans. J Cancer Res Clin Oncol. May 2009;135(5):653–665. doi: 10.1007/s00432-009-0550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HL. Atypical lipomatous tumor, its variants, and its combined forms: a study of 61 cases, with a minimum follow-up of 10 years. Am J Surg Pathol. Jan 2007;31(1):1–14. doi: 10.1097/01.pas.0000213406.95440.7a. [DOI] [PubMed] [Google Scholar]
- Estourgie SH, Nielsen GP, Ott MJ. Metastatic patterns of extremity myxoid liposarcoma and their outcome. J Surg Oncol. Jun 2002;80(2):89–93. doi: 10.1002/jso.10093. [DOI] [PubMed] [Google Scholar]


