Abstract
Background
Intracranial-pressure monitoring is considered the standard of care for severe traumatic brain injury and is used frequently, but the efficacy of treatment based on monitoring in improving the outcome has not been rigorously assessed.
Methods
We conducted a multicenter, controlled trial in which 324 patients 13 years of age or older who had severe traumatic brain injury and were being treated in intensive care units (ICUs) in Bolivia or Ecuador were randomly assigned to one of two specific protocols: guidelines-based management in which a protocol for monitoring intra-parenchymal intracranial pressure was used (pressure-monitoring group) or a protocol in which treatment was based on imaging and clinical examination (imaging–clinical examination group). The primary outcome was a composite of survival time, impaired consciousness, and functional status at 3 months and 6 months and neuro-psychological status at 6 months; neuropsychological status was assessed by an examiner who was unaware of protocol assignment. This composite measure was based on performance across 21 measures of functional and cognitive status and calculated as a percentile (with 0 indicating the worst performance, and 100 the best performance).
Results
There was no significant between-group difference in the primary outcome, a composite measure based on percentile performance across 21 measures of functional and cognitive status (score, 56 in the pressure-monitoring group vs. 53 in the imaging–clinical examination group; P = 0.49). Six-month mortality was 39% in the pressure-monitoring group and 41% in the imaging–clinical examination group (P = 0.60). The median length of stay in the ICU was similar in the two groups (12 days in the pressure-monitoring group and 9 days in the imaging–clinical examination group; P = 0.25), although the number of days of brain-specific treatments (e.g., administration of hyperosmolar fluids and the use of hyperventilation) in the ICU was higher in the imaging–clinical examination group than in the pressure-monitoring group (4.8 vs. 3.4, P = 0.002). The distribution of serious adverse events was similar in the two groups.
Conclusions
For patients with severe traumatic brain injury, care focused on maintaining monitored intracranial pressure at 20 mm Hg or less was not shown to be superior to care based on imaging and clinical examination. (Funded by the National Institutes of Health and others; ClinicalTrials.gov number, NCT01068522.)
Although the monitoring of intracranial pressure is widely recognized as standard care for patients with severe traumatic brain injury, its use in guiding therapy has incomplete acceptance, even in high-income countries.1–3 Successive editions of the guidelines for the management of severe traumatic brain injury4–7 have documented the inadequate evidence of efficacy, calling for randomized, controlled trials while also noting the ethical issues that would be posed if the control group consisted of patients who did not undergo monitoring. The identification of a group of intensivists in Latin America who routinely managed severe traumatic brain injury without using available monitors and for whom there was equipoise regarding its efficacy eliminated that ethical constraint and led to the implementation of the randomized, controlled trial described here.
Data from rigorous randomized, controlled trials of intracranial-pressure monitoring in the management of traumatic brain injury are lacking, and few high-quality, prospective case–control or cohort studies have been conducted.7 Historically, the use of monitoring-based management has been confounded by several factors. These include the involvement of intensivists and the development of the subspecialty of neuro-critical care; the vast improvements in the resuscitation of patients with trauma (and those with brain injury, in particular); myriad developments in the management of traumatic brain injury during prehospital emergency care, emergency department care, and rehabilitation; and marked improvements in monitoring and management techniques in the intensive care unit (ICU). Such confounding can be rigorously addressed only in a randomized, controlled trial. Here we report the results of such a trial.
The primary objective of the Benchmark Evidence from South American Trials: Treatment of Intracranial Pressure (BEST:TRIP) trial was to determine whether the information derived from the monitoring of intracranial pressure in patients with severe traumatic brain injury improves medical practice and patient outcomes. Our primary hypothesis was that a management protocol based on the use of intracranial-pressure monitoring would result in reduced mortality and improved neuropsychological and functional recovery at 6 months. Our secondary hypothesis was that incorporating intracranial-pressure monitoring into the management of severe traumatic brain injury would have benefits for the health care system, including a reduced risk of complications and a shorter ICU stay.
METHODS
STUDY DESIGN
The study was a multicenter, parallel-group trial, with randomized assignment to intracranial-pressure monitoring (the pressure-monitoring group) or imaging and clinical examination (the imaging–clinical examination group). Randomization was stratified according to study site, severity of injury, and age. The study was started at three Bolivian hospitals (for details, see the Supplementary Appendix, available with the full text of this article at NEJM.org); an additional Bolivian hospital and two Ecuadorian hospitals were subsequently recruited to increase enrollment. All six sites had ICUs staffed with intensivists, 24-hour computed tomographic (CT) services and neurosurgery coverage, and high volumes of patients with trauma.
ELIGIBILITY
All patients presenting with traumatic brain injury were screened for eligibility on admission at the study hospitals. To be included in the study, patients had to be 13 years of age or older and have a score on the Glasgow Coma Scale (GCS) of 3 to 8 (with a score on the GCS motor component of 1 to 5 if the patient was intubated) or a higher score on admission that dropped to the specified range within 48 hours after injury. (The GCS ranges from 3 to 15, with higher scores indicating higher levels of consciousness; the motor score ranges from 1 to 6.) Patients with a GCS score of 3 and bilateral fixed and dilated pupils and those with an injury believed to be unsurvivable were excluded. The complete list of inclusion and exclusion criteria has been reported previously8 and is available in the Supplementary Appendix. Informed consent was obtained for all participants.
GROUP ASSIGNMENTS AND INTERVENTIONS
Randomization sequences were computer-generated by a data-center biostatistician and were stratified according to site, severity of injury (GCS score of 3 to 5, or GCS motor score of 1 to 2 if the patient was intubated, vs. GCS score of 6 to 8, or GCS motor score of 3 to 5 if the patient was intubated), and age (<40 years vs. ≥40 years), with a block size of 2 or 4 (see the Supplementary Appendix).
The study was conducted in accordance with the protocol (available at NEJM.org), which specified that three CT scans be obtained (at baseline, 48 hours, and 5 to 7 days) and standard supportive care provided for each patient, with care to include mechanical ventilation, sedation, and analgesia. Non-neurologic problems were managed aggressively in both groups.
Patients randomly assigned to the pressure-monitoring group had an intraparenchymal monitor placed as soon as possible and were treated to maintain an intracranial pressure of less than 20 mm Hg, in accordance with the guidelines for the management of severe traumatic brain injury4–7 (for more information see the description of treatment protocols in the Supplementary Appendix). Drainage of cerebrospinal fluid required ventriculostomy placement. The care for patients randomly assigned to the imaging–clinical examination group was provided in accordance with a protocol based on the pretrial standard for care at the three original participating hospitals (see the Supplementary Appendix). In the absence of intracranial mass lesions requiring surgery, signs of intracranial hypertension on imaging or clinical examination were treated first with hyperosmolar therapies with the use of protocol-specified doses on a fixed schedule of administration, optional mild hyperventilation (at a partial pressure of arterial carbon dioxide of 30 to 35 mm Hg), and optional ventricular drainage. Continuing edema prompted consideration of the administration of high-dose barbiturates. Additional treatments were required for patients with “neuroworsening,”9 persistent edema, or clinical signs of intracranial hypertension. (More information on the interventions provided and on operational definitions — including the definition of neuroworsening — is available in the Supplementary Appendix.)
OUTCOMES
The primary outcome, assessed within 6 months after the study onset, was a composite of 21 components: measures of survival (survival time, counted as 1 component), duration and level of impaired consciousness (time to follow commands, sum of errors on the orientation questions from the Galveston Orientation and Amnesia Test [GOAT] on discharge from the hospital — 2 components), functional status and orientation 3 months after injury (assessed with the use of the Extended Glasgow Outcome Scale [GOS-E], the Disability Rating Scale, and GOAT — 3 components), and functional and neuropsychological status 6 months after injury (15 components). The battery of tests included measures of mental status, working memory, information-processing speed, episodic memory and learning, verbal fluency, executive function, and motor dexterity (information on the range and direction of scores for each measure is provided in Table S2 in the Supplementary Appendix). Trained examiners who were unaware of the group assignments administered the tests at 3 and 6 months. Data quality and monitoring are discussed in the Supplementary Appendix.
For the primary outcome, each participant’s percentile was determined separately for each of the 21 measures; the overall outcome was the average of the 21 percentiles10 (on a scale from 0 to 100, with lower percentiles representing worse outcomes); for details, see the outcomes section in the Supplementary Appendix. Protocol-specified secondary outcomes were the length of stay in the ICU (measured as the total number of days in the ICU and the number of days in the ICU on which the patient received at least one brain-specific treatment) and systemic complications. Brain-specific treatments were those directed at intracranial hypertension and included the administration of hyperosmolar agents and pressors and the use of hyperventilation but excluded ventilation, sedation, and analgesia. Additional, post hoc secondary outcomes were the hospital length of stay, the number of days of mechanical ventilation, treatment with high-dose barbiturates or decompressive craniectomy, and therapeutic intensity (for details, see the Supplementary Appendix). For some analyses focused specifically on interventions for intracranial hypertension, we defined the duration of therapy as the number of days from injury until the last brain-specific treatment. Data for patients who survived for more than 1 day after the last brain-specific treatment (collectively referred to as the brain-treatment survivors subgroup) were also analyzed. We integrated brain-specific treatments by summing the number of treatments delivered per hour over the course of the treatment interval.
STUDY OVERSIGHT
The study was approved by the institutional review board at the University of Washington and the ethics committees at all study centers. All authors vouch for the accuracy and completeness of the data and data analyses and for the fidelity of this report to the study protocol. Integra Life Sciences donated the catheters used in monitoring intracranial pressure and provided additional unrestricted support for this project. Integra had no role in the design or conduct of the study, the data analysis, or the writing of the manuscript.
STATISTICAL ANALYSIS
The planned sample size of 324 was determined by means of simulation to provide 80% power to detect an increase of 10 percentage points in the percentage of patients with a good outcome or with moderate disability according to the GOS-E (odds ratio with imaging and clinical examination vs. pressure monitoring, 1.5), and a corresponding improvement on other measures (see the Supplementary Appendix). One planned interim efficacy analysis was conducted when half the participants had undergone the 6-month assessment.
The primary hypothesis was tested with the use of the blocked Wilcoxon test,11 with blocking on stratification factors, and a two-sided significance level of 0.05. We obtained odds ratios and confidence intervals from a logistic proportional-odds model, accounting for the same factors (see the Supplementary Appendix).10 This analysis was supplemented by similar analyses of individual measures and composite analyses of subgroup measures. Cox models were used to analyze survival. A significance level of 0.01 was used to test secondary hypotheses. The main analyses included data on all participants randomly assigned to a treatment group (intention-to-treat population). Sensitivity analyses included analyses restricted to patients who survived, those who received the assigned treatment, and those who survived for at least 24 hours after receiving brain-specific treatments.
RESULTS
STUDY PARTICIPANTS
Patients were recruited between September 2008 and October 2011, with the last follow-up visit occurring in May 2012 (see Fig. S1 in the Supplementary Appendix for information on screening, randomization, and follow-up). The trial ended when the planned sample size was attained. Of 528 eligible patients, 204 (39%) were excluded before randomization (see Table S4 in the Supplementary Appendix for a comparison of the baseline characteristics of enrolled patients and excluded patients). Of the patients who underwent randomization, 92% were followed for 6 months or until death (Table S4 in the Supplementary Appendix). Protocol violations were few (Table S5 in the Supplementary Appendix). The two treatment groups were similar at baseline with regard to all baseline characteristics (Table 1, and Table S6 in the Supplementary Appendix).
Table 1.
Baseline Characteristics of the Study Participants.*
| Variable | Pressure-Monitoring Group (N = 157) | Imaging–Clinical Examination Group (N = 167) |
|---|---|---|
| Age — yr | ||
| Median | 29 | 29 |
| Interquartile range | 22–44 | 22–44 |
| Male sex — no. (%) | 143 (91) | 140 (84) |
| Transferred from another hospital — no./total no. (%) | 97/157 (62) | 101/166 (61) |
| Time to admission to study hospital — hr | ||
| Median | 3.5 | 2.9 |
| Interquartile range | 1.1–8.3 | 1.0–6.5 |
| Direct admissions | ||
| Median | 1.0 | 1.0 |
| Interquartile range | 0.5–1.5 | 0.5–2.0 |
| Transfers | ||
| Median | 6.3 | 5.0 |
| Interquartile range | 3.3–12.2 | 2.8–9.8 |
| Time to admission to first hospital | ||
| Median | 3.0 | 2.5 |
| Interquartile range | 1.1–6.6 | 1.3–6.3 |
| Glasgow Coma Scale at randomization — motor score† | ||
| Median | 5 | 4 |
| Interquartile range | 3–5 | 3–5 |
| Marshall classification on initial CT — no. (%)‡ | ||
| Diffuse injury I | 1 (1) | 0 |
| Diffuse injury II | 24 (15) | 20 (12) |
| Diffuse injury III | 70 (45) | 68 (41) |
| Diffuse injury IV | 10 (6) | 12 (7) |
| Evacuated mass lesion | 48 (31) | 58 (35) |
| Nonevacuated mass lesion | 4 (3) | 7 (4) |
| Abbreviated Injury Scale — score for head§ | ||
| Median | 5 | 5 |
| Interquartile range | 4–5 | 4–5 |
| Mesencephalic cisterns compressed or absent on initial CT — no./total no. (%) | 131/157 (83) | 143/165 (87) |
| Midline shift (≥5 mm) detected on initial CT — no./total no. (%) | 53/157 (34) | 64/164 (39) |
| Signs of intracranial hypertension detected on initial CT — no./total no. (%)¶ | 140/156 (90) | 146/164 (89) |
There were no significant differences between the groups. Additional data are available in Table S6 in the Supplementary Appendix.
The range of scores for the motor component of the Glasgow Coma Scale is 1 to 6, with higher scores indicating a higher level of consciousness.
The Marshall classification of traumatic brain injury is based on a review of CT scans, with diffuse injury I indicating no visible pathology, diffuse injury II indicating the presence of cisterns, with a midline shift of 0 to 5 mm, diffuse injury III indicating pathology similar to that in diffuse injury II, but with swelling, and diffuse injury IV indicating pathology similar to that seen in diffuse injuries II or III, with a midline shift of more than 5 mm. For more detailed information see the Definitions section in the Supplementary Appendix and Marshall et al.12 Percentages for this variable exclude unknown values.
Scores on the Abbreviated Injury Scale range from 1 to 6, with higher values representing more severe injury.
Data on signs of intracranial hypertension are based on the impression of the interpreting physician.
Traffic incidents were the primary cause of injury. Only 45% of participants were transported to the first hospital by ambulance. Most were transferred to study hospitals from another center; the median time to arrival at the first hospital was 1.0 hour for direct admissions and 2.7 hours for transfers. The median time from injury to arrival at study centers for all patients was 3.1 hours. We were unable to acquire accurate information on prehospital interventions or early secondary insults (i.e., hypoxemia or hypotension) because they were not uniformly assessed and recorded.
INITIAL INJURY
Of the study participants who underwent randomization, 24% had a GCS score that was higher on admission but subsequently dropped to the specified range for enrollment. The median GCS motor score at randomization was 4.0; 49% of participants had localizing brain injuries, with none of the participants following commands. One or both pupils were nonreactive in 44% of participants. On the Abbreviated Injury Scale (ranging from 0 to 6, with higher scores indicating more severe injury), the median score for head injury was 5; 82% of participants had a score of 4 or higher. Initial CT revealed a high severity of injury overall, with grade III diffuse injury2–14 (swelling of the brain causing compression of the basal cisterns, without a mass lesion or a midline shift of >5 mm) in 43% of the participants and mass lesions requiring surgical treatment in 33%. Mesencephalic cisterns were compressed or absent in 85% of the participants, and the midline was shifted by more than 5 mm in 36%.
CLINICAL OUTCOMES
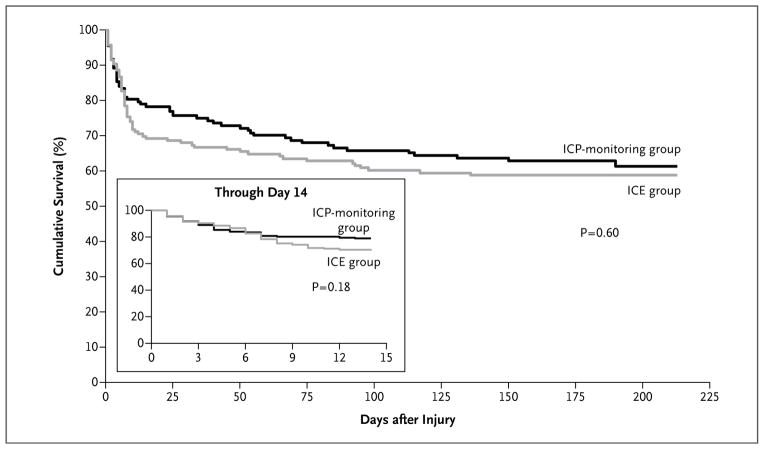
Table 2 (and Table S7A in the Supplementary Appendix) shows the results for the primary (composite) outcome, individual measures, and sensitivity analyses. There were no significant differences between groups. The survival rates for the two study groups are shown in Figure 1. The 14-day mortality was 30% in the imaging–clinical examination group as compared with 21% in the pressure-monitoring group (hazard ratio, 1.36; 95% confidence interval [CI], 0.87 to 2.11; P = 0.18); the 6-month mortality was 41% and 39% in the two groups, respectively (hazard ratio, 1.10; 95% CI, 0.77 to 1.57; P = 0.60). The results for the primary outcome were similar in an analysis restricted to survivors and in analyses of subgroups defined by sex (prespecified subgroup analysis), site, CT findings, and age (Tables S7B and S8 in the Supplementary Appendix).
Table 2.
Clinical Outcomes.*
| Variable | Pressure-Monitoring Group (N = 157) | Imaging–Clinical Examination Group (N = 167) | P Value | Proportional Odds Ratio (95% CI)† |
|---|---|---|---|---|
| Patients assessed at 6 mo — no. (%) | 144 (92) | 153 (92) | ||
| Primary outcome‡ | 0.49§ | 1.09 (0.74–1.58) | ||
| Median | 56 | 53 | ||
| Interquartile range | 22–77 | 21–76 | ||
| Cumulative mortality at 6 mo — % | 39 | 41 | 0.60¶ | 1.10 (0.77–1.57) |
| GOS-E scale at 6 mo — no. (%)|| | ||||
| Death | 56 (39) | 67 (44)** | 0.40§ | 1.23 (0.77–1.96) |
| Unfavorable outcome | 24 (17) | 26 (17) | ||
| Favorable outcome | 63 (44) | 60 (39) |
Additional outcomes are listed in Table S7A in the Supplementary Appendix. Outcomes for survivors only are in listed Table S7B in the Supplementary Appendix.
Proportional odds ratios were adjusted for site, age, and severity of injury. A value of more than 1 indicates a better outcome for the pressure-monitoring group. The study was designed to detect a difference corresponding to an odds ratio of 1.5. CI denotes confidence interval.
The primary outcome was based on a composite measure and calculated as an average percentile over 21 elements. The range is 0 to 100, and a higher percentile indicates a better outcome. A detailed description of the composite outcome appears in the outcomes section in the Supplementary Appendix; individual elements are listed in Table S2 in the Supplementary Appendix.
Statistical significance was determined by means of a blocked Wilcoxon test stratified according to site, age, and severity of injury at randomization.
Statistical significance was determined by means of Cox model regression with adjustment for site, age, and severity of injury at randomization.
The Extended Glasgow Outcome Scale (GOS-E) ranges from 1 to 8, with 1 indicating death and 8 indicating the most favorable recovery. Patients with scores ranging from 2 to 4 were classified as having an unfavorable outcome, and those with scores ranging from 5 to 8 were classified as having a favorable outcome.
Mortality for the 6-month GOS-E assessment was higher than cumulative mortality because data for participants who were lost to follow-up were excluded from the 6-month GOS-E assessment but were included as censored data for the calculation of cumulative mortality.
Figure 1. Cumulative Survival Rate According to Study Group.
A Kaplan–Meier survival plot based on the prespecified analysis shows the cumulative survival rate at 6 months among patients assigned to imaging and clinical examination (ICE) as compared with those assigned to intracranial-pressure (ICP) monitoring (hazard ratio for death, 1.10; 95% confidence interval [CI], 0.77 to 1.57). The inset shows the results of the post hoc analysis at 14 days (hazard ratio, 1.36; 95% CI, 0.87 to 2.11).
PROCESSES OF CARE
Table 3 (and Table S9A in the Supplementary Appendix) shows the between-group comparisons for variables reflecting processes of care. The hospital length of stay was marginally shorter in the imaging–clinical examination group than in the pressure-monitoring group only when all participants who underwent randomization were included in the analysis. There were no significant differences between groups with respect to the ICU length of stay, in either the intention-to-treat population or the brain-treatment survivors subgroup (Table S9B in the Supplementary Appendix). For this subgroup, the median length of stay was 13 days in the ICU and 26 days in the hospital. There were no significant between-group differences in the number of days of mechanical ventilation. The evaluation of non-neurologic complications also revealed no significant differences between treatment groups, except that patients in the pressure-monitoring group had a significantly higher rate of decubitus ulcers (12%, vs. 5% in the imaging–clinical examination group; P = 0.03).
Table 3.
Processes of Care.*
| Variable | Pressure-Monitoring Group (N = 157) | Imaging–Clinical Examination Group (N = 167) | P Value† | Proportional Odds Ratio (95% CI)‡ |
|---|---|---|---|---|
| Duration of ICP monitoring — days | — | — | — | |
| Median | 3.6 | |||
| Interquartile range | 2.0–6.6 | |||
| Initial ICP ≥20 mm Hg — no./total no. (%) | 55/147 (37) | — | — | — |
| ICP ≥20 mm Hg — % of readings | — | — | — | |
| Median | 7 | |||
| Interquartile range | 1–31 | |||
| CPP ≤60 mm Hg — % of readings | — | — | — | |
| Median | 6 | |||
| Interquartile range | 2–21 | |||
| Protocol-specified comparisons | ||||
| Length of stay in ICU — days | 0.25 | 0.81 (0.55–1.18) | ||
| Median | 12 | 9 | ||
| Interquartile range | 6–17 | 6–16 | ||
| Length of stay in ICU with brain-specific treatment — days§ | 0.002 | 1.87 (1.28–2.75) | ||
| Median | 3.4 | 4.8 | ||
| Interquartile range | 1.1–7.0 | 2.3–7.4 | ||
| Respiratory complications — no. (%) | 93 (59) | 108 (65) | 0.36 | 1.00 (0.63–1.59) |
| Sepsis — no. (%) | 16 (10) | 12 (7) | 0.43 | 0.61 (0.27–1.41) |
| Decubitus ulcers — no. (%) | 19 (12) | 8 (5) | 0.03 | 0.35 (0.15–0.85) |
| Non-neurologic complications — no. (%) | 134 (85) | 147 (88) | 0.52 | 1.20 (0.62–2.34) |
| Post hoc comparisons¶ | ||||
| Integrated brain-specific treatment intensity | <0.001 | 2.36 (1.60–3.47) | ||
| Median | 69 | 125 | ||
| Interquartile range | 13–181 | 45–233 | ||
| Individual treatments — no./total no. (%) | ||||
| Mannitol | 80/157 (51) | 94/166 (57) | 0.25 | 1.32 (0.82–2.13) |
| Hypertonic saline | 90/156 (58) | 119/166 (72) | 0.008 | 1.95 (1.19–3.22) |
| Furosemide | 6 (4) | 13 (8) | 0.11 | 2.53 (0.82–7.81) |
| Hyperventilation | 93 (60) | 122 (73) | 0.003 | 2.16 (1.29–3.61) |
| Cerebrospinal fluid drainage | 1 (1) | 3 (2) | 0.37 | 2.84 (0.29–27.78) |
| Barbiturates | 38 (24) | 22 (13) | 0.02 | 0.46 (0.25–0.83) |
| Neurosurgical procedures — no./total no. (%) | ||||
| Craniotomy for mass lesion | 63/157 (40) | 74/166 (45) | 0.50 | 1.19 (0.76–1.86) |
| Craniectomy | 44/157 (28) | 49/166 (30) | 0.81 | 1.04 (0.63–1.69) |
| Alone | 9 (6) | 9 (5) | 1.00 | 0.93 (0.35–2.42) |
| With other neurosurgical procedure | 35 (22) | 40 (24) | 0.79 | 1.07 (0.63–1.80) |
Additional variables measured as part of the processes of care are listed in Table S9A in the Supplementary Appendix for all patients who underwent randomization. Processes of care for brain-specific treatment for survivors only are listed in Table S7B in the Supplementary Appendix. CPP denotes cerebral perfusion pressure, ICP intracranial pressure, and ICU intensive care unit.
P values for comparisons in which the median and interquartile range are provided were calculated with the use of a blocked Wilcoxon test11; all other P values were calculated with the use of Fisher’s exact test.
For proportional odds ratios, a value greater than 1 indicates a more favorable assessment for the pressure-monitoring group.
The length of stay in the ICU with brain-specific treatment was defined as the time up to last use of a treatment for intracranial hypertension other than ventilation, sedation, or analgesia.
The treatment intensity for post hoc comparisons was defined as the number of different treatments for intracranial hypertension (other than ventilation, sedation, or analgesia) per hour, summed over the duration of brain-specific treatment, and counting high-dose mannitol, hypertonic saline, or hyperventilation as two treatments. See Table S9A in the Supplementary Appendix for details.
The median time during which intracranial pressure was monitored was 3.6 days in the entire pressure-monitoring group and 4.0 days in the brain-treatment survivors subgroup (Table 3, and Tables S9A and S9B in the Supplementary Appendix). The median and mean percentages of readings that were 20 mm Hg or higher were 7 and 20%, respectively, in the entire study population and 5 and 13%, respectively, in the brain-treatment survivors subgroup. For these respective groups, the intracranial pressure was 20 mm Hg or higher initially in 37% and 29% of patients and at any time during monitoring in 79% and 76% of patients. The incidence of neuroworsening after randomization was 25% for the entire study population and did not differ significantly between the two treatment groups.
The median interval during which patients received brain-specific treatment was significantly longer in the imaging–clinical examination group than in the pressure-monitoring group. In addition, post hoc analyses of integrated treatment intensity (see the definition in the outcomes section in the Supplementary Appendix) revealed that the total number of treatments was significantly greater for the imaging–clinical examination group as a whole and for the brain-treatment survivors subgroup than for the pressure-monitoring group. Table 3, and Table S9A in the Supplementary Appendix, show that the use of high-dose barbiturates was greater in the pressure-monitoring group than in the imaging–clinical examination group (24% vs. 13%). There was no significant between-group difference in the number of patients who underwent craniectomy. The proportion of patients treated with hypertonic saline and the proportion treated with hyperventilation were significantly higher in the imaging–clinical examination group than in the pressure-monitoring group (72% vs. 58% and 73% vs. 60%, respectively). Among patients who received treatment with mannitol or hypertonic saline, the duration of treatment was longer in the imaging–clinical evaluation group than in the pressure-monitoring group (21 hours vs. 13 hours for mannitol and 21 hours vs. 10 hours for hypertonic saline).
ADVERSE EVENTS
The distributions of serious adverse events, adverse events, complications, and catheter-related adverse events are shown in Table 4, as well as in Tables S10A and S10B in the Supplementary Appendix. There were no serious catheter-related adverse events in either study group.
Table 4.
Catheter-Related or Serious Adverse Events.*
| Adverse Event | Pressure-Monitoring Group (N = 157) | Imaging–Clinical Examination Group (N = 167) | P Value† |
|---|---|---|---|
| number (percent) | |||
| Events related to ICP catheter‡ | 10 (6) | — | — |
|
| |||
| Infection | 0 | — | — |
|
| |||
| Catheter malfunction | 4 (3) | — | — |
|
| |||
| Unplanned catheter removal | 4 (3) | — | — |
|
| |||
| Hemorrhage | 2 (1) | — | — |
|
| |||
| Any serious adverse event | 70 (45) | 76 (46) | 0.91 |
|
| |||
| Infections | 13 (8) | 10 (6) | 0.52 |
|
| |||
| Nervous system events, excluding infections | 19 (12) | 29 (17) | 0.21 |
|
| |||
| Respiratory system events, excluding infections | 9 (6) | 8 (5) | 0.81 |
|
| |||
| Cardiovascular system events | 17 (11) | 13 (8) | 0.44 |
|
| |||
| Death from an unspecified cause | 12 (8) | 12 (7) | 1.00 |
Additional adverse events are listed in Tables S10A and S10B in the Supplementary Appendix.
Statistical significance was calculated with the use of Fisher’s exact test.
None of the catheter-related adverse events met the criteria for a serious adverse event.
DISCUSSION
Our results do not support the hypothesized superiority of management guided by intracranial-pressure monitoring over management guided by neurologic examination and serial CT imaging in patients with severe traumatic brain injury. Intracranial-pressure monitoring is the cornerstone of treatment for severe traumatic brain injury. The principle guiding additional interventions, such as the monitoring of cerebral perfusion pressure or tissue-perfusion modification, is the maintenance of intracranial pressure below 20 mm Hg.
Most of the data from nonrandomized, controlled trials support the association of treatment based on monitored intracranial pressure with improved recovery, which has led to the recommendation of this approach in successive editions of published guidelines for the management of severe traumatic brain injury4–7 (although there have been calls for a randomized, controlled trial). Dissenting literature does exist. In two retrospective studies, there was no association15 or a negative association16 between monitoring-based treatment and outcome, and in an older, small, low-quality study of the usefulness of monitoring in guiding mannitol dosing, monitoring was not found to be useful.17
Since our study was conducted in Bolivia and Ecuador, the extent to which the findings can be generalized to other patient populations warrants discussion. Our data suggest that the care provided in the study hospitals adhered to the fundamentals of ICU care and was consistent with the study design. Prehospital resuscitation is less developed in Bolivia and Ecuador than in higher-income countries, and the more severely injured patients in those two countries may not survive long enough to reach the hospital. Thus, the study population may have had less severe brain injury than comparable ICU populations in higher-income countries. On the other hand, less advanced prehospital resuscitation may result in secondary insults (e.g., hypoxemia and hypotension), which would serve to increase the severity of the injury. In our study, the initial and subsequent readings of intracranial pressure, findings on CT, and pupillary responses were all consistent with very severe injury. The early outcome curves in our study appear to be consistent with what would be expected for young adults with severe brain injury whose care was being well managed in ICUs in wealthier countries. The results we report on early mortality were also similar to those reported in higher-income countries.14 Survival at 6 months is confounded by high mortality (35% of the deaths) after the first 14 days, which is probably related to the limited resources available after discharge from the ICU. None of the study participants received rehabilitation or extensive medical care after hospital discharge. The elderly population with traumatic brain injury, which is prominent in high-income countries, was not represented in this study.
Between-group differences in the individual treatments delivered (with greater use of hyper-tonic saline, mannitol, and hyperventilation in the imaging–clinical examination group than in the pressure-monitoring group) reflect differences in approaches to treatment: scheduled treatment in the imaging–clinical examination protocol and treatment as indicated in the pressure-monitoring protocol. The quantitative measurement of intracranial pressure and the consequent fixed treatment threshold probably explains the more frequent administration of high-dose barbiturates and high-dose hypertonic saline in the pressure-monitoring group.
There was a need to standardize the type of monitoring used. Intraparenchymal monitoring was chosen for its accuracy,7 ease of insertion, safety profile,18 and low maintenance requirements. The alternative — a transduced ventricular catheter, which is accepted worldwide and was available but rarely used at the study sites before the start of the study — was not believed to be as compatible with our study setting, even though it offers the inherently useful therapeutic option of draining cerebrospinal fluid. Cerebrospinal-fluid drainage was a treatment option that would have required separate ventriculostomy placement — an approach to monitoring that is similar to that specified in the protocol for the ongoing Brain Tissue Oxygen Monitoring in Traumatic Brain Injury (BOOST 2) trial (ClinicalTrials.gov number, NCT00974259). Drainage of cerebrospinal fluid is consistent with guidelines-based management.7 Although it is effective as a means of lowering elevated intracranial pressure temporarily,19 drainage has not been shown to improve the outcome of severe traumatic brain injury.20
At issue here is not the question of whether intracranial pressure is important — both groups were treated for intracranial hypertension. We investigated whether the guidelines-based7 protocol used in this study significantly improved the outcome. Our results do not support the superiority of treatment based on intracranial-pressure monitoring7 over treatment guided by neurologic testing and serial CT imaging in improving short-term or long-term recovery in the general population of patients with severe traumatic brain injury. This finding does not argue against the use of intracranial–pressure monitoring. Only the monitoring-based interventional algorithm was tested here. It is possible that the imaging–clinical examination protocol provided superior control of intracranial pressure.17 Alternatively, the lack of efficacy may be attributable to other factors, such as the use of a universal threshold for intracranial pressure or the efficacies and toxic effects of the therapeutic agents used, individually or in combination. Additional reasons for the lack of efficacy may include the interpretation of the data on intracranial pressure (a focus on instantaneous values rather than trends or on intracranial pressure rather than cerebral compliance), the lack of identification of subtypes of traumatic brain injury requiring different approaches to management (subtype identification may evolve over the course of treatment), the universal primacy of manipulation of intracranial pressure as opposed to consideration of other physiological interventions (e.g., management of cerebral perfusion pressure), or even the consideration of intracranial pressure as a treatment variable rather than merely an indication of disease severity.
The value of knowing the precise intracranial pressure is not being challenged here, nor is the value of aggressively treating severe traumatic brain injury being questioned. Rather our data suggest that a reassessment of the role of manipulating monitored intracranial pressure as part of multimodality monitoring and targeted treatment of severe traumatic brain injury is in order.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health and the Fogarty International Center, the National Institute of Neurological Disorders and Stroke (ROINS058302), and Integra Life Sciences.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Myburgh JA, Cooper DJ, Finfer SR, et al. Epidemiology and 12-month outcomes from traumatic brain injury in Australia and New Zealand. J Trauma. 2008;64:854–62. doi: 10.1097/TA.0b013e3180340e77. [DOI] [PubMed] [Google Scholar]
- 2.Sahjpaul R, Girotti M. Intracranial pressure monitoring in severe traumatic brain injury — results of a Canadian survey. Can J Neurol Sci. 2000;27:143–7. [PubMed] [Google Scholar]
- 3.Stocchetti N, Penny KI, Dearden M, et al. Intensive care management of head-injured patients in Europe: a survey from the European brain injury consortium. Intensive Care Med. 2001;27:400–6. doi: 10.1007/s001340000825. [DOI] [PubMed] [Google Scholar]
- 4.Bullock R, Chesnut R, Clifton G, et al. Guidelines for the management of severe head injury — revision 1. New York: Brain Trauma Foundation; 1998. [DOI] [PubMed] [Google Scholar]
- 5.Guidelines for the management of severe head injury. J Neurotrauma. 1996;13:639–734. doi: 10.1089/neu.1996.13.643. [DOI] [PubMed] [Google Scholar]
- 6.The Brain Trauma Foundation, the American Association of Neurological Surgeons, the Joint Section on Neurotrauma and Critical Care. Guidelines for the management of severe head injury — revision. J Neurotrauma. 2000;17:457–62. [Google Scholar]
- 7.Bratton SL, Chesnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl):S1–S106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 8.Carney N, Lujan S, Dikmen S, et al. Intracranial pressure monitoring in severe traumatic brain injury in Latin America: process and methods for a multi-center randomized controlled trial. J Neurotrauma. 2012;29:2022–9. doi: 10.1089/neu.2011.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris GF, Juul N, Marshall SB, Benedict B, Marshall LF. Neurological deterioration as a potential alternative endpoint in human clinical trials of experimental pharmacological agents for treatment of severe traumatic brain injuries. Neurosurgery. 1998;43:1369–72. [PubMed] [Google Scholar]
- 10.O’Brien PC. Procedures for comparing samples with multiple end points. Biometrics. 1984;40:1079–87. [PubMed] [Google Scholar]
- 11.van Elteren P. On the combination of independent two-sample tests of Wilcoxon. Bull Inst Internat Stat. 1960;37:351–61. [Google Scholar]
- 12.Marshall LF, Bowers-Marshall S, Klauber MR, et al. A new classification of head injury based on computerized tomography. J Neurosurg. 1991;75(Suppl):S14–S20. [Google Scholar]
- 13.Association for the Advancement of Automotive Medicine. Abbreviated Injury Scale (AIS) Barrington, IL: AAAM; 2001. [Google Scholar]
- 14.Marshall LF, Gautille T, Klauber MR, et al. The outcome of severe head injury. J Neurosurg. 1991;75(Suppl):S28–S36. [Google Scholar]
- 15.Cremer OL, van Dijk GW, van Wensen E, et al. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Crit Care Med. 2005;33:2207–13. doi: 10.1097/01.ccm.0000181300.99078.b5. [DOI] [PubMed] [Google Scholar]
- 16.Shafi S, Diaz-Arrastia R, Madden C, Gentilello L. Intracranial pressure monitoring in brain-injured patients is associated with worsening of survival. J Trauma. 2008;64:335–40. doi: 10.1097/TA.0b013e31815dd017. [DOI] [PubMed] [Google Scholar]
- 17.Smith HP, Kelly D, Jr, McWhorter JM, et al. Comparison of mannitol regimens in patients with severe head injury undergoing intracranial monitoring. J Neurosurg. 1986;65:820–4. doi: 10.3171/jns.1986.65.6.0820. [DOI] [PubMed] [Google Scholar]
- 18.Gelabert-González M, Ginesta-Galan V, Sernamito-García R, Allut AG, Bandin-Diéguez J, Rumbo RM. The Camino intracranial pressure device in clinical practice: assessment in a 1000 cases. Acta Neurochir (Wien) 2006;148:435–41. doi: 10.1007/s00701-005-0683-3. [DOI] [PubMed] [Google Scholar]
- 19.Kerr ME, Weber BB, Sereika SM, Wilberger J, Marion DW. Dose response to cerebrospinal fluid drainage on cerebral perfusion in traumatic brain-injured adults. Neurosurg Focus. 2001;11(4):E1. doi: 10.3171/foc.2001.11.4.2. [DOI] [PubMed] [Google Scholar]
- 20.Meyer MJ, Megyesi J, Meythaler J, et al. Acute management of acquired brain injury part II: an evidence-based review of pharmacological interventions. Brain Inj. 2010;24:706–21. doi: 10.3109/02699051003692126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.