Abstract
BACKGROUND & AIMS
Variations in genes that regulate bile acid (BA) synthesis are associated with colonic transit in patients with irritable bowel syndrome (IBS). We investigated features of BA synthesis and excretion and genetic features of patients with different types of IBS.
METHODS
In 26 healthy volunteers, 26 patients with IBS and constipation (IBS-C), and 26 with IBS and diarrhea (IBS-D), we measured serum levels of 7α-hydroxy-4-cholesten-3-one (C4; a surrogate for BA synthesis) and fibroblast growth factor (FGF) 19 (an ileal hormone that downregulates BA synthesis). For stool samples, we measured concentration of BA, weight, and amount of fat when participants were given high-fat diets. Spearman correlations were used to explore relationships among factors. We analyzed 1 polymorphism in Klotho-β (KLB) and 3 in fibroblast growth factor receptor-4 (FGFR4) for all members of each group using analysis of covariance.
RESULTS
The concentration of BA in stool was associated with group (for a comparison of 3 groups; P = .057); it was higher in patients with IBS-D than IBS-C (P = .017). The serum level of C4 was higher in patients with IBS-D than IBS-C (P = .02) or healthy volunteers (P = .01); 38% of patients with IBS-D had increased serum levels of C4, compared with healthy volunteers. Serum level of C4 correlated with stool concentration of BA (rs = 0.606; P < .001), serum FGF19 (rs = −0.324; P = .007), and stool weight (rs = 0.366; P = .003). Stool concentration of BA correlated with weight (rs = 0.737; P < .001) and level of fat (rs = 0.528; P < .001). Body mass index correlated with serum level of C4 (rs = 0.423, P < .001) and stool concentration of BA (rs = 0.507, P < .001), and was higher in patients with IBS-D compared with other groups (overall P = .036). FGFR4 rs1966265 was associated with stool level of BA (P = .032).
CONCLUSIONS
Patients with IBS-D have greater body mass index and synthesize and excrete higher levels of BA than individuals with IBS-C or healthy volunteers. Serum levels of C4 might be used to identify patients with IBS-D who have BA malabsorption; studies are needed to determine if some patients have a genetic predisposition to this disorder.
Keywords: Colon, Malabsorption, Liver, Metabolism
Irritable bowel syndrome (IBS) is characterized by recurrent abdominal pain or discomfort associated with altered bowel function that are unexplained by overt structural or tissue abnormalities.1 Diarrhea-predominant IBS (IBS-D) may have specific etiologies, including bile acid (BA) malabsorption (BAM). Colonic exposure to BA, as in idiopathic BAM, stimulates colonic motility and secretion and is associated with functional diarrhea and IBS-D.2–6 In a systematic review, BAM was reported in 32% of patients with unexplained chronic diarrhea; these patients’ diarrhea responds to BA sequestration therapy.5 The BA sequestrant, colesevelam, may lead to constipation in diabetic patients7 and delayed ascending colon emptying in patients with IBS-D with evidence of increased BA synthesis.8
Hepatic BA synthesis is partly controlled by feedback inhibition by fibroblast growth factor (FGF) 19, a hormone secreted by ileal enterocytes into the portal circulation in response to high intracellular concentration of BA in ileal enterocytes. FGF19 binds to FGF receptor 4 (FGFR4) and the coreceptor Klotho-β (KLB) on the hepatocyte cell membrane, leading to suppression of the rate-limiting enzyme in BA synthesis, cytochrome P450 7A1.9 Chronic diarrhea patients with idiopathic BAM may have reduced FGF19 secretion, leading to high hepatic BA synthesis and excessive BA entry into the colon and diarrhea.10
Impaired apical reuptake of BA into ileal enterocytes is rarely caused by loss-of-function mutations in the apical sodium-dependent BA transporter gene (ASBT),11,12 although this impaired ileal BA reabsorption can be used to accelerate colonic transit and treat chronic idiopathic constipation with the small molecule apical sodium-dependent BA transporter inhibitor A3309.13,14 Ileal BA transport inhibition by A3309 was associated with increased serum 7α-hydroxy-4-cholesten-3-one (C4), a surrogate for increased hepatic BA synthesis.13,14 These data suggest that BAs are natural laxatives functioning mainly in the colon.
We previously showed that nonsynonymous variations in genes coding for proteins involved in regulation of BA synthesis, specifically a single nucleotide polymorphism (SNP) in the KLB gene (KLB rs17618244), are associated with colonic transit in IBS-D.15 Two genetic variations in FGFR4 rs1966265 and rs351855 modulate KLB rs17618244’s association with colonic transit in IBS-D. In addition, a pharmacogenomics study showed that SNPs in KLB (rs17618244) and FGFR4 (rs376618) influence the degree of acceleration of ascending colonic emptying in constipation-predominant IBS (IBS-C) treated with the primary bile salt, chenodeoxycholate.16 FGFR4 (rs351855) is also associated with the deceleration of ascending colonic emptying in IBS-D patients treated with the BA sequestrant colesevelam.17
The mechanism of the association between BA metabolic regulation and the pathogenesis of IBS remains incompletely defined. The relationships of BA synthesis and excretion to stool fat and weight in IBS patients are also poorly characterized. In this study, we aimed to: (1) correlate the degree of BA synthesis and excretion with stool fat and weight in IBS patients and healthy controls; and (2) assess for potential relationships between stool BA excretion and nonsynonymous genetic variations in KLB and FGFR4.
Methods
Participants
The study was reviewed and approved by Mayo Clinic’s Institutional Review Board, and each participant provided signed informed consent. From a cohort of approximately 700 previously genotyped IBS patients (based on Rome II criteria) and healthy volunteers (HV), we invited 78 randomly selected participants: 26 HV, 26 IBS-C, and 26 IBS-D patients. These sample sizes were expected to provide approximately 80% power (based on a 2-sample t test using a 2-sided α level of .05 to detect the effect sizes shown in Supplementary Table 1 for the end points of interest (ie, associations with group status corresponding to differences between pairs of groups’ end points of interest), ranging from 18% (stool weight) to 45% (serum C4 and FGF19).
Protocol
Participants received Mayo Clinic instructional pamphlets to follow a high-fat (approximately 100 g) diet per day for 4 days: 2 before and 2 during stool collection. A food diary used to record the high-fat diet was dispensed and reviewed with patients by desk personnel. Stool samples were collected over the last 48 hours of the high-fat diet for measuring stool weight, fat content, and BA concentrations. Fasting blood samples were collected and used for measuring serum C4 and FGF19 levels.
Biochemical Measurements
We measured serum C4 by liquid chromatography-mass spectrometry18 and serum FGF19 by a commercial enzyme-linked immunosorbent assay.8 Fat in a 48-hour stool collection was measured by nuclear magnetic resonance spectrometry at Mayo’s Department of Laboratory Medicine and Pathology.
Extraction of Bile Acids From Stool and Estimation of Total Stool 3α Hydroxy BAs
The homogenized stool samples (100–120 mg) were used for extraction of total BAs: 1.5 mL of a solution of 200 mM NaOH and 150 mM NaCl were added to the sample and incubated at room temperature for 10 minutes on a shaker. The samples were centrifuged at 8000 rpm for 2 minutes, and supernatant was collected. The aqueous extraction step was repeated 2 more times for a total of 3 extractions. The pellet was finally resuspended in 500 μL of 100% methanol and then centrifuged. The supernatant was added to previously collected aqueous extracts.
The BA extracts were further purified by using solid-phase extraction on C18 3 cc to 60 mg Oasis HLB columns (Waters Corporation, Milford, MA). The columns were activated by adding 1 mL of 100% methanol, washed with 2 mL of water, and equilibrated with 1 mL of a solution of 200 mM NaOH and 150 mM NaCl. The supernatants were applied to columns at 1–2 mL/min using a Cerex-48 N2 gas positive pressure solid phase extraction manifold (SPEware, Baldwin Park, CA). Columns were washed with 1 mL of aqueous extraction buffer and with 2 mL of water. The BAs were eluted in 1 mL of 100% methanol, and the eluates were dried under N2 gas at 45°C for 35 minutes. The dried extracts, which were resuspended in 20 μL of 100% methanol and 480 μL of 50% stripped serum in 150 mM NaCl, were then added to each sample.
The quantitative determination of total 3α hydroxy BAs (henceforth referred to as “BAs”) was performed on a Cobas c311 Analyzer (Roche Diagnostics, Pleasanton, CA) using Diazyme Laboratory Total Bile Acids Assay Kit (Diazyme Lab, Poway, CA). If the concentration of BAs exceeded the linear range of 1–180 μmol/L, samples were diluted with 150 mM NaCl before assay. The final BAs concentration was expressed in μmol/g of homogenized stool and then converted to μmol/24 hours.
Genotyping
Genotyping of 4 nonsynonymous SNPs in KLB and FGFR4 with minor allele frequencies (MAF) >9% (Supplementary Table 1) was performed as in previous studies, and is summarized in the Supplementary Methods.
Statistical Analysis
The sample size assessments for specific end points are detailed in Supplementary Table 2 and are based on the variations observed in the data obtained for this study. Most of the end points were not normally distributed and thus the effect sizes listed are only approximate. Because the distribution for most end points was positively skewed, data are presented as median and interquartile range (IQR). In general, nonparametric statistical analyses were used. In view of the fact that we included 26 HV in this study, we assessed the proportion of IBS-D patients with abnormally high indexes of BA synthesis or excretion relative to the 90th percentile of the HV. The IQR data also provide the 25th and 75th percentile distributions.
Spearman correlations were used to explore relationships of BA synthesis and excretion with serum FGF19, stool fat, and stool weight. We tested the associations of the measured responses obtained from blood and stool samples with KLB rs17618244 and FGFR4 rs351855, rs1966265, and rs376618 using the Wilcoxon rank-sum test based on a dominant genetic model. In particular, the associations with phenotype subgroup were assessed univariately (Kruskal–Wallis test).
Results
Participant Demographics
Table 1 shows the age, body mass index (BMI), and estimated daily fat intake during the study for each of the 3 subgroups. Previous cholecystectomy was recorded in 23% of IBS-D, approximately 4% of IBS-C, and approximately 4% of HV (P = .05). All patients’ symptoms anteceded the cholecystectomy and were confirmed by study subjects as not significantly aggravated or changed by subsequent cholecystectomy.
Table 1.
Subgroup Demographics, Estimated Daily Fat Intake, Serum FGF19, and 48-Hour Stool Weight
| IBS-D | IBS-C | Healthy controls | |
|---|---|---|---|
| n | 22 | 26 | 23 |
| Cholecystectomy, n | 5 | 1 | 1 |
| Age (y) | 40 (29–51) | 47 (43–49) | 43 (36–48) |
| BMIa (kg/m2) | 29.5 (24.3–36.4) | 26.0 (22.2–30.1) | 24.6 (23.4–27.3) |
| Daily fat intake (g) | 104.3 (100–108.4) | 102.5 (96.8–106.8) | 100 (97.8–103.9) |
| Serum FGF19 (pg/mL) | 115.1 (62.4–202.3) | 107.2 (68.6–170.2) | 88.5 (79.3–131.1) |
| 48-Hour stool weight (g) | 271 (182–481) | 160 (101–259) | 193 (125–320) |
NOTE. Data show median (IQR).
Overall associations with subgroup: P = .036.
BMI was associated with subgroup (overall P = .036 where the BMI in IBS-D was approximately 4 higher than in IBS-C [P = .026] and HV [P = .022]). Serum FGF19 and stool weight are also shown in Table 1.
Measurements of BA Synthesis and Excretion, Stool Fat, and Weight
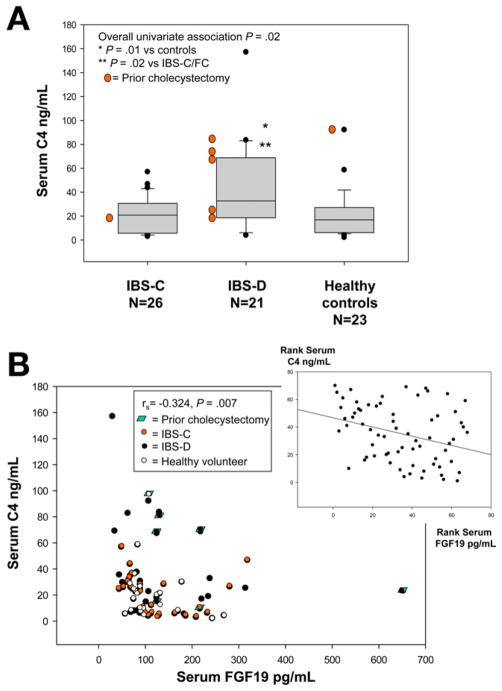
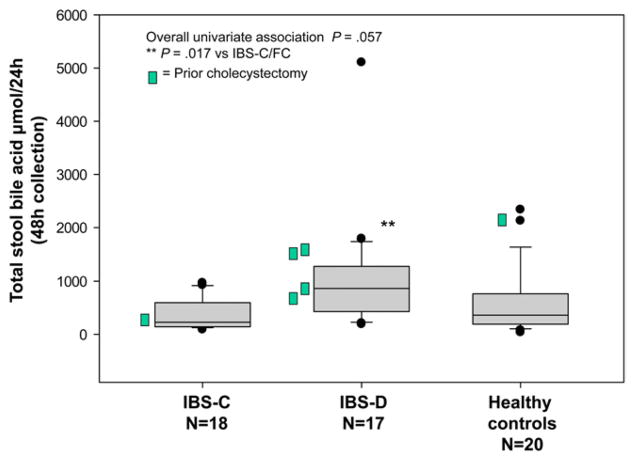
An overall univariate association of serum C4 with subgroup was identified (overall P = .02) with higher serum C4 (Figure 1) in IBS-D than in IBS-C (P = .02) and HV (P = .01). There were also overall associations of 48-hour stool BA (Figure 2) with subgroup (P = .057), with significantly higher 48-hour stool BA in IBS-D compared with IBS-C (P = .017), but not compared with HV (P = .152). Previous cholecystectomy was also associated with serum C4 (P = .04) and stool BA (P = .05).
Figure 1.
(A) Association of fasting serum C4 and subgroup. There was a significant overall univariate association with group and a significant difference between IBS-D and both HV and IBS-C groups. (B) Association of fasting serum C4 and serum FGF19; note the significant negative association, based on Spearman correlation. Inset, the same data are plotted based on rank transformation, and the line demonstrates the inverse correlation between serum FGF19 and C4.
Figure 2.
Association of total 48-hour stool BA and subgroup. There was a borderline overall univariate association with group and a significant difference between IBS-D and IBS-C groups.
We estimated that 2 out of 17 IBS-D patients (12%; 0/2 had prior cholecystectomy) had elevated stool BA, and 8 out of 21 (38%; 3/8 had prior cholecystectomy) had elevated serum C4, relative to the 90th percentile in the HV. One patient had elevated stool BA and serum C4. Thus, 9 of 21 patients (43%) had increased synthesis or excretion of BA.
There were numerical differences between subgroups in the 48-hour stool weight and 48-hour stool fat that were not statistically significant, with higher stool weight and stool fat in IBS-D compared with HV, and lower values in IBS-C compared with HV (Supplementary Table 3).
There was no association of fasting serum FGF19 levels with subgroups.
Rank Correlations Between BA Synthesis, Excretion, and Stool Fat and Weight
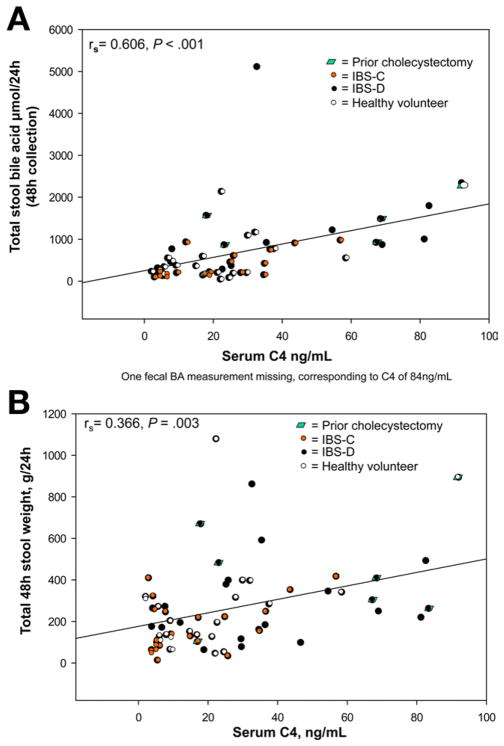
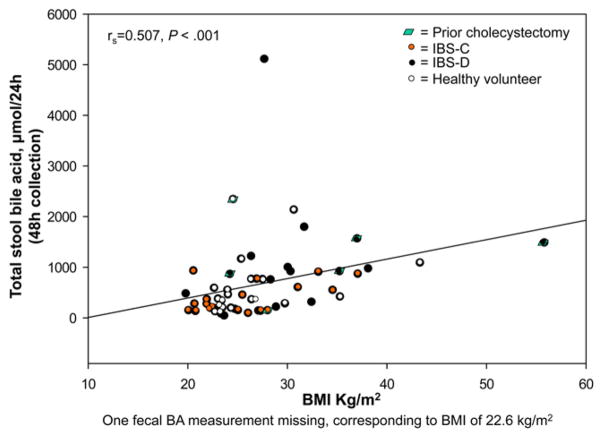
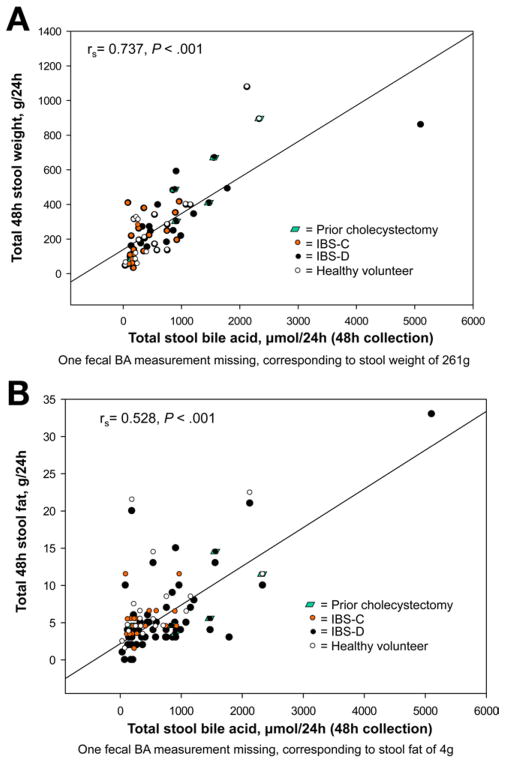
These are shown in Figures 3–5. There were significant Spearman correlations between serum C4 and FGF19 (rs = −0.324, P = .007), stool weight (rs = 0.366, P = .003), and total stool BA (rs = 0.606, P < .001). Total stool BA correlated with stool weight (rs = 0.737, P < .001) and fat (rs = 0.528, P < .001). BMI correlated with serum C4 (rs = 0.423, P < .001) and total stool BA (rs = 0.507, P < .001).
Figure 3.
Relationship between fasting serum C4 and total 48-hour stool BA excretion (A) and total 48-hour stool weight (B).
Figure 5.
Relationship between BMI and total stool BA excretion.
Relationship of Candidate Genetic Variants to BA Synthesis and Stool Excretion
FGFR4 rs1966265 was marginally associated with stool BA (P = .032, not statistically significant after correction for multiple comparisons with 4 different SNPs tested), but not with serum C4. There were no significant associations of serum C4 or stool BA with KLB rs17618244, FGFR4 rs351855, or FGFR4 rs376618 tested.
Discussion
This study demonstrates that IBS-D patients, unselected for clinical response to BA binding, have evidence of higher hepatic BA synthesis and stool BA excretion compared with control subjects and patients with IBS-C. In IBS-D, the upper quartile for 48-hour stool weight and fat were, respectively, 481 g and 8 g, and the 90th percentile for stool fat was 15 g/48 hours. These data suggest that about 25% of IBS-D patients had increased 48-hour stool weight, but that the stool fat was within the normal range in most patients (14 g/48 hours). Overall, these data confirm that a subgroup of patients with IBS-D have increased stool weight that is associated with high BA synthesis and excretion. In fact, the median serum C4 was 32.7 pg/mL in IBS-D, while the 90th percentile for HV was 37.6 pg/mL, suggesting that almost 40% of IBS-D patients in this cohort have increased serum C4, a marker of BA synthesis rate. Similarly, median stool BA in IBS-D was 864 μmol/24 hours and the 75th percentile in health was 762 μmol/24 hours. In contrast to the deficiency of BA and sulfation of BA in a subset of children with constipation,19 we did not observe deficiencies of BA in patients with IBS-C. However, it is important to note that some patients had extremely low stool BA excretion, and that the BA assay used does not detect sulfated BAs. Therefore, it is possible that we may have missed identifying a subgroup with normal stool BA output, but in the chemical form of sulfated BAs among the IBS-C group. Retardation of colonic transit by octreotide in acromegalic patients is associated with increased serum deoxycholic acid, which may result from the increased bacterial 7α-dehydroxylation of the primary BA, cholic acid, by colonic bacteria, and with greater colonic absorption of the secondary BA.20
The BA synthesis and excretion were also significantly associated with higher stool weight and stool fat across the 3 groups of participants. We have confirmed the reciprocal relationship between fasting serum C4 and FGF19 levels originally demonstrated by Walters et al.10
One interpretation of our findings is that the differences in BA biosynthesis or stool excretion cause the increased stool weight and the clinical presentation of IBS-D. However, an alternative interpretation is that rapid colonic transit may decrease colonic BA absorption, whereas slow transit may increase colonic BA absorption, with these differences in absorption then resulting in increased BA synthesis in IBS-D and decreased BA synthesis in IBS-C.
Stool BA measurement is the gold standard in the diagnosis of BAM and BA diarrhea; however, the demanding nature of stool collection and processing led to the development of a radionuclide-based synthetic BA retention test, the 75selenium homocholic acid taurine retention test. This is the preferred diagnostic modality in countries where the test is available. In countries where 75selenium homocholic acid taurine retention test is not available, such as the United States, stool BA remains the best choice for ruling out BAM because it provides direct assessment of the amount of BA transiting and exiting through the lower gastrointestinal tract.
An alternative, nonspecific marker of BAM is serum C4, which reflects the rate of hepatic BA synthesis; however, serum C4 may be elevated with alcohol use, chronic liver disease, and hypertriglyceridemia,21–23 and paradoxically, very high serum C4 has also been described in constipated patients with slow oral-anal transit time.24 Another test proposed for BAM is serum FGF19; this may be elevated in cholestatic liver disease and decreased in obesity.
In view of known diurnal variations in serum C4 and FGF19 levels, we collected blood in the morning, with patients fasting. We did not detect differences in serum FGF19 between the 3 subgroups. We anticipated reduced serum FGF19 in IBS-D patients, based on studies by Walters et al10 in 17 patients, of whom approximately 25% had values within the range observed in 19 HV. We have confirmed the overall significant inverse correlation between serum C4 and FGF19 (rs = −0.324, P = .007) reported in the literature.8,10 These data suggest that mechanisms other than ileal FGF19 deficiency may result in BAM in patients with IBS-D.
In contrast to our previous study in which the functional KLB variation rs17618244 mediating KLB protein stability was associated with accelerated colonic transit in patients with IBS-D, there was no significant association between markers of BAM and this KLB variation.
On the other hand, our finding that a genetic variation in FGFR4, 1 of the 2 receptor proteins located on hepatocytes, had borderline significant association with stool BA excretion (P = .032) and hence with BAM, supports the hypothesis that genetic variation in the pathways of BA synthesis may be etiologically important. We regard the current study as hypothesis-generating from the perspective of understanding the mechanisms of BAM in IBS-D patients.
We observed significant positive correlations between serum C4, stool BA, and stool weight. These are expected physiologically, as high hepatic synthesis and delivery to the colon are expected to increase colonic motility and secretion and, hence, stool weight. The positive correlation between stool BA and stool fat may reflect the impairment of BA absorption in the ileum, resulting in increased stool BAs and ultimately resulting in BA depletion and abnormal micelle formation and fat mal-absorption. This has been classically described in patients with ileal resection.25 However, mild steatorrhea has also been observed in patients with “nonorganic” BAM and functional diarrhea.26 Further studies of BA balance are required to explore the hypothesis that BA loss in patients with IBS-D may eventually exceed the hepatic BA synthesis rate and lead to steatorrhea.
We also identified significant overall associations of higher BMI with elevations in both BA synthesis and excretion. Higher BMI has previously been described in healthy subjects with accelerated colonic transit.27 Higher BMI has also been observed in those with idiopathic BAM compared with HV.28 In our current study, BMI was significantly and positively correlated with hepatic BA synthesis and stool BA excretion, as reflected by serum C4 and stool BA, respectively. BMI was higher by approximately 4 in IBS-D patients relative to both IBS-C patients and HV in our current cohort. These results provide support for the hypothesis that excess body mass is linked to accelerated colonic transit and diarrhea by a mechanism of excessive BA synthesis and excretion, as seen in the IBS-D group. Further investigation is required to clarify the exact pathogenetic mechanism; specifically, whether derangements in BA metabolism precede and contribute to gain in body mass, or the opposite temporal sequence.
Accelerated colonic transit has previously been described in healthy subjects with higher BMI.27 Higher BMI has also been observed in those with idiopathic BAM compared with HV.28 In our current study, higher BMI was significantly correlated with both increased hepatic BA synthesis and luminal BA excretion, as reflected by higher serum C4 and stool BA, respectively. BMI was higher by approximately 4 in IBS-D patients relative to both IBS-C patients and HV in our current cohort. Our results support the hypothesis that obesity is linked to accelerated colonic transit and diarrhea by excessive BA synthesis and excretion.
In summary, our study provides important confirmation that BAM is a potential mechanism for the diarrhea in patients with IBS-D, and that total stool BA or, perhaps more conveniently, serum C4 may be used to identify patients with BAM. Based on our current study, we estimate that serum C4 would identify 43% (9 of 21) of IBS-D patients with BAM, for whom more specific therapy with BA sequestrants such as cholesty-ramine5,29,30 or the more easily tolerated agent, colesevelam,8,31 may be effective. Total stool BA excretion is marginally associated with FGFR4 genetic variation. Further studies of BA synthesis and excretion, colonic transit, and genotype in larger numbers of IBS-D are under way.
Supplementary Material
Figure 4.
Relationship between total 48-hour stool weight (A) and stool fat (B) and total 48-hour stool BA excretion.
Acknowledgments
The authors thank Mrs Cindy Stanislav for excellent secretarial assistance.
Funding
This study was supported in part by NIH grants 1RC1-DK086182 and R01-DK092179 (to Dr Camilleri).
Abbreviations used in this paper
- BA
bile acid
- BAM
bile acid malabsorption
- BMI
body mass index
- C4
7α-hydroxy-4-cholesten-3-one
- FGF
fibroblast growth factor
- FGFR4
fibroblast growth factor receptor 4
- HV
healthy volunteers
- IBS
irritable bowel syndrome
- IBS-C
constipation-predominant irritable bowel syndrome
- IBS-D
diarrhea-predominant irritable bowel syndrome
- IQR
interquartile ranges
- KLB
Klotho-β
- MAF
minor allele frequencies
- SNP
single nucleotide polymorphism
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at doi:10.1016/j.cgh.2012.05.006.
References
- 1.Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 2.Eusufzai S. Bile acid malabsorption in patients with chronic diarrhoea. Scand J Gastroenterol. 1993;28:865–868. doi: 10.3109/00365529309103126. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Bañares F, Esteve M, Salas A, et al. Systematic evaluation of the causes of chronic watery diarrhea with functional characteristics. Am J Gastroenterol. 2007;102:2520–2528. doi: 10.1111/j.1572-0241.2007.01438.x. [DOI] [PubMed] [Google Scholar]
- 4.Sciarretta G, Furno A, Morrone B, et al. Absence of histopathological changes of ileum and colon in functional chronic diarrhea associated with bile acid malabsorption, assessed by SeHCAT test: a prospective study. Am J Gastroenterol. 1994;89:1058–1061. [PubMed] [Google Scholar]
- 5.Wedlake L, A’Hern R, Russell D, et al. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707–717. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 6.Wildt S, Nørby Rasmussen S, Lysgård Madsen J, et al. Bile acid malabsorption in patients with chronic diarrhoea: clinical value of SeHCAT test. Scand J Gastroenterol. 2003;38:826–830. doi: 10.1080/00365520310004461. [DOI] [PubMed] [Google Scholar]
- 7.Zieve FJ, Kalin MF, Schwartz SL, et al. Results of the glucose-lowering effect of Welchol study (GLOWS): a randomized, double-blind, placebo-controlled pilot study evaluating the effect of co-lesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin Ther. 2007;29:74–83. doi: 10.1016/j.clinthera.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159–165. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walters JR, Tasleem AM, Omer OS, et al. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1189–1194. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Oelkers P, Kirby LC, Heubi JE, et al. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2) J Clin Invest. 1997;99:1880–1887. doi: 10.1172/JCI119355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montagnani M, Love MW, Rössel P, et al. Absence of dysfunctional ileal sodium-bile acid cotransporter gene mutations in patients with adult-onset idiopathic bile acid malabsorption. Scand J Gastroenterol. 2001;36:1077–1080. doi: 10.1080/003655201750422693. [DOI] [PubMed] [Google Scholar]
- 13.Wong BS, Camilleri M, McKinzie S, et al. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol. 2011;106:2154–2164. doi: 10.1038/ajg.2011.285. [DOI] [PubMed] [Google Scholar]
- 14.Chey WD, Camilleri M, Chang L, et al. A randomized placebo-controlled phase IIb trial of a3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. Am J Gastroenterol. 2011;106:1803–1812. doi: 10.1038/ajg.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong BS, Camilleri M, Carlson PJ, et al. A Klothoβ variant mediates protein stability and associates with colon transit in irritable bowel syndrome with diarrhea. Gastroenterology. 2011;140:1934–1942. doi: 10.1053/j.gastro.2011.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao AS, Wong BS, Camilleri M, et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmaco-dynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549–1558. doi: 10.1053/j.gastro.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong BS, Camilleri M, Carlson PJ, et al. Pharmacogenetics of the effects of colesevelam on colonic transit in irritable bowel syndrome with diarrhea. Dig Dis Sci. 2012;57:1222–1226. doi: 10.1007/s10620-012-2035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camilleri M, Nadeau A, Tremaine WJ, et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009;21:734–e43. doi: 10.1111/j.1365-2982.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann AF, Loening-Baucke V, Lavine JE, et al. Altered bile acid metabolism in childhood functional constipation: inactivation of secretory bile acids by sulfation in a subset of patients. J Pediatr Gastroenterol Nutr. 2008;47:598–606. doi: 10.1097/MPG.0b013e31816920a6. [DOI] [PubMed] [Google Scholar]
- 20.Thomas LA, Veysey MJ, Murphy GM, et al. Octreotide induced prolongation of colonic transit increases faecal anaerobic bacteria, bile acid metabolising enzymes, and serum deoxycholic acid in patients with acromegaly. Gut. 2005;54:630–635. doi: 10.1136/gut.2003.028431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Axelson M, Mörk B, Sjövall J. Ethanol has an acute effect on bile acid biosynthesis in man. FEBS Lett. 1991;281:155–159. doi: 10.1016/0014-5793(91)80382-d. [DOI] [PubMed] [Google Scholar]
- 22.Brydon WG, Nyhlin H, Eastwood MA, et al. Serum 7 alpha-hydroxy-4-cholesten-3-one and selenohomocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea. Eur J Gastroenterol Hepatol. 1996;8:117–123. doi: 10.1097/00042737-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Duane WC. Abnormal bile acid absorption in familial hypertriglyceridemia. J Lipid Res. 1995;36:96–107. [PubMed] [Google Scholar]
- 24.Abrahamsson H, Ostlund-Lindqvist AM, Nilsson R, et al. Altered bile acid metabolism in patients with constipation-predominant irritable bowel syndrome and functional constipation. Scand J Gastroenterol. 2008;43:1483–1488. doi: 10.1080/00365520802321212. [DOI] [PubMed] [Google Scholar]
- 25.Poley JR, Hofmann AF. Role of fat maldigestion in pathogenesis of steatorrhea in ileal resection. Fat digestion after two sequential test meals with and without cholestyramine. Gastroenterology. 1976;71:38–44. [PubMed] [Google Scholar]
- 26.Ung KA, Kilander AF, Lindgren A, et al. Impact of bile acid malabsorption on steatorrhoea and symptoms in patients with chronic diarrhoea. Eur J Gastroenterol Hepatol. 2000;12:541–547. doi: 10.1097/00042737-200012050-00011. [DOI] [PubMed] [Google Scholar]
- 27.Delgado-Aros S, Camilleri M, Garcia MA, et al. High body mass alters colonic sensory-motor function and transit in humans. Am J Physiol Gastrointest Liver Physiol. 2008;295:G382–G388. doi: 10.1152/ajpgi.90286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadik R, Abrahamsson H, Ung KA, et al. Accelerated regional bowel transit and overweight shown in idiopathic bile acid malabsorption. Am J Gastroenterol. 2004;99:711–718. doi: 10.1111/j.1572-0241.2004.04139.x. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann AF, Poley JR. Cholestyramine treatment of diarrhea associated with ileal resection. N Engl J Med. 1969;281:397–402. doi: 10.1056/NEJM196908212810801. [DOI] [PubMed] [Google Scholar]
- 30.Sciarretta G, Fagioli G, Furno A, et al. 75Se HCAT test in the detection of bile acid malabsorption in functional diarrhoea and its correlation with small bowel transit. Gut. 1987;28:970–975. doi: 10.1136/gut.28.8.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wedlake L, Thomas K, Lalji A, et al. Effectiveness and tolerability of colesevelam hydrochloride for bile-acid malabsorption in patients with cancer: a retrospective chart review and patient questionnaire. Clin Ther. 2009;31:2549–2558. doi: 10.1016/j.clinthera.2009.11.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.