Abstract
Study Design/Setting
Randomized, controlled study in a laboratory setting. Blinded observations/assessment of study outcomes.
Objective
The Purpose of this study is to determine the performance characteristics of MASTERGRAFT® STRIP with bone marrow aspirate (BMA) as a bone graft extender in a rabbit posterolateral spine fusion model.
Summary of Background Data
The rabbit posterolateral fusion model is an established environment for testing of fusion concepts. It offers the opportunity to obtain radiographic, histological, and biomechanical data on novel fusion materials.
Methods
Thirty six rabbits were entered into the study with 34 used for analysis. Bilateral posterolateral lumbar intertransverse fusions were performed at L5-L6. the lateral two thirds of the transverse processes were decorticated and covered with graft material: autograft only (2.5 – 3.0 cc/side), 75% MASTERGRAFT® STRIP + 5.0cc BMA / 25% autograft (3.0cc total per side), or 50% MASTERGRAFT® STRIP + 5.0cc BMA and 50% autograft (3.0cc total per side). Animals were humanely euthanized at 8 weeks post surgery.
Results
The autograft group had a 60% radiographic fusion rate (6/10) and a manual palpation fusion rate of 50% (5/10). the 50% MASTERGRAFT® STRIP group had a 75% radiographic and manual palpation fusion rate (9/12). the 75% MASTERGRAFT® STRIP group demonstrated a 58% (7/12) radiographic and manual palpation fusion rate. Histologically, no adverse inflammatory reactions of significant size were present. The two MASTERGRAFT® STRIP groups demonstrated a tendency towards more bone development across the fusion bed.
Conclusions
In this commonly used rabbit posterolateral fusion model, MASTERGRAFT® STRIP with BMA in an autograft extender mode produces biomechanical and radiographic results similar to autograft fusion alone.
Introduction
Iliac crest autograft is considered the gold standard graft material despite limitations in the quantity available and complications associated with the harvesting procedure1–12. These disadvantages have motivated investigators to seek alternative graft materials to extend, enhance, and/or substitute for autograft. Examples of such alternatives include: allografts, synthetic materials, and recombinant human bone morphogenetic proteins (BMPs).
Many such products have been in clinical use for years. As the number and complexity of these options grow, so does the need for scientific studies that examine evidence for methods of use, appropriate volume/percentages of graft material, and local biocompability/fusion results. Such studies have the opportunity to add to the collective knowledge of safe and efficacious use.
The current study was performed to assess MASTERGRAFT® STRIP as an autograft extender in the rabbit lumbar posterolateral fusion model. Fusion rates of iliac crest autograft (approximately 3cc/side) were compared to lesser amounts of autograft extended with the investigational “STRIP.”
Methods
The rabbit fusion model is a well accepted tool for evaluating bone graft performance. The surgical procedure involves exposure of the transverse processes between L5/L6, limiting decortication to the lateral regions and grafting with the same material bilaterally. Autograft is harvested from the bilateral iliac crests yielding between 2.5–3. 0cc's per crest, such that approximately 3cc's of graft is placed on each posterolateral graft bed. This procedure typically results in fusion rates around 65%13–18. To evaluate MASTERGRAFT® STRIP as an extender, two groups were compared to autograft alone, STRIP 50:50 (1.5cc STRIP + 1.5cc autograft) and STRIP 75:25 (2.25cc STRIP + 0.75cc Autograft). Each volume of MASTERGRAFT® STRIP was soaked with the same amount of bone marrow aspirate (BMA) which was acquired from the rabbit's proximal tibia.
Skeletally mature New Zealand White Rabbits weighing 4.5–5.5 kg were entered into the study. All procedures were approved by the Institutional Animal Care Use Committee (#0708172) and conducted at The University of Iowa Department of Orthopaedics, Bone Healing Research Lab-Iowa Spine Research Center. Throughout the study, animals were individually caged and monitored daily for signs of pain and discomfort.
Surgical Procedure
All operative procedures were performed in a surgical suite using inhalation anesthesia and aseptic techniques. A preanesthetic dose of Ketamine HCL 26mg/kg, Acepromazine Maleate 0.15mg/kg, and Xylazine HCL 0.78 mg/kg was administered intramuscularly. Surgical anesthesia was maintained with 1.5–2.5% isoflurane delivered in O2. Cardiorespiratory monitoring was continued throughout the procedure.
A parenteral dose of cefazolin (13 mg/kg) was administered for infection prophylaxis preoperatively and then BID for 48 hours post-op.
Rabbits were placed prone on the operating table and surgically prepped with 70% Betadine solution. A single level posterolateral intertransverse process fusion was performed in 36 rabbits (Table 1). A dorsal midline incision, approximately 15 centimeters long, was made from L1 to the sacrum and the soft-tissues overlying the transverse processes (TP) were dissected via separate bilateral fascial incisions. The transverse processes were decorticated with a high-speed burr. At no time were the vertebral bodies decorticated in the gutter of the motion segment.
Table 1.
Experimental Design
| Group | Graft type | Manual Palpation n= | Radiographic Fusion | Histology n= | Sacrifice |
|---|---|---|---|---|---|
| Autograft | Autograft (3.0 cc) | 12 | 12 | 3 | 8 Weeks |
| STRIP 50:50 | STRIP 50% (1.5 cc) soaked with 1.5 cc BMA + Autograft 50% (1.5 cc) | 12 | 12 | 3 | 8 Weeks |
| STRIP 75:25 | STRIP 75% (2.25 cc) soaked with 1.5 cc BMA + Autograft 25% (0.75 cc) | 12 | 12 | 3 | 8 Weeks |
Approximately 3.0 ml of corticocancellous bone graft from the iliac crest was obtained bilaterally as needed depending on the study group. This volume of graft is the maximum amount which can be harvested from the rabbit iliac crest without significant animal morbidity13;19–23. Investigational implant preparation of the MASTERGRAFT® STRIP was done by hydrating the STRIP with corresponding amounts of BMA. In the BMA groups, a 1 mm hole was drilled in the tibial tuberosity. An 18g needle and syringe were used to obtain 5.0 cc of BMA. The hole was then filled with surgical bone wax and the surgical incision closed in a routine manner.
The morselized cancellous bone graft or the combination of STRIP/BMA + autograft was then placed between the transverse processes in the paraspinal bed (3.0 ml per side). The lateral two thirds of the transverse processes were covered with the graft. The MASTERGRAFT® STRIP + autograft combination was 75% MASTERGRAFT® STRIP / 25% autograft or 50% MASTERGRAFT® STRIP and 50% autograft. In each of these groups, the autograft was implanted first in the paraspinal bed and the MASTERGRAFT® STRIP placed over the autograft.
Animals were housed and monitored throughout the study. Animals were humanely euthanized at 8 weeks post surgery, a time point consistent with the published literature of this model19,23–26.
Manual Palpation
The primary outcome used to determine fusion was manual palpation13. After removing the spines, fusion was graded by three independent blinded observers as “fused” if no detectable motion was present at the treated segment when tested in flexion and extension. The fusion was graded as “not fused” if motion was present. Final results were determined by agreement of at least 2 of the 3 observers.
Radiographic Assessment
At time of euthanasia, high resolution images of removed spines were judged by three ‘blinded’ observers for radiographic fusion by evaluating for continuous trabecular bridging between the grafted transverse processes. Density of the grafts limited observers from accurately grading the fusion sites and false positive grades of union was prevalent.
Histology
Three specimens from each group were randomly selected for histological evaluation (Figures. 1–3). Non-decalcified slides were prepared and stained with hematoxylin and eosin. Slides were evaluated for presence of inflammation, extent of graft remodeling, and general observations relevant to bone formation activity. New Bone Formation (NBF) was scored on a 0–3 scale (0 = none detected, 1= small uncommon foci, 2= moderate sized, multiple foci, and 3 = extensive multiple foci). Fusion was scored using a 1–10 scale where a score of 10 was complete bridging of the TPs with mature bone (Table 2).
Figure 1. Autograft Control Histological Analysis.
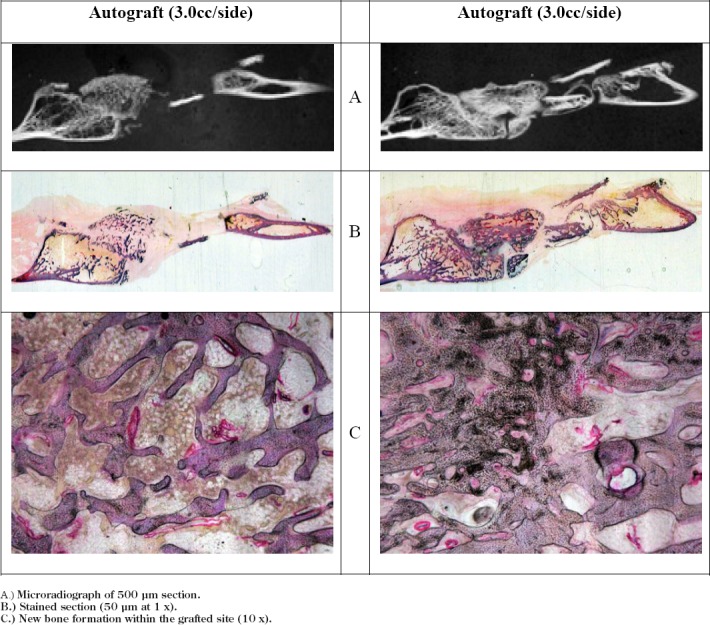
A.) Microradiograph of 500 μm section.
B.) Stained section (50 μm at 1 x).
C.) New bone formation within the grafted site (10 x).
Figure 3. 75%/25% Histological Analysis.
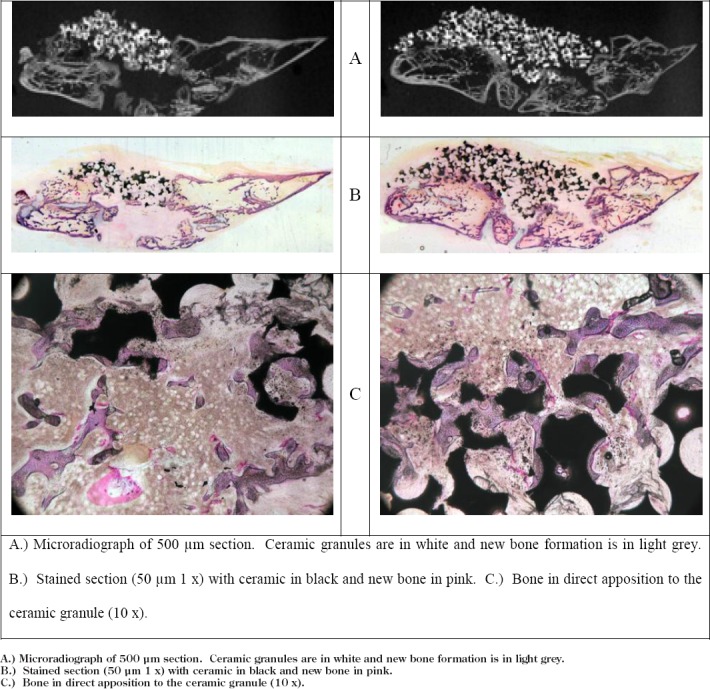
A.) Microradiograph of 500 μm section. Ceramic granules are in white and new bone formation is in light grey.
B.) Stained section (50 μm 1 x) with ceramic in black and new bone in pink.
C.) Bone in direct apposition to the ceramic granule (10 x).
Table 2.
Histologic Scoring Scale for Fusion
| Fusion | Score |
|---|---|
| Union of TPs by mature bone; complete bridge | 10 |
| Union of TPs by immature bone and cartilage; complete bridge | 9 |
| Union of TPs by cartilage with little fibrocartilage | 8 |
| Partial union with more bone (> 75%) than cartilage and fibrocartilage | 7 |
| Partial union with more bone (56% – 75%) than other tissues (i.e., cartilage, fibrocartilage and fibrous tissue) | 6 |
| Partial bridge; ∼equal amounts of bone (45% – 55%) and other tissues (i.e., cartilage, fibrocartilage and fibrous tissue) | 5 |
| Minimal bridge with less bone (25% – 44%) than other tissue (i.e., cartilage, fibrocartilage and fibrous tissue) | 4 |
| Minimal bone (<25%) with predominantly other tissue (i.e., fibrocartilage, predominantly fibrous tissue) | 3 |
| Little new bone with predominantly fibrous tissue | 2 |
| Fibrous tissue only between TP; full (across the defect) | 1 |
Complications
Two (2) rabbits were omitted from the study due to complications.
Figure 2. 50%/50% Histological Analysis.
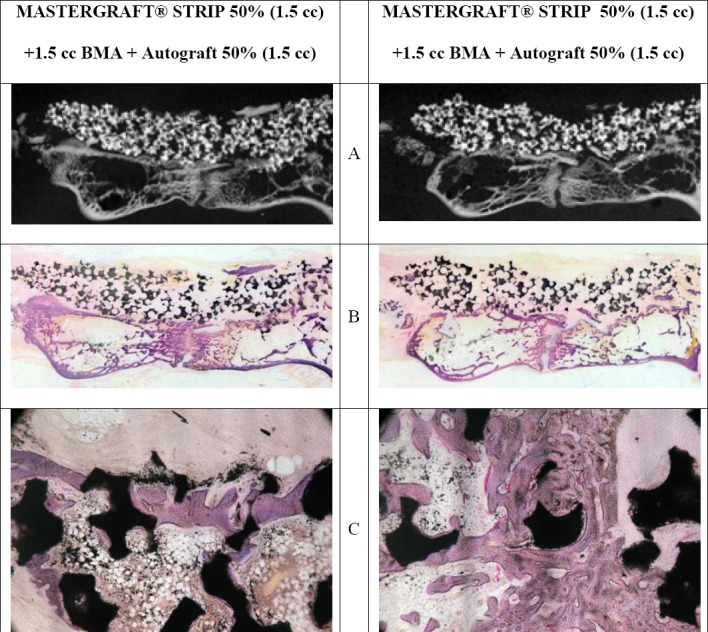
A.) Microradiograph of 500 μm section. Ceramic granules are in white and new bone formation is in light grey.
B.) Stained section (50 μm 1 x) with ceramic in black and new bone in pink.
C.) Bone in direct apposition to the ceramic granule (10 x).
Results
The rabbits tolerated the surgery and study period well and regained normal activity within 24 hours of surgery. Two (2) rabbits were omitted from the study due to post-op complications associated with graft harvest. Necropsy of the animals was unremarkable regardless of treatment. Macroscopic analysis of the paraspinal bed area demonstrated healthy tissue with no apparent adverse effects such as inflamed, necrotic, or decreased vascularized tissue.
Animals were evaluated by manual palpation, radiographic and histological criteria (Table 3 & 4). The autograft group had a 60% radiographic fusion rate (6/10) and a 50% (5/10) fusion rate when scored by manual palpation. The STRIP 50:50 group had a 75% fusion rate by manual palpation and radiographic scoring. The STRIP 75:25 group had a 58% fusion rate by manual palpation and radiographic assessment.
Table 3.
Fusion Results
| Treatment | Manual Palpation Fusion rate | radiographic Fusion rate |
|---|---|---|
| Autograft | 50% (5/10) | 60% (6/10) |
| STRIP 50:50 | 75% (9/12) | 75% (9/12) |
| STRIP 75:25 | 58% (7/12) | 58% (7/12) |
Table 4.
Histological assessment of NBF, inflammation and fusion
| New bone formation (NBF) | Inflammation | Fusion | |
|---|---|---|---|
| MASTERGRAFT ® STRIP / Autograft 50/50 | 2.33 | 3.83 | 7.00 |
| MASTERGRAFT ® STRIP / Autograft 75/25 | 2.17 | 4.00 | 7.00 |
| Autograft | 2.17 | 3.67 | 6.50 |
NBF was scored on a 0–3 scale. Inflammation was scored on a 0-4 scale were a score of 4 indicated no inflammatory response. Fusion was scored using a 1-10 scale where a score of 10 was complete bridging of the TPs with mature bone.
Histology
Blinded histological assessments were made on each histological specimen by a board-certified veterinary pathologist (Table 4, Figures 1–3). There were no adverse inflammatory reactions to the MASTERGRAFT® STRIP regardless of volume of test article. In sections where a mild inflammatory response was noted (2 autograft and 1 MASTERGRAFT® STRIP 50%/autograft 50%), there were uncommon, locally extensive foci devoid of new bone formation with fibrous or loose connective tissue often in an unusual stromal pattern suggestive of resolving or chronic inflammation.
The STRIP treatment groups tended to have more histological evidence of mature/immature bone development across the inter-transverse process spaces than did the autograft controls. In all groups, most new bone growth was regularly seen adjacent to the transverse processes and variably extended across intertransverse process space.
The histologic scores suggested the STRIP 50:50 group had similar bone formation and fusion compared to the STRIP 25/75 group, while both implant groups had increased fusion scores over autograft.
Discussion:
Optimal bone graft substitutes should demonstrate biocompatibility be of consistent quality, be able to promote osseous formation, be cost-effective, and be readily available. MASTERGRAFT® STRIP is a resorbable, malleable, osteoconductive scaffold composed of biphasic calcium phosphate (hydroxyapatite and beta-tricalcium phosphate) ceramic granules and purified fibrillar bovine type I collagen. It is designed to create a favorable environment for bony ingrowth. The objective of this study was to evaluate the efficacy of MASTERGRAFT® STRIP as a bone graft extender in a rabbit bilateral lumbar posterolateral spine fusion model.
This study demonstrates the efficacy of MASTERGRAFT® STRIP with BMA in two separate autograft extender ratios in a commonly used rabbit spinal fusion model. The ability of MASTERGRAFT® STRIP with BMA to be combined with lesser amounts of autograft and achieve similar fusion rates as autograft alone meets the definition for an autograft extender in this model27. The fusion rate of 50% (manual palpation) observed in the Autograft group is consistent with prior studies performed in this laboratory as well as other published studies13–18. The manual palpation fusion rates for both investigational STRIP + BMA extender groups demonstrated similar or slightly better results when compared to Autograft control. As with other radiodense calcium-based bone void fillers, the initial, interval, and final radiographs could over predict the rate of biomechanical fusion as the density of non-remodeled graft material may cause the appearance of fusion by typical radiographic criteria in this model. This finding has been noted in other studies with some calcium-based filler formulations in our lab28–29. CT-based assessments (not performed in the current study) and/or histological assessment may have the potential to be more consistent with the final biomechanical status of the fusion.
Histologically, the results between the 3 groups were similar and not significantly different from one another, remodeling was underway in all groups, and that there was no evidence of adverse inflammatory reactions.
The results of this rabbit study suggest that MASTERGRAFT® STRIP with BMA is effective in producing a posterolateral fusion by radiographic and manual palpation criteria in an extender mode. While animal models cannot be translated into clinically successful human applications, the results of this study suggest that further investigation into use of MASTERGRAFT® STRIP with BMA as an autograft extender in a clinical setting may be appropriate.
Conclusions:
In this well-established rabbit lumbar posterolateral fusion model, MASTERGRAFT® STRIP with BMA was an effective extender of autograft, allowing reduction of autograft by up to 75% while achieving fusion rates equal to or slightly better than autograft alone.
Footnotes
Funding: Portions of this study were funded by a grant to the University of Iowa from Medtronic Sofamor Danek, Memphis, TN.
Device Status: MASTERGRAFT® STRIP is FDA approved as a bone void filler for human use.
Disclosures: The authors report no other conflicts of interest – consultancy agreements, royalties, gifts received, intellectual property with regard to the products (MASTERGRAFT® STRIP) or company (Medtronic) involved in this scientific investigation.
Reference List
- 1.Arrington ED, Smith WJ, Chambers HG, et al. Complications of iliac crest bone graft harvesting. Clin.Orthop.Relat Res. 1996:300–9. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 2.Ebraheim NA, Elgafy H, Xu R. Bone-graft harvesting from iliac and fibular donor sites: techniques and complications. J.Am.Acad.Orthop.Surg. 2001;9:210–8. doi: 10.5435/00124635-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Hu R, Hearn T, Yang J. Bone graft harvest site as a determinant of iliac crest strength. Clin.Orthop.Relat Res. 1995:252–6. [PubMed] [Google Scholar]
- 4.Hu RW, Bohlman HH. Fracture at the iliac bone graft harvest site after fusion of the spine. Clin.Or-thop.Relat Res. 1994:208–13. [PubMed] [Google Scholar]
- 5.Kahn B. Superior gluteal artery laceration, a complication of iliac bone graft surgery. Clin.Orthop.Relat Res. 1979:204–7. [PubMed] [Google Scholar]
- 6.Kurz LT, Garfin SR, Booth RE., Jr Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14:1324–31. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Lim EV, Lavadia WT, Roberts JM. Superior gluteal artery injury during iliac bone grafting for spinal fusion. A case report and literature review. Spine. 1996;21:2376–8. doi: 10.1097/00007632-199610150-00015. [DOI] [PubMed] [Google Scholar]
- 8.Sasso RC, Williams JI, Dimasi N, et al. Postoperative drains at the donor sites of iliac-crest bone grafts. A prospective, randomized study of morbidity at the donor site in patients who had a traumatic injury of the spine. J.Bone Joint Surg.Am. 1998;80:631–5. doi: 10.2106/00004623-199805000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28(2):134–9. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 10.St John TA, Vaccaro AR, Sah AP, et al. Physical and monetary costs associated with autogenous bone graft harvesting. American Journal of Orthopedics (Chatham, NJ) 2003;32(1):18–23. [PubMed] [Google Scholar]
- 11.Summers BN, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J.Bone Joint Surg.Br. 1989;71:677–80. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 12.Xu R, Ebraheim NA, Yeasting RA, et al. Anatomic considerations for posterior iliac bone harvesting. Spine. 1996;21:1017–20. doi: 10.1097/00007632-199605010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine. 1995:412–20. doi: 10.1097/00007632-199502001-00003. [DOI] [PubMed] [Google Scholar]
- 14.Bozic KJ, Glazer PA, Zurakowski D, et al. In vivo evaluation of coralline hydroxyapatite and direct current electrical stimulation in lumbar spinal fusion. Spine. 1999;24(20):2127–33. doi: 10.1097/00007632-199910150-00012. [DOI] [PubMed] [Google Scholar]
- 15.Long J, Lewis S, Kuklo T, et al. The effect of cyclooxygenase-2 inhibitors on spinal fusion. Journal of Bone & Joint Surgery - American Volume. 2002;84-A(10):1763–8. doi: 10.2106/00004623-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Liao SS, Guan K, Cui FZ, et al. Lumbar spinal fusion with a mineralized collagen matrix and rhBMP-2 in a rabbit model. Spine. 2003;28(17):1954–60. doi: 10.1097/01.BRS.0000083240.13332.F6. [DOI] [PubMed] [Google Scholar]
- 17.Lehman RA, Jr., Kuklo TR, Freedman BA, et al. The effect of alendronate sodium on spinal fusion: a rabbit model. Spine Journal: Official Journal of the North American Spine Society. 2004;4(1):36–43. doi: 10.1016/s1529-9430(03)00427-3. -Feb. [DOI] [PubMed] [Google Scholar]
- 18.Tay BK, Le AX, Heilman M, et al. Use of a collagen-hydroxyapatite matrix in spinal fusion. A rabbit model. Spine. 1998;23(21):2276–81. doi: 10.1097/00007632-199811010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Grauer JN, Erulkar JS, Patel TC, et al. Biomechanical evaluation of the New Zealand white rabbit lumbar spine: a physiologic characterization. Eur. Spine J. 2000;9:250–5. doi: 10.1007/s005860000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grauer JN, Vaccaro AR, Kato M, et al. Development of a New Zealand white rabbit model of spinal pseudarthrosis repair and evaluation of the potential role of OP-1 to overcome pseudarthrosis. Spine. 2004;29:1405–12. doi: 10.1097/01.brs.0000129028.25671.96. [DOI] [PubMed] [Google Scholar]
- 21.Boden SD, Schimandle JH, Hutton WC. Lumbar intertransverse-process spinal arthrodesis with use of a bovine bone-derived osteoinductive protein. A preliminary report. Journal of Bone & Joint Surgery-American Volume. 1995;77(9):1404–17. doi: 10.2106/00004623-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Boden SD, Schimandle JH, Hutton WC, et al. In vivo evaluation of a resorbable osteoinductive composite as a graft substitute for lumbar spinal fusion. Journal of Spinal Disorders. 1997;10(1):1–11. [PubMed] [Google Scholar]
- 23.Boden SD, Martin GJ, Jr, Morone M, et al. The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar spine fusion. Spine. 1999;24(4):320–7. doi: 10.1097/00007632-199902150-00003. [DOI] [PubMed] [Google Scholar]
- 24.Kraiwattanapong C, Boden SD, Louis-Ugbo J, et al. Comparison of Healos/bone marrow to INFUSE(rhBMP-2/ACS) with a collagen-ceramic sponge bulking agent as graft substitutes for lumbar spine fusion. Spine. 2005;30:1001–7. doi: 10.1097/01.brs.0000160997.91502.3b. [DOI] [PubMed] [Google Scholar]
- 25.Grauer JN, Patel TC, Erulkar JS, et al. 2000 Young Investigator Research Award winner. Evaluation of OP-1 as a graft substitute for intertransverse process lumbar fusion. Spine. 2001;26(2):127–33. doi: 10.1097/00007632-200101150-00004. [DOI] [PubMed] [Google Scholar]
- 26.Magit DP, Maak T, Trioano N, et al. Healos/recombinant human growth and differentiation factor-5 induces posterolateral lumbar fusion in a New Zealand white rabbit model. Spine. 2006;31:2180–8. doi: 10.1097/01.brs.0000232823.82106.0a. [DOI] [PubMed] [Google Scholar]
- 27.boden SD. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine. 2002;27:S26–S31. doi: 10.1097/00007632-200208151-00007. [DOI] [PubMed] [Google Scholar]
- 28.Smucker JD, et al. B2A peptide on ceramic granules enhance posterolateral spinal fusion in rabbits compared with autograft. Spine. 2008;33(12):1324–1329. doi: 10.1097/BRS.0b013e3181732a74. May 20. [DOI] [PubMed] [Google Scholar]
- 29.Smucker JD, et al. Assesment of Mastergraft Putty as a Graft Extender in a Rabbit Posterolateral Fusion Model. Spine. 2011 doi: 10.1097/BRS.0b013e31824444c4. Dec 13 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


