Abstract
Background
Uncomplicated urinary tract infections (UTI) in dogs usually are treated with antimicrobial drugs for 10–14 days. Shorter duration antimicrobial regimens have been evaluated in human patients.
Hypothesis
A high dose short duration (HDSD) enrofloxacin protocol administered to dogs with uncomplicated UTI will not be inferior to a 14-day treatment regimen with amoxicillin-clavulanic acid.
Animals
Client-owned adult, otherwise healthy dogs with aerobic bacterial urine culture yielding ≥103 CFU/mL of bacteria after cystocentesis.
Methods
Prospective, multicenter, controlled, randomized blinded clinical trial. Enrolled dogs were randomized to group 1 (enrofloxacin 18–20 mg/kg PO q24h for 3 days) or group 2 (amoxicillin-clavulanic acid 13.75–25 mg/kg PO q12h for 14 days). Urine cultures were obtained at days 0, 10, and 21. Microbiologic and clinical cure rates were evaluated 7 days after antimicrobial treatment was discontinued. Lower urinary tract signs and adverse events also were recorded.
Results
There were 35 dogs in group 1 and 33 in group 2. The microbiologic cure rate was 77.1 and 81.2% for groups 1 and 2, respectively. The clinical cure rate was 88.6 and 87.9% for groups 1 and 2, respectively. Cure rates between groups did not differ according to the selected margin of noninferiority.
Conclusions and Clinical Importance
HDSD enrofloxacin treatment was not inferior to a conventional amoxicillin-clavulanic acid protocol for the treatment of uncomplicated bacterial UTI in dogs. Further research is warranted to determine if this protocol will positively impact owner compliance and decrease the emergence of antimicrobial resistance.
Keywords: Amoxicillin-clavulanic acid, Bladder, Enrofloxacin, Lower urinary tract infection
Bacterial urinary tract infections (UTIs) have been estimated to occur in approximately 14% of dogs during their lifetime,1 with a variable age of onset. The clinical signs of bacterial cystitis include stranguria, pollakiuria, inappropriate urinations, dysuria, and hematuria. Clinically occult UTIs in dogs and cats also have been reported.2,3 Although there are no studies in dogs to determine the appropriate duration of treatment for simple, uncomplicated UTIs, they usually are treated for 7–14 days.4–6 Uncomplicated UTI is defined as a UTI without structural, metabolic, or functional urinary tract abnormalities, as well as the absence of other comorbidities, such as cystic calculi, bladder neoplasia, and systemic disorders, such as uncontrolled diabetes mellitus or hyperadrenocorticism7 that could favor a more serious adverse outcome. A longer duration of treatment has been suggested for humans8 and dogs6 with complicated UTIs.
In human patients, uncomplicated UTIs often are treated for a maximum of 3 days; however, single dose protocols also have been described.9,10 Various antimicrobial regimens for UTI, including fluoroquinolones, have been evaluated; relapsing infections were reported more often in single dose protocols.10–12 A relapsing or persistent infection occurs when the initial infection was not resolved despite treatment. In contrast, reinfections also may occur, but imply that a new bacterial species or strain infects the patient after a period of sterility.13 Efficacy of short duration treatment regimens has been variable, but some higher dose protocols appear to result in longer short- and long-term cure rates.14 Fluoroquinolones are commonly used15,16 because of their spectrum of activity against uropathogens and the high concentrations that are achieved in urine.17,18 In humans, short durations of treatment and decreased dosing frequency of antimicrobials have been reported to increase compliance, lower costs, decrease adverse effects, and may be as effective as conventional protocols.7,19
Fluoroquinolones also have been used to treat UTI in dogs. Enrofloxacina is a fluoroquinolone antimicrobial drug that was developed specifically for veterinary use, and approved dosages range from 5 to 20 mg/kg PO q24h. The efficacy of a high dose, short duration (HDSD) antimicrobial regimen in naturally occurring UTI in dogs has not been evaluated. Therefore, the purpose of this study was to evaluate the efficacy of a 3-day course of HDSD enrofloxacin compared with a 14-day period of treatment with amoxicillin-clavulanic acidb for uncomplicated UTI in dogs. Amoxicillin-clavulanic acid was chosen because this antibiotic is approved for treatment of UTI in dogs, has a spectrum of activity against common uropathogens, reaches high concentrations in the urine, and is commonly prescribed by primary care veterinarians.4,20 We hypothesized that in naturally occurring uncomplicated UTI, neither microbiologic cure rate nor clinical resolution rate would be significantly different between the 2 treatment groups.
Materials and Methods
Study Design
This was a prospective, multicenter, controlled, randomized, and blinded clinical trial. The trial was conducted in accordance with Guidance for Industry #85, Good Clinical Practice.c The study was performed at 2 university teaching hospitals (William R. Pritchard, Veterinary Medical Teaching Hospital at University of California, Davis, and The Ohio State University Veterinary Medical Center) as well as 3 veterinary private practice sites located in Missouri, Louisiana, and Pennsylvania.
Client-owned, adult dogs that weighed 5–50 kg and that were suspected to have an uncomplicated clinical UTI at each study site were eligible for enrollment. Juvenile dogs were excluded from the study because articular cartilage lesions have been reported with the use of fluoroquinolones in rapidly growing dogs.21 Young age was defined as <9 months of age for dogs that weighed 5.0–25.9 kg at maturity, <1 year for dogs that weighed 26–45 kg, and <18 months for dogs that weighed >45 kg. A complete medical history was collected. Dogs were excluded from the study if they had a history of persistent or recurrent UTI (defined as >3 UTIs in 1 year with or without a period of sterility), or uncontrolled systemic comorbid diseases that could predispose the dog to recurrent infections. Dogs with diabetes mellitus, hyperadrenocortocism, and urinary incontinence also were excluded. Antimicrobial drug administration within 7 days or corticosteroid administration within 14 days of initial presentation or other compounds containing metal cations or administration of antacids also were criteria for exclusion, the latter because these cations can bind to enrofloxacin and decrease its absorption.20
On day 0, a physical examination, which included bladder palpation, was performed on all dogs by 1 of the study investigators. The presence or absence of lower urinary tract signs (LUTS) was recorded. LUTS were defined as pollakiuria, stranguria, and hematuria. A complete blood count (CBC) and serum biochemical analysis also were performed on day 0. In addition, urine was collected by cystocentesis and subjected to urinalysis and quantitative aerobic bacterial urine culture with susceptibility testing by broth microdilution using minimum inhibitory concentration (MIC) testing.
To be enrolled in the study, dogs had to be deemed otherwise healthy based on the history, physical examination, and absence of abnormalities on the CBC and serum biochemical analysis. Ultrasound examination of the urinary bladder was performed only in dogs seen at the university sites. Only dogs with aerobic bacterial urine cultures that yielded ≥103 CFU/mL of at least 1 of the isolates or a mixture of up to 2 target pathogenic bacteria were definitively eligible for inclusion. Pathogenic bacteria were defined as Escherichia coli, Proteus spp., Klebsiella spp., Pseudomonas aeruginosa, Enterobacter spp., Staphylococcus aureus, Staphylococcus intermedius group (S. pseudintermedius, S. intermedius, S. delphini), alpha-hemolytic Streptococcus spp., and Enterococcus spp. Dogs were included if nontargeted bacteria were isolated in conjunction with the targeted pathogens. Dogs with suspected or confirmed pyelonephritis, prostatitis, urinary tract neoplasia, urinary calculi, uncontrolled systemic disease, spinal cord injuries, and suspected CNS disorders were not eligible for enrollment.
Enrolled dogs then were randomized to group 1 or group 2 by random number generation to ensure an equal number of dogs in each group. Dogs in group 1 received enrofloxacin,a 18–20 mg/kg PO q24h for 3 days. Dogs in group 2 received amoxicillin-clavulanic acid,b 13.75–25 mg/kg PO q12h for 14 days. An unblinded veterinary technician enrolled dogs and dispensed medications for each study site. The time of day the antimicrobial was to be administered was not specified to the client. The technician instructed all pet owners not to discuss the treatment regimen with anyone other than the dispenser, but did not otherwise participate in the clinical observations. All veterinarians who performed clinical observations were blinded to treatment group. During the study period, dog owners were asked to record concomitant medications and adverse events that occurred. They were instructed to return their dog to the study site on day 10 (±2 days) and day 21 (±2 days) and bring the prescribed antimicrobial drug with them. During these visits, a complete history was obtained, pills were counted, and a physical examination, urinalysis, and quantitative aerobic bacterial urine culture and susceptibility testing were performed.
Microbiologic cure and clinical cure were evaluated as study endpoints 7 days after discontinuation of antimicrobial treatment, which corresponded to day 10 for group 1 and day 21 for group 2. Long-term microbiologic and clinical cure rates also were determined for group 1 dogs on day 21, 18 days after completing enrofloxacin treatment. Only dogs that had urine cultures performed at those times points were included in the statistical analysis for microbiologic cure. If an animal was removed because of persistent clinical signs, it was considered a clinical failure. Microbiologic cure was defined as a negative aerobic bacterial urine culture, or a culture that yielded <103 CFU/mL of bacteria. Microbiologic failure was defined as an aerobic bacterial urine culture that yielded >103 CFU/mL of the same pathogen as detected in the pretreatment urine specimen. Clinical cure was defined as a lack of any LUTS.
Culture and Susceptibility Testing
Urine cultures and antimicrobial susceptibility testing were performed at the hospital microbiology laboratories (university sites) or at an independent laboratoryd for the private practice sites. Culture and susceptibility testing procedures were performed in a similar manner at each laboratory. Quantitative urine cultures were performed by streaking 5% defibrinated sheep blood and MacConkey agarse each with 10 μL of urine using a calibrated loop. Plates were incubated at 35°C in 5% CO2 or ambient air. Organisms were identified using routine microbiologic biochemical testing, including gram stain, spot testing (indole, oxidase), tubed biochemical media, and bacterial identification kits.f22 Cultures were considered negative when there was no growth after 3 days. Susceptibility testing was performed by broth microdilution according to Clinical Laboratory Standards Institute (CLSI) methods using a commercially available panel of antimicrobial drugs.g23 Interpretive breakpoints were based on CLSI standards for small animals when available, otherwise standards for human patient isolates were used.24
Statistical Analysis
Noninferiority tests were performed using the Proc Freq procedureh on the dependent variables (proportion of successes) for microbiologic cure as the primary parameter, and clinical outcome as the secondary parameter. The lower bound 1-sided 95% confidence interval was calculated for the success rate difference between group 1 and group 2, and was compared with −20% margin of difference to determine noninferiority.
Body weight, sex, and the results of urinalysis on day 0 were compared between groups. For continuous data, an analysis of variance method was used that included treatment group, sex, the interaction between treatment group and sex, and, where applicable, a baseline covariate. For categorical data, Fisher's exact test was used.
Statistical analysis was performed using a statistical analysis software packageg and an alpha level of 0.05 was used for statistical significance. Data were expressed as means ± standard deviations if normally distributed; otherwise, the median and range were provided.
Results
Dogs
A total of 92 dogs were evaluated for entry into the study on day 0 across all 5 study sites. Twenty-four dogs ultimately were excluded. Nine dogs were excluded because growth on urine culture was <103 CFU/mL (n = 7), or growth on urine culture was a nontargeted pathogen (n = 2). An additional 15 dogs were excluded after enrollment because either the organism grown on day 10 or day 21 was not the same organism as day 0 (n = 2), owner noncompliance was identified (n = 5), a concurrent disorder that may have predisposed the dog to a UTI was documented (n = 4), the incorrect dosage of medication was prescribed (n = 2), the dog was removed from trial because of an owner-perceived adverse event (n = 1), or a medication was administered that was not allowed according to the study protocol (n = 1).
Of the 68 remaining dogs included in the statistical analysis, 35 (51.4%) were allocated to group 1 and 33 (48.5%) were allocated to group 2. Group 1 was comprised of 30 females and 5 males and group 2 was comprised of 27 females and 6 males. There was no difference in sex distribution between the 2 groups (P = .75). All dogs in both groups were spayed or neutered. Group 1 was comprised of 6 Labrador Retrievers, 3 Boxers, 3 mixed breed dogs, 3 Siberian Huskies, 2 Golden Retrievers, 2 Dachshunds, and 1 each of various other breeds. Group 2 consisted of 6 mixed breed dogs, 5 Labrador Retrievers, 3 Dachshunds, 3 Pugs, 2 Golden Retrievers, and 1 each of various other breeds. The median body weight for group 1 dogs was 25.4 kg (range, 5.0–48.8 kg) and for group 2 dogs was 19.0 kg (range, 5.1–50.0 kg) (P = .42). The median age for group 1 and group 2 dogs was 7.4 and 9.4 years, respectively (P = .42).
No differences in clinical signs were noted between the groups (Table 1). Ten dogs from group 1 and 8 dogs from group 2 had all 3 LUTS. Bladder pain was identified in 6 dogs from group 1 and 7 dogs from group 2.
Table 1.
Number and percentage of dogs with lower urinary tract signs as reported by owners on day 0. Group 1 dogs were treated with enrofloxacin for 3 days and group 2 dogs were treated with clavulanic acid-amoxicillin for 14 days
| Clinical Sign | Group 1 (n = 35) | Group 2 (n = 33) | P Value |
|---|---|---|---|
| Pollakiuria | 33 (94.3) | 33 (100) | .5 |
| Stranguria | 21 (60.0) | 19 (57.6) | 1.0 |
| Macroscopic hematuria | 16 (45.7) | 14 (42.4) | .8 |
The mean urine specific gravity of group 1 and group 2 dogs was 1.028 ± 0.012 (range, 1.005–1.050) and 1.026 ± 0.013 (range, 1.002–1.052), respectively. There was no difference in urine specific gravity between the 2 groups (P = .92). The median urine pH of group 1 and group 2 dogs was 7.5 (range, 5.0–9.0) and 7.0 ± 1.1 (range, 5.0–8.5), respectively. There was no difference in urine pH between groups (P = .72).
The most common pathogen isolated from both groups was E. coli (Fig 1). Among group 1 dogs, urine culture yielded ≥103 CFU/mL of a single pathogen in 33/35 (94.3%) dogs. In 1 dog, both E. coli and a Staphylococcus spp. were isolated and in another, both a Proteus spp. and Enterococcus spp. were isolated. Among group 2 dogs, urine culture yielded ≥103 CFU/mL of a single pathogen in 28/33 (84.8%) dogs. From 5 dogs, significant growth occurred for 2 bacterial species. These were Staphylococcus spp., and a Streptococcus spp., E. coli and a Proteus spp., a Streptococcus spp. and a Citrobacter spp., a Proteus spp. and Staphylococcus spp., and S. intermedius and Staphylococcus felis.
Fig. 1.
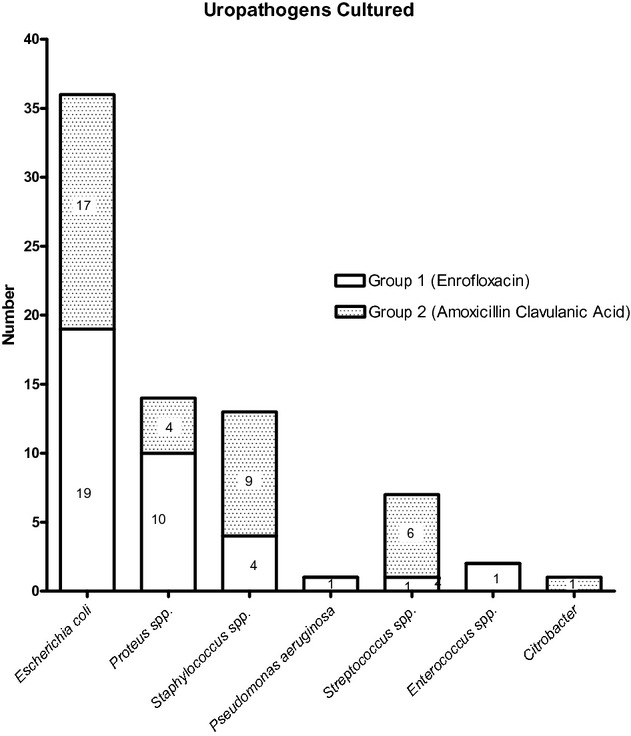
All uropathogens cultured from all dogs at day 0. Group 1 dogs were treated with enrofloxacin for 3 days and group 2 dogs were treated with clavulanic acid-amoxicillin for 14 days.
Treatment Efficacy
Only 32 dogs in group 2 were included in the statistical analysis of microbiologic cure because 1 dog was withdrawn by the owner on day 7 because of persistent LUTS, and follow-up urine culture could not be performed. This dog was considered a clinical failure in the analysis of clinical cure (see below). The microbiologic cure rate for dogs in group 1 and group 2 was 27/35 (77.1%) and 26/32 (81.2%), respectively. The lowest end of the 95% confidence interval for this difference was −20.0%, which is equal to the −20% margin of difference and indicated noninferiority of the group 1 treatment compared with that for group 2.
Of the 35 group 1 dogs, 8 (22.9%) had ≥103 CFU/mL bacteria present on urine culture on day 10. Three of these 8 dogs were males. E. coli was isolated initially and on day 10 from 5 dogs, and in 3 of those 5 dogs a second organism was present (2 had a Streptococcus spp. and 1 an Enterobacter spp. infection). One dog each had persistent positive cultures for a Staphylococcus spp., Enterobacteriaceae spp., and an Enterococcus spp. Less than 103 CFU/mL of bacteria were isolated from 2 additional dogs. These dogs were not considered treatment failures according to the study definition. On day 21, 1 of these 2 dogs had a negative culture, and Enterococcus spp. was persistently isolated from the urine of the second dog. Two of the dogs that were treatment failures on day 10 (1 dog with an E. coli and 1 with a Enterobacteriaceae spp.) subsequently were withdrawn from the study and no follow-up cultures were obtained from these dogs on day 21. Only 1 of these 2 dogs had LUTS. Furthermore, an additional dog was withdrawn after day 10 because of persistent LUTS. All of the remaining dogs completed the study. The microbiologic cure rate on day 21 for dogs in group 1 was 22/32 (68.8%).
Of the 32 group 2 dogs that were included in the statistical analysis, 6 (18.8%) had positive urine cultures on day 21. One of these dogs was male. E. coli was isolated initially and at day 21 from 4 dogs, and 1 dog each had persistent positive cultures for a Proteus spp. and Staphylococcus spp. One of the dogs with a positive culture on day 21 had pollakiuria. No other dogs with positive cultures had LUTS.
The clinical cure rate for group 1 and group 2 was 31/35 (88.6%) and 29/33 (87.9%), respectively. The lowest end of the 95% confidence interval for this difference was −12.18%, which was higher than the −20% margin of difference and indicated noninferiority.
Of all the group 1 dogs, 4/35 (11.4%) had LUTS on day 10. All 4 dogs had pollakiuria and 1 of the 4 dogs also had hematuria. Only 1 of the 4 dogs in group 1 with clinical signs on day 10 also had a positive urine culture. The dog with pollakiuria and hematuria had a negative urine culture at day 10, but, as mentioned, was withdrawn from the study and no follow-up culture was obtained on day 21. At day 21, the clinical cure rate for dogs in group 1 was 28/32 (87.5%).
Of all the group 2 dogs, 3/32 (9.4%) had LUTS on day 21. One dog had stranguria, 1 had pollakiuria, and 1 had stranguria, pollakiuria, and pain on bladder palpation. Only the dog with pollakiuria alone had a positive aerobic bacterial urine culture. One additional dog in group 2 also was considered a clinical failure because LUTS recurred and the dog returned for an unscheduled recheck on day 7. Aerobic bacterial urine culture was negative at that time.
Susceptibility Test Results
Of the group 1 dogs, 3/35 (8.6%) had positive cultures on day 0 for organisms that were resistant to enrofloxacin, and 1 additional dog had a positive culture for an organism with intermediate susceptibility to enrofloxacin. One of these 4 dogs had a positive culture for E. coli, and on day 10, this dog had a positive culture for an E. coli as well as a Streptococcus spp. and was considered a microbiologic failure. On day 21, aerobic bacterial urine culture for this dog was negative despite no additional treatment. The 2nd dog had a positive culture for a Streptococcus spp. on day 0, but on day 10, <103 CFU/mL of a Staphylococcus spp. were isolated, so this dog was not considered a microbiologic failure. This dog also had a negative bacterial urine culture on day 21. The 3rd dog had a positive culture for E. coli on day 0, and on day 10, ≥103 CFU/mL of an Enterobacteriaceae were isolated. This was 1 of the 2 dogs withdrawn from the study because of persistent positive culture. The 4th dog had a positive culture for an Enterococcus spp. on day 0 with intermediate susceptibility to enrofloxacin. An Enterococcus spp. again was isolated (≥103 CFU/mL) on days 10 and 21 with intermediate susceptibility to enrofloxacin. Clinical cures occurred in all 4 of the dogs. All of the other pathogens isolated from dogs in group 1 on day 10 were susceptible to enrofloxacin. When evaluating MIC values for amoxicillin-clavulanic acid for group 1 dogs, only 1 dog had a uropathogen (Pseudomonas spp.) that was resistant to amoxicillin-clavulanic acid; this Pseudomonas spp. was susceptible to enrofloxacin.
Of the group 2 dogs, 2/33 (6.1%) had positive cultures for organisms on day 0 that were resistant to amoxicillin-clavulanic acid. One of the dogs had a positive culture for both a resistant E. coli and a susceptible Proteus spp. On day 21, <103 CFU/mL of an unidentified bacteria was isolated from this dog, which was considered a microbiologic cure. Clinical cure also occurred in this dog. The other dog was positive for a Staphylococcus spp. on day 0, but had a negative culture on day 10 and day 21 and was free of LUTS. All of the bacteria isolated from dogs on day 21 were susceptible to amoxicillin-clavulanic acid. When evaluating enrofloxacin MIC results for group 2 dogs, 2 dogs had pathogens resistant to enrofloxacin, and 1 had a pathogen with intermediate susceptibility; all 3 isolates were Streptococcus spp. In all 3 dogs, the pathogens were susceptible to amoxicillin-clavulanic acid.
Adverse Events
For group 1 dogs, owners of 15 dogs reported a total of 22 adverse events. In 4 dogs, the owners reported adverse events on multiple days. Based on the history, the attending clinician decided if the adverse event was related to the antimicrobial administration. The assessment was subjective because diagnostics and serum drug concentrations were not obtained for these dogs. The clinicians deemed 9 of the 22 events to be unrelated to administration of the drug and included such clinical signs as lethargy (n = 3), and 1 report each of inappropriate urination, agitation, constipation, diarrhea, abdominal pain, and a small follicular cyst that ruptured on the dog's dorsum. Eleven events were thought to be possibly (n = 8) or probably (n = 3) related to administration of the enrofloxacin and included vomiting (n = 3), lethargy (n = 3), diarrhea (n = 2), anorexia (n = 1), inappetence (n = 1), and hypersalivation after drug administration (n = 1). In 1 dog with lethargy and inappetence, the attending clinician could not determine if the events were related to administration of the antimicrobial because it remained unclear if the dog had these clinical signs before the study. Another dog that was treated with enrofloxacin was removed from the study on day 2 as a consequence of vomiting that occurred 1 and 3 hours after administration of the antimicrobial drug. The dog also was lethargic and inappetent, but responded well to supportive care.
For the group 2 dogs, owners of 14 dogs reported a total of 21 adverse events. In 2 dogs, the owners reported adverse events on multiple days. Ten of the 21 events were thought to be unrelated to administration of the amoxicillin-clavulanic acid and included anxiety (n = 3), polyphagia (n = 2), urinary incontinence (n = 2), inappropriate urination (n = 1), polydipsia (n = 1), and an epidermal inclusion cyst (n = 1). Ten events were thought to be possibly (n = 8) or probably (n = 2) related to the administration of the antimicrobial and included a decreased appetite (n = 5), vomiting (n = 3), diarrhea (n = 1), and constipation (n = 1). In 1 case, the attending clinician could not determine if the event (vomiting) was related to administration of the antimicrobial because the owner reported the clinical sign >5 days after it had occurred.
Discussion
In this study, we demonstrated that an HDSD enrofloxacin protocol was not inferior to a 14-day antibiotic treatment regimen using amoxicillin-clavulanic acid. The latter is commonly used in clinical practice for treatment of uncomplicated UTI in dogs.4 A total of 77.1% of dogs that received HDSD enrofloxacin had negative urine cultures 7 days after completing antimicrobial treatment compared with 81.2% of dogs receiving the amoxicillin-clavulanic acid protocol. The HDSD enrofloxacin protocol also was not inferior to amoxicillin-clavulanic acid with regard to clinical cure rates.
We chose to study enrofloxacin and amoxicillin-clavulanic acid because both drugs are used frequently in clinical practice, are excreted in the urine, and have good activity against many common urinary pathogens, including those isolated in this study.25 Trimethoprim-sulfamethoxazole also is recommended for treatment of UTI in dogs,6,26 and its use is reported frequently in the human literature.15 However, trimethoprim-sulfamethoxazole can cause hypersensitivity reactions in dogs, including keratoconjunctivitis sicca.27 A number of studies in humans have evaluated the efficacy of fluoroquinolones for treatment of uncomplicated UTI in women.15,16 We chose to study 1 of the more commonly used fluoroquinolones, enrofloxacin, which is labeled for veterinary use. A higher dose of enrofloxacin was prescribed because HDSD fluoroquinolone protocols in human medicine were reported in some studies to have slightly higher cure rates than low dose protocols.14
Our study only examined dogs diagnosed with uncomplicated UTI, but the pathogens isolated were similar to those previously reported for dogs with UTI, some of which were recurrent or relapsing infections.13,28–30 E. coli, Proteus spp., and Staphylococcus spp. accounted for the majority of our isolates. In contrast to other studies, we did not isolate Klebsiella spp. from any of the dogs in our study. However, we would expect either drug to have good activity against most Klebsiella spp.25 As has been reported by others,31 only a single bacterial species was isolated from most dogs in our study.
Group 2 dogs had more gram positive uropathogens cultured and no dog in group 2 had a Pseudomonas spp. isolated. This study was randomized, and, although a difference in the distribution of gram positive and gram negative pathogens existed between groups (Fig 1), no difference in the prevalence of E. coli was found between groups, which is the most common uropathogen reported in dogs. Clinical and microbiologic outcomes for dogs that had growth of only E. coli (18 dogs in group 1 and 15 dogs in group 2) were not different, although the limited sample size did not allow us to test for noninferiority (data not shown). We designed our study as a clinical field trial and chose to evaluate data for all pathogens because evidence for differing treatment outcomes for different pathogens is lacking and other variables, such as the possession of virulence factors by different isolates of the same bacterial species also may have the potential to influence outcome.
For those dogs classified as a microbiologic failure, we were unable to definitively identify relapse because molecular investigations of the pathogens using pulse field gel electrophoresis would be needed32 and this testing was beyond the scope of this study. Even susceptibility patterns do not reliably distinguish between relapsing or persisting infections and reinfections, because antibiotic resistance patterns do not reflect the genetic composition of the whole chromosome.33
Both groups of dogs in this study contained a high percentage of female dogs, which is similar to previous studies of UTI in dogs.13,34 The microbiologic and clinical cure rate for both groups was similar to short-term cure rates reported for women with uncomplicated UTI when given ciprofloxacin administered as a 250 or 750 mg single dose,35 which resulted in microbiologic cure rates of 81.1 and 82.6%, respectively, and clinical cure rates of 89 and 92%, respectively. However, an absence of clinical signs did not imply negative culture results in our study, and likewise, the presence of clinical signs did not predict a positive culture after antimicrobial treatment was instituted. It appears that even in dogs suspected to have uncomplicated UTI, urine culture after antimicrobial treatment may be indicated to be certain that bacteria have been eradicated.
Few studies have evaluated the efficacy of any dosing regimen of antimicrobial treatment for UTI in dogs. No studies of short duration oral antimicrobial protocols for treatment of naturally occurring canine UTI have been published. One-day and 3-day duration treatment protocols were evaluated in an induced E. coli canine cystitis model using either injectable amikacin or oral trimethoprim-sulfadiazine.36 One-day treatment with either of these drugs was not effective in effecting bacteriological cure after treatment was stopped. The 3-day treatment protocol using trimethoprim-sulfadiazine resulted in bacteriologically sterile urine 14 days after treatment in 4 of 4 female dogs, but in none of 4 male dogs. Cefovecin has been evaluated for the treatment of uncomplicated UTI in dogs when given as a single subcutaneous injection of 8 mg/kg of body weight.30 Although this treatment was reported to be effective, it is difficult to compare the bacteriological results in this study to those in our study because different time points and methods of urine collection were used, as well as different definitions for microbiologic or clinical cure. Furthermore, cefovecin is not currently approved for the treatment of UTI in dogs in the United States.
In human medicine, short-duration antimicrobial protocols increase compliance, and result in lower costs of treatment.19 In our study, owners were required to keep a log of the drugs administered and compliance was good in both groups we evaluated, although participation in a study may have increased owner compliance. Further studies would be required to determine if owners who use short-duration protocols are more inclined to administer all medications prescribed.
In our study, 91.4% of the isolates from dogs in group 1 were susceptible to enrofloxacin and 93.9% of the isolates from dogs in group 2 were susceptible to amoxicillin-clavulanic acid. The microbiologic and clinical cure rate in our study was lower than that predicted by the organisms' susceptibility for dogs receiving either of the drug regimens used in this study. When evaluating all dogs with persistent positive cultures, none had pathogens resistant to the antimicrobial originally prescribed. The reasons for these differences remain unclear. Although all dogs were screened carefully for underlying diseases that could result in complicated UTI, not all dogs had bladder imaging studies performed and it is possible some dogs with more complicated infections could have been included in the study. Studies that investigate the resistance patterns of urine bacterial isolates from dogs for which the doses of the drugs administered and owner compliance are evaluated also are warranted.
All potential adverse events were reported by owners, but it was up to the attending to clinician to decide if the event was associated with the antimicrobial administered. Adverse events were minimal and only 1 dog that received HDSD enrofloxacin was removed from the study because of gastrointestinal signs. One dog in group 2 had persistent inappetence during treatment, but remained in the study. All other owners felt adverse effects encountered with either antimicrobial protocol were not severe enough to warrant removal from the study.
In summary, an HDSD enrofloxacin protocol was not inferior when compared with a standard antimicrobial regimen using amoxicillin-clavulanic acid for uncomplicated UTI in dogs. The drug regimen was well tolerated by most dogs and can be considered as an alternative for treating uncomplicated UTIs in dogs. Because antimicrobial resistance of canine uropathogens to fluoroquinolones has been reported,37,38 further research is warranted to determine what impact this protocol will have on the selection for antimicrobial-resistant bacteria. Additional research also should be undertaken to determine if a shortened protocol will positively impact owner compliance. Subsequent studies can help develop guidelines for antimicrobial dosing protocols for uncomplicated UTI.
Acknowledgments
Study supported by a grant from Bayer HealthCare LLC, Animal Health Division, Shawnee Mission, KS.
The authors acknowledge the assistance of the faculty, staff, clinicians, and pet owners that participated in the study.
Glossary
- CFU
colony forming units
- CLSI
Clinical Laboratory Standards Institute
- CNS
central nervous system
- HDSD
high dose short duration
- LUTS
lower urinary tract signs
- MIC
minimum inhibitory concentration
- UTI
urinary tract infection
Footnotes
Baytril®, Bayer HealthCare LLC, Animal Health Division, Shawnee Mission, KS
Clavamox®, Pfizer Animal Health, New York, NY
VICH GL9, July 1, 2001
Microbial Research Incorporated, Fort Collins, CO
Hardy Diagnostics, Santa Maria, CA
API 20 Strep® and API20E®, bioMérieux, Durham, NC
eCMV1BURF Plates, Trek Diagnostic Systems, Westlake, OH
SAS Institute Inc, Cary, NC
Conflict of Interest Disclosure
Study supported by a grant from Bayer HealthCare LLC, Animal Health Division, Shawnee Mission, KS.
Drs Westropp and Chew had travel and accommodations covered or reimbursed by Bayer HealthCare LLC, Animal Health Division.
Drs Westropp and Chew have lectured for Bayer HealthCare LLC, Animal Health Division.
References
- 1.Ling GV. Therapeutic strategies involving antimicrobial treatment of the canine urinary tract. J Am Vet Med Assoc. 1984;185:1162–1164. [PubMed] [Google Scholar]
- 2.McGuire NC, Schulman R, Ridgway MD, Bollero G. Detection of occult urinary tract infections in dogs with diabetes mellitus. J Am Anim Hosp Assoc. 2002;38:541–544. doi: 10.5326/0380541. [DOI] [PubMed] [Google Scholar]
- 3.Litster A, Moss S, Platell J, Trott DJ. Occult bacterial lower urinary tract infections in cats—urinalysis and culture findings. Vet Microbiol. 2009;136:130–134. doi: 10.1016/j.vetmic.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Pressler BM, Bartges J. Urinary tract infections. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 7th ed. St. Louis, MO: Saunders Elsevier; 2010. pp. 2036–2047. [Google Scholar]
- 5.Grauer GF. Urinary tract infections. In: Nelson RW, Couto CG, editors. Small Animal Internal Medicien. St. Louis, MO: Mosby Elsevier; 2009. pp. 664–665. [Google Scholar]
- 6.Weese JS, Blondeau JM, Boothe D, et al. Antimicrobial use guidelines for treatment of urinary tract disease in dogs and cats: Antimicrobial guidelines working group of the international society for companion animal infectious diseases. Vet Med Int. 2011;2011:1–9. doi: 10.4061/2011/263768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagenlehner FM, Weidner W, Naber KG. An update on uncomplicated urinary tract infections in women. Curr Opin Urol. 2009;19:368–374. doi: 10.1097/MOU.0b013e32832ae18c. [DOI] [PubMed] [Google Scholar]
- 8.Llor C, Rabanaque G, Lopez A, Cots JM. The adherence of GPs to guidelines for the diagnosis and treatment of lower urinary tract infections in women is poor. Fam Pract. 2011;28:294–299. doi: 10.1093/fampra/cmq107. [DOI] [PubMed] [Google Scholar]
- 9.Bleidorn J, Gagyor I, Kochen MM, et al. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection? Results of a randomized controlled pilot trial. BMC Med. 2010;8:30. doi: 10.1186/1741-7015-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arav-Boger R, Leibovici L, Danon YL. Urinary tract infections with low and high colony counts in young women. Spontaneous remission and single-dose vs multiple-day treatment. Arch Intern Med. 1994;154:300–304. [PubMed] [Google Scholar]
- 11.Leibovici L, Wysenbeek AJ. Single-dose antibiotic treatment for symptomatic urinary tract infections in women: A meta-analysis of randomized trials. Q J Med. 1991;78:43–57. [PubMed] [Google Scholar]
- 12.Karachalios GN, Georgiopoulos AN, Nasopoulou-Papadimitriou DD, Adracta DJ. Value of single-dose ciprofloxacin in the treatment of acute uncomplicated urinary tract infection in women. Drugs Exp Clin Res. 1991;17:521–524. [PubMed] [Google Scholar]
- 13.Seguin MA, Vaden SL, Altier C, et al. Persistent urinary tract infections and reinfections in 100 dogs (1989-1999) J Vet Intern Med. 2003;17:622–631. doi: 10.1111/j.1939-1676.2003.tb02492.x. [DOI] [PubMed] [Google Scholar]
- 14.Nicolle LE. Short-term therapy for urinary tract infection: Success and failure. Int J Antimicrob Agents. 2008;31(Suppl 1):S40–S45. doi: 10.1016/j.ijantimicag.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 15.Zalmanovici Trestioreanu A, Green H, Paul M, et al. Antimicrobial agents for treating uncomplicated urinary tract infection in women. Cochrane Database of Syst Rev (Online) 2010;10:CD007182. doi: 10.1002/14651858.CD007182.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naber KG. Which fluoroquinolones are suitable for the treatment of urinary tract infections? Int J Antimicrob Agents. 2001;17:331–341. doi: 10.1016/s0924-8579(00)00362-9. [DOI] [PubMed] [Google Scholar]
- 17.Wagenlehner FM, Naber KG. Treatment of bacterial urinary tract infections: Presence and future. Eur Urol. 2006;49:235–244. doi: 10.1016/j.eururo.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Keitzmann M. Overview of the pharmacokinetic properties of fluoroquinolones in companion animals. Suppl Compend Contin Educ Pract Vet. 1999;21(12):7–11. [Google Scholar]
- 19.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 20.Plumb DC, editor. Veterinary Drug Handbook. 6th ed. Stockholm, WI: Pharma Vet Inc; 2008. [Google Scholar]
- 21.Burkhardt JE, Hill MA, Turek JJ, Carlton WW. Ultrastructural changes in articular cartilages of immature beagle dogs dosed with difloxacin, a fluoroquinolone. Vet Pathol. 1992;29:230–238. doi: 10.1177/030098589202900307. [DOI] [PubMed] [Google Scholar]
- 22.Murray PR, Baron EJ, Jorgensen JH. Manual of Clinical Microbiology. 9th ed. Washington DC: ASM; 2007. [Google Scholar]
- 23.Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. CLSI document M31-A3. [Google Scholar]
- 24.Performance Standards for Antimicrobial Susceptibility Testing: Nineteenth Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2009. Vol 29; No. 3; CLSI document M100-S20. [Google Scholar]
- 25.Barsanti J. Genitourinary infections. In: Infectious Diseases of the Dog and Cats. 3rd ed. St Louis, MO: Saunders: Elsevier; 2006. pp. 935–961. [Google Scholar]
- 26.Rohrich PJ, Ling GV, Ruby AL, et al. In vitro susceptibilities of canine urinary bacteria to selected antimicrobial agents. J Am Vet Med Assoc. 1983;183:863–867. [PubMed] [Google Scholar]
- 27.Lavergne SN, Danhof RS, Volkman EM, Trepanier LA. Association of drug-serum protein adducts and anti-drug antibodies in dogs with sulphonamide hypersensitivity: A naturally occurring model of idiosyncratic drug toxicity. Clin Exp Allergy. 2006;36:907–915. doi: 10.1111/j.1365-2222.2006.02506.x. [DOI] [PubMed] [Google Scholar]
- 28.Tivapasi MT, Hodges J, Byrne BA, Christopher MM. Diagnostic utility and cost-effectiveness of reflex bacterial culture for the detection of urinary tract infection in dogs with low urine specific gravity. Vet Clin Pathol. 2009;38:337–342. doi: 10.1111/j.1939-165X.2009.00147.x. [DOI] [PubMed] [Google Scholar]
- 29.Ling GV, Rohrich PJ, Ruby AL, et al. Canine urinary tract infections: A comparison of in vitro antimicrobial susceptibility test results and response to oral therapy with ampicillin or with trimethoprim-sulfa. J Am Vet Med Assoc. 1984;185:277–281. [PubMed] [Google Scholar]
- 30.Passmore CA, Sherington J, Stegemann MR. Efficacy and safety of cefovecin (Convenia) for the treatment of urinary tract infections in dogs. J Small Anim Pract. 2007;48:139–144. doi: 10.1111/j.1748-5827.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- 31.Ling GV, Norris CR, Franti CE, et al. Interrelations of organism prevalence, specimen collection method, and host age, sex, and breed among 8,354 canine urinary tract infections (1969–1995) J Vet Intern Med. 2001;15:341–347. [PubMed] [Google Scholar]
- 32.Drazenovich N, Ling GV, Foley J. Molecular investigation of Escherichia coli strains associated with apparently persistent urinary tract infection in dogs. J Vet Intern Med. 2004;18:301–306. doi: 10.1892/0891-6640(2004)18<301:mioecs>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Freitag T, Squires RA, Schmid J, et al. Antibiotic sensitivity profiles do not reliably distinguish relapsing or persisting infections from reinfections in cats with chronic renal failure and multiple diagnoses of Escherichia coli urinary tract infection. J Vet Intern Med. 2006;20:245–249. doi: 10.1892/0891-6640(2006)20[245:aspdnr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Bartges JW. Diagnosis of urinary tract infections. Vet Clin North Am Small Anim Pract. 2004;34:923–933. doi: 10.1016/j.cvsm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Raz R, Rottensterich E, Hefter H, et al. Single-dose ciprofloxacin in the treatment of uncomplicated urinary tract infection in women. Eur J Clin Microbiol Infect Dis. 1989;8:1040–1042. doi: 10.1007/BF01975166. [DOI] [PubMed] [Google Scholar]
- 36.Rogers KS, Lees GE, Simpson RB. Effects of single-dose and three-day trimethoprim-sulfadiazine and amikacin treatment of induced Escherichia coli urinary tract infections in dogs. Am J Vet Res. 1988;49:345–349. [PubMed] [Google Scholar]
- 37.Cooke C, Singer RS, Jang SS, Hirsh DC. Enrofloxacin resistance in Escherichia coli isolated from dogs with urinary tract infections. J Am Vet Med Assoc. 2002;220:190–192. doi: 10.2460/javma.2002.220.190. [DOI] [PubMed] [Google Scholar]
- 38.Cohn LA, Gary AT, Fales WH, Madsen RW. Trends in fluoroquinolone resistance of bacteria isolated from canine urinary tracts. J Vet Diagn Invest. 2003;15:338–343. doi: 10.1177/104063870301500406. [DOI] [PubMed] [Google Scholar]


