Abstract
Objectives
Vascular endothelial growth factor (VEGF) is a potent angiogenic factor that plays an important role during skeletal development and fracture healing. Previous experimental studies have shown that VEGF applied immediately after injury can stimulate bone repair in animal fracture nonunion models. However, the effectiveness of VEGF on an established fracture non-union has not been determined. the goal of this work was to test the ability of VEGF applied at a later stage on the healing of fracture nonunions.
Methods
In this study, a murine non-union model was induced by rapid distraction of a tibia osteotomy. this model exhibits radiological and histological evidence of impaired fracture healing at 7 days after the completion of distraction. VEGF (10 µg in 20 µl Pbs/day, n=10) or control (20 µl Pbs/day, n=10) was injected directly into the distraction gap through the posterior musculature on three consecutive days (7, 8, and 9 days after completing distraction). A third group of animals (n=10) with rapid distraction, but no injections, served as non-treated controls. Fracture healing was analyzed by x-ray, histology, and histomorphometry at 27 days after the last round of distraction. results: radiographs showed that half of the VEGF treated animals (5/10) achieved bony healing whereas the majority of Pbs treated (7/10) and non-treated controls (8/10) did not exhibit bone bridging. Histological and histomorphometric analyses demonstrated that VEGF increased, but not significantly, the amount of bone formed in the distraction gap (1.35 ± 0.35 mm3), compared to the saline treated (0.77 ± 0.25 mm3, p=0.19) and non-treated animals (0.79 ± 0.23mm3, p=0.12).
Conclusions
Results from this study demonstrate that VEGF potentially promotes bone repair, warranting further research in this direction.
Keywords: Vascular Endothelial Growth Factor, VEGF, fracture, delayed union, non-union, distraction osteogenesis
Introduction
In the United States, there are over six million cases of fractures occurring each year, with about 5–10% of them do not heal on a timely manner 1. Blood supply is crucial for fracture healing and lack of perfusion is one of the most important factors that cause delayed fracture healing or non-union. In a mouse model, femoral artery resection prior to the creation of tibia fractures leads to a large amount of cell death and delayed bone repair 2.
During fracture healing, recovery of blood supply to the fracture site relies on angiogenesis, whereby new blood vessels form from preexisting ones. Inhibiting angiogenesis decreases bone formation, resulting in fracture non-union 3. Thus, improving angiogenesis and increasing blood supply are promising targets to treat delayed fracture healing or non-unions.
The process of angiogenesis is regulated by many growth factors. Among them, vascular endothelial growth factor (VEGF) is one of the most potent angiogenic factors. In fracture calluses, VEGF is expressed by hypertrophic chondrocytes and may be released from cartilage matrix by MMP9-mediated matrix degradation, which induces vascular invasion of the hypertrophic cartilage 4. The ability of VEGF to enhance bone regeneration has been established in several animal models 5-7. VEGF delivered as a protein or through transgenic approaches can promote healing of femoral fractures in mice 5, radius segmental defects in rabbits 5, 6, and bone drilling defects in rats7. While these studies have established the effects of VEGF on bone repair, the timing of VEGF administration in the treatment of fracture non-unions needs to be further determined for at least two reasons. First, in previous studies, VEGF protein or genes were delivered at the time that bone injuries were created. Clinically, fractures that eventually develop into delayed healing or non-union frequently cannot be identified at the time of injury, and are recognized by radiography weeks or months later. Therefore, results from studies that administer VEGF immediately after bone injury are not directly applicable to those conditions where impaired healing presents at a later stage. Second, the development of fracture non-union is a complex process in which cellular and molecular environments are constantly evolving, and VEGF administered at different stages could have different effects. In the current study, we hypothesized that VEGF delivered to the site of an established bone non-union can improve bone repair. To address this question, we used a murine non-union model induced by rapid distraction 8. In this model, radiological and histological signs of impaired fracture healing, such as fibrosis which is commonly seen in human non-unions, are evident at as early as seven days after the completion of rapid distraction 8. We tested whether VEGF delivered at this time can stimulate bone formation in the distraction gaps.
Materials and Methods
Animals and surgical procedures
All protocols have been approved by the Institutional Animal Care and Use Committee (IACUC) at our university. Male 129J/B6 mice (10–12 weeks old) were used in this study. Animals were anesthetized with an intraperitoneal injection of 0.02 ml/g of 2% Avertin (2-2-2-Tribromoethanol, Fluka, Buchs, Switzerland). One dose of Ancef (Cefazolin, 25mg/kg) was provided before surgery for prophylaxis. The left hind leg was shaved and prepared in a sterile manner. Two insect pins (0.25mm in diameter, Fine Science Tools, Foster City, CA) were placed perpendicularly to each other in the proximal and distal metaphyses. The tibia was centered within custom-made aluminum rings both proximally and distally, and bolts were tightened to secure the pins to the frame. The two rings were connected with three threaded rods (2/56 × 3/4 inch). An anterior longitudinal incision was then made over the mid-shaft of the tibia and an osteotomy was created 9. Incisions were closed with a running 5–0 nylon suture. Rapid distraction (0.36 mm/12 hrs) was performed immediately after surgery and then every 12 hours during the first 4 days by turning the nuts on the threaded rods. There were 9 rounds of distraction and the total distraction gap created was about 3.2mm. After recovery, animals were allowed to ambulate ad libitum. Buprenorphine (Sigma, St. Louis, MO) was given subcutaneously as needed for analgesia.
Delivery of VEGF
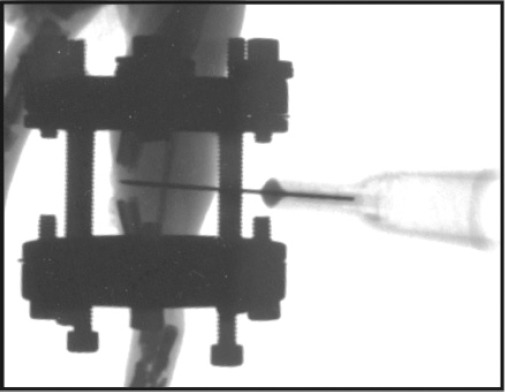
Our previous work demonstrated that by 7 days after completing rapid distraction, signs of non-union, including lack of new bone formation on radiological and histological examinations and presence of abundant fibrous tissue in the distraction gap on histology, can be observed in this model8. Hence, this time was chosen to perform VEGF treatment and x-ray was used to confirm the possibility of nonunion. Animals were anesthetized by breathing isofluorene, human recombinant VEGF (Genentech, South San Francisco, CA. 10 µg in 20 µl PBS) or control (20 µl PBS) was directly injected into the distraction gap through the posterior musculature with a 30 gauge needle (Fig. 1). The position of the needle was confirmed by radiographs. Another group of mice receiving rapid distraction without injection were also used as controls.
Figure 1. Orthogonal radiograph of the distraction gap of a mouse tibia demonstrates placement of a 30G needle through the posterior musculature into the distraction gap.
Tissue Preparation
Animals were sacrificed on day 27 after completing rapid distraction (i.e. 31.5 days after bone osteotomy and 20 days after first injection). Fractured legs were collected, fixed in 4% paraformaldehyde (PFA) at 4°C overnight, and decalcified in 19% EDTA for 10–12 days at 4°C. Decalcification was confirmed radiographically, and then the pins were removed. Tissues were dehydrated with graded ethanol solutions and embedded in paraffin. Sagittal sections (10 µm) through the tibia were collected throughout the cortex of the distal osteotomy site.
Histological and histomorphometric analyses
Every fifth slide was dewaxed, rehydrated, and stained with modified Trichrome staining to visualize bone in the fracture calluses. The distraction gap on every 15th section was digitized under a Leica microscope. For each sample, 5–7 sections 300mm apart were analyzed. The area of new bone was measured if it fell between the osteotomized bone ends using Photoshop. Bone tissue was selected based on the blue staining and morphology and the pixels of selected bone were converted into area. The total volume of bone (BV) was calculated using the equation for a conical frustum: BV =1/3h ∑ (Ai+Ai+1+√AiAi+1). Ai and Ai+1 are the area of bone in the sequential sections; h is distance between sections (300mm), and n is total number of sections analyzed for each specimen10. Histomorphometric data were analyzed in SAS (version 6.12, SAS Institute, Inc, Cary, NC) using one-way Analysis of Variance (ANOVA).
Results
Rapid distraction induces non-union
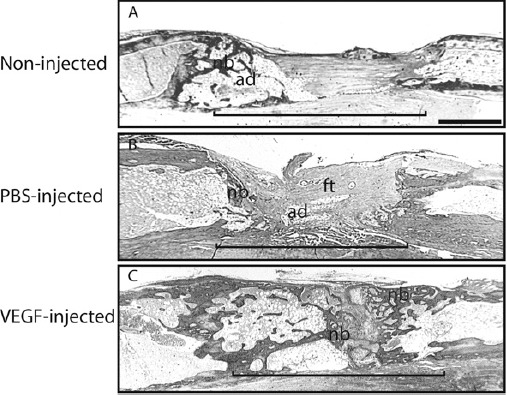
In this mouse model of tibia distraction osteogenesis, distraction at a rate of 0.36 mm/12 hours significantly impaired bone formation in the distraction gap, and this agrees with our previous data 8. By 27 days of maturation, bridging calluses were absent from radiographs in 8 out of 10 animals. Some radiographic signs of bony union were observed in the other 2 animals. In the 8 animals exhibiting radiographic non-union, histological analysis demonstrated a small amount of new bone present at the fracture ends but not in the gap (Fig. 2A). Instead, the distraction gap was occupied by muscle, fibrous tissue, and fatty tissue (Fig. 2A), which are common histological findings in other models of delayed fracture healing or non-union 2, 3.
Figure 2. Histological analysis at 27 days after completing rapid distraction. Tissue is stained by trichrome staining. (A) A non-injected distraction gap exhibits a small amount of new bone (nb) at the osteotomy ends and adipose (ad) and fibrous tissues (ft) in the distraction gap (bracket). (B) Similar histological findings are observed in a saline-injected distraction gap. (C) A VEGF-treated distraction gap is bridged by new bone (nb). Recanalization of the regenerated bone is also seen in VEGF injected animals. ad = adipose tissue, ft = fibrous tissue, nb = new bone, bracket = distraction gap. scale bar = 1mm.
VEGF decreases the rate of non-union induced by rapid distraction
By 27 days of maturation, most (7 out of 10) of the saline injected control animals failed to heal their distraction gaps, as evidenced by the lack of bony bridging callus on radiographs and stained histologic sections. Similar to the non-injected controls described above, distraction gaps in the saline injected animals were mostly occupied by fibrous and adipose tissues (Fig. 2B).
In comparison, VEGF treatment decreased the rate of non-union. By 27 days, half (5 out of 10) of the animals in this group exhibited bridging bony calluses on radiographs. Histological analysis on these 5 animals demonstrated that a large amount of new bone formed in the distraction gap connecting the two fracture ends (Fig. 2C). A small amount of fibrous tissue and cartilage was also observed in the gap (Fig. 2C).
VEGF mildly increases the amount of bone in the distraction gap
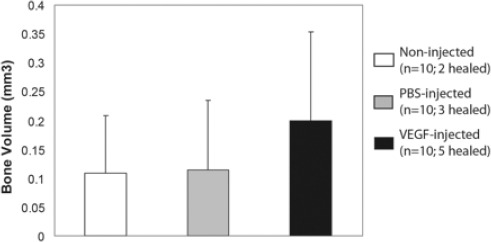
Histomorphometric analysis quantifying new bone formation in the distraction gaps (Fig. 3) showed that similar amount of new bone was present in the non-injected controls (0.77 ± 0.25 mm3) and saline injected animals (0.79 ± 0.23mm3). In comparison, VEGF treated animals exhibited more bone formation in the distraction gaps (1.35 ± 0.35 mm3), however, the differences are not statistically significant (p = 0.12 for VEGF injected vs. non-injected, and p = 0.19 for VEGF vs. saline injected).
Figure 3. Histomorphometric analysis of the effect of VEGF on osteogenesis in distraction gaps. Data is shown as mean ± SD. VEGF-injected vs. non-injected (p=0.12); VEGF-injected vs. salineinjected (p=0.19).
Discussion
In this study we found that exogenous VEGF, applied at a time when radiological and histological signs of nonunion are evident, potentially enhances bone formation. The mouse model of fracture non-union, resulting from osteotomy and rapid distraction, exhibits some histological signs of an atrophic non-union, including absence of a callus and the presence of fibrous and adipose tissues in fracture gaps. Results from the current study suggest that VEGF could be used to treat delayed fracture healing and non-union.
The molecular and cellular environments are constantly changing as bone repair progresses through its various stages. Many genes regulating osteogenesis, chondrogenesis, angiogenesis, and renervation are temporally expressed during fracture healing 11-13. In distraction osteogenesis, the strongest expression of VEGF is found during the first several days after the completion of distraction 14. Expression of VEGF transcripts is upregulated after injury and remains prevalent throughout distraction osteogenesis, while expression of MMP (i.e. MMP2, MMP9, and MMP13) transcripts are increased during the phase of distraction, but to a lesser extent during the phase of consolidation 15. In fracture non-unions, osteoblasts exhibit abnormal expression profiles of genes that are related to mineralization 16. Therefore, the timing of VEGF treatment may significantly affect the outcome of healing bone. It is well established that VEGF delivered at the time of injury can improve bone repair. In the current study, VEGF (10 µg) was delivered to the distraction gaps at 7 days after completing distraction when radiographic and histological signs of delayed fracture healing were evident. VEGF treatment at this stage appeared to stimulate bone formation and decrease the rate of non-union. These results suggest that VEGF treatment can be considered for problematic fracture healing diagnosed weeks or months after injury.
Multiple mechanisms may underlie the stimulating effect of VEGF on bone repair. VEGF is a potent angiogenic factor and it may improve fracture healing by promoting angiogenesis. Street et al. 5 treated mouse femur fractures with VEGF and found VEGF treated fractures had 26% more vascularity than carrier treated ones. In the model of fracture non-union used in the current study, avascular fibrous tissue was present in the distraction gap at 7–8 days after completion of rapid distraction8, which was also the time point we chose to administer VEGF. The presence of avascular fibrous tissue in the distraction gap denotes the benefits of the pro-angiogenic properties of VEGF. However, there is literature suggesting that pro-angiogenesis might not be the only mechanism through which VEGF enhances bone formation. VEGF may have direct effects on osteoblasts and osteoclasts. VEGF or its receptors are expressed by osteoblasts 5, 17, 18, and VEGF can enhance the activity of cultured osteoblasts by increasing the formation of bone nodules and alkaline phosphatase expression 5. VEGF also plays a role in osteoclastogenesis by upregulating RANK expression in osteoclast precursors 19. VEGF, by binding to its receptors present on osteoclasts 20, can increase the resorption activity of mature osteoclasts 21, 22. Therefore, the effect of VEGF on osteogenesis in our current study could result from synergistic effects that include angiogenesis, osteoblastogenesis, and osteoclastogenesis.
We observed only a mild pro-osteogenic effect of VEGF, which could be due to our delivery method. In our study, VEGF was suspended in phosphate saline buffer and directly injected into the bone defect. It is possible that administered VEGF might have diffused from the injection site too quickly, compromising the outcome. Techniques that retain the exogenous VEGF at the site, such as using the appropriate scaffolds or controlled release techniques, could significantly improve its function. As an example, Eckardt et al. applied VEGF locally to a rabbit model of distraction osteogenesis using osmotic pumps but failed to enhance bone formation23. In another study, the same group of researchers found that an equal amount of VEGF delivered with a carrier was able to enhance bone repair in an experimental rabbit non-union model 6. Recently, other researchers have tried to combine VEGF with osteogenic factors or mesenchymal stem cells to achieve even better outcome. In a rat cranial critical size defect model, dual delivery of VEGF and bone morphogenetic protein-2 (BMP-2) showed synergistic effects on bone formation at 4 weeks after treatment. However, this effect was not detected by 12 weeks 24. In another study, Kumar et al. transplanted mesenchymal stem cells that co-express VEGF and BMP-2 to a segmental bone defect in mice and found that the combination improved the biomechanical properties of newly formed bone 25.
In conclusion, results from the current study demonstrate that VEGF may have a role in stimulating bone repair. While the current study shows a trend towards the positive effects of VEGF on bone healing in this murine rapid distraction non-union model, the results are not statistically significant. From the results, however, it is clear that many of the animals administered VEGF showed a robust response to the factor. Larger numbers of animals per group are required to further evaluate the significance of this response. Further research is also required to optimize the dosage and delivery method to maximize the effects of VEGF.
Acknowledgments
This work was funded by National Institutes of Health-NIAMS (KO8-AR002164 and RO1-AR053645 to TM.), Orthopaedic Trauma Association (a research grant to CL.), Orthopaedic Research and Education Foundation (a grant to CMO., and Zimmer Inc. We would like to thank Dr. Zena Werb for her helpful comments on the manuscript. Drs. Stuart Gansky and Sara Shain provided statistical analysis.
References
- 1.Einhorn T.A. Enhancement of fracture-healing. The Journal of bone and joint surgery. American volume. 1995;77(6):940–56. doi: 10.2106/00004623-199506000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Lu C., Miclau T., Hu D., et al. Ischemia leads to delayed union during fracture healing: a mouse model. J Orthop Res. 2007;25(1):51–61. doi: 10.1002/jor.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hausman M.R., Schaffler M.B., Majeska R.J. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29(6):560–4. doi: 10.1016/s8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 4.Colnot C., Thompson Z., Miclau T., et al. Altered fracture repair in the absence of MMP9. Development. 2003;130(17):4123–33. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Street J., Bao M., Deguzman L., et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656–61. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckardt H., Ding M., Lind M., et al. Recombinant human vascular endothelial growth factor enhances bone healing in an experimental nonunion model. J Bone Joint Surg Br. 2005;87(10):1434–8. doi: 10.1302/0301-620X.87B10.16226. [DOI] [PubMed] [Google Scholar]
- 7.Tarkka T., Sipola A., Jamsa T., et al. Adenoviral VEGF-A gene transfer induces angiogenesis and promotes bone formation in healing osseous tissues. J Gene Med. 2003;5(7):560–6. doi: 10.1002/jgm.392. [DOI] [PubMed] [Google Scholar]
- 8.Choi P., Ogilvie C., Thompson Z., et al. Cellular and molecular characterization of a murine non-union model. J Orthop Res. 2004;22(5):1100–7. doi: 10.1016/j.orthres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Tay B.K., Le A.X., Gould S.E., et al. Histochemical and molecular analyses of distraction osteogenesis in a mouse model. J Orthop Res. 1998;16(5):636–42. doi: 10.1002/jor.1100160518. [DOI] [PubMed] [Google Scholar]
- 10.Lu C., Miclau T., Hu D., et al. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23(6):1300–7. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heiner D.E., Meyer M.H., Frick S.L., et al. Gene expression during fracture healing in rats comparing intramedullary fxation to plate fxation by DNA microarray. J Orthop Trauma. 2006;20(1):27–38. doi: 10.1097/01.bot.0000184143.90448.aa. [DOI] [PubMed] [Google Scholar]
- 12.Desai B.J., Meyer M.H., Porter S., et al. The effect of age on gene expression in adult and juvenile rats following femoral fracture. J Orthop Trauma. 2003;17(10):689–98. doi: 10.1097/00005131-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Meyer M.H., Etienne W., Meyer R.A., Jr Altered mRNA expression of genes related to nerve cell activity in the fracture callus of older rats: A randomized, controlled, microarray study. BMC Musculoske-let Disord. 2004;5:24. doi: 10.1186/1471-2474-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu J., Zou S., Li J., et al. Temporospatial expression of vascular endothelial growth factor and basic fbroblast growth factor during mandibular distraction osteogenesis. J Craniomaxillofac Surg. 2003;31(4):238–43. doi: 10.1016/s1010-5182(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho R.S., Einhorn T.A., Lehmann W., et al. The role of angiogenesis in a murine tibial model of distraction osteogenesis. Bone. 2004;34(5):849–61. doi: 10.1016/j.bone.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Lawton D.M., Andrew J.G., Marsh D.R., et al. Expression of the gene encoding the matrix gla protein by mature osteoblasts in human fracture non-unions. Mol Pathol. 1999;52(2):92–6. doi: 10.1136/mp.52.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlandini M., Spreafco A., Bardelli M., et al. Vascular endothelial growth factor-D activates VEGFR-3 expressed in osteoblasts inducing their differentiation. J Biol Chem. 2006;281(26):17961–7. doi: 10.1074/jbc.M600413200. [DOI] [PubMed] [Google Scholar]
- 18.Steinbrech D.S., Mehrara B.J., Saadeh P.B., et al. VEGF expression in an osteoblast-like cell line is regulated by a hypoxia response mechanism. Am J Physiol Cell Physiol. 2000;278(4):C853–60. doi: 10.1152/ajpcell.2000.278.4.C853. [DOI] [PubMed] [Google Scholar]
- 19.Yao S., Liu D., Pan F., et al. Effect of vascular endothelial growth factor on RANK gene expression in osteoclast precursors and on osteoclastogenesis. Arch Oral Biol. 2006;51(7):596–602. doi: 10.1016/j.archoralbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Niida S., Kondo T., Hiratsuka S., et al. VEGF receptor 1 signaling is essential for osteoclast development and bone marrow formation in colony-stimulating factor 1-defcient mice. Proc Natl Acad Sci U S A. 2005;102(39):14016–21. doi: 10.1073/pnas.0503544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aldridge S.E., Lennard T.W., Williams J.R., et al. Vascular endothelial growth factor receptors in osteoclast differentiation and function. Biochem Biophys Res Commun. 2005;335(3):793–8. doi: 10.1016/j.bbrc.2005.07.145. [DOI] [PubMed] [Google Scholar]
- 22.Sipola A., Nelo K., Hautala T., et al. Endostatin inhibits VEGF-A induced osteoclastic bone resorption in vitro. BMC Musculoskelet Disord. 2006;7:56. doi: 10.1186/1471-2474-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckardt H., Bundgaard K.G., Christensen K.S., et al. Effects of locally applied vascular endothelial growth factor (VEGF) and VEGF-inhibitor to the rabbit tibia during distraction osteogenesis. J Orthop Res. 2003;21(2):335–40. doi: 10.1016/S0736-0266(02)00159-6. [DOI] [PubMed] [Google Scholar]
- 24.Patel Z.S., Young S., Tabata Y., et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931–40. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S., Wan C., Ramaswamy G., et al. Mesenchymal Stem Cells Expressing Osteogenic and Angiogenic Factors Synergistically Enhance Bone Formation in a Mouse Model of Segmental Bone Defect. Mol Ther. 2010;18(5):1026–1034. doi: 10.1038/mt.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]


