Abstract
Background
Few studies have applied multiple imaging modalities to examine cognitive correlates of white matter. We examined the utility of T2-weighted MRI-derived white matter hyperintensities (WMH) and diffusion tensor imaging-derived fractional anisotropy (FA) to predict cognitive functioning among older adults.
Methods
Quantitative MRI and neuropsychological evaluations were performed in 112 older participants from an ongoing study of the genetics of Alzheimer’s disease (AD) in African Americans. Regional WMH volumes and FA were measured in multiple regions of interest. We examined the association of regional WMH and an FA summary score with cognitive test performance. Differences in WMH and FA were compared across diagnostic groups (i.e., normal controls, mild cognitive impairment, and probable AD).
Results
Increased WMH volume in frontal lobes was associated with poorer delayed memory performance. FA did not emerge as a significant predictor of cognition. White matter hyperintensity volume in the frontal and parietal lobes was increased in MCI participants and more so in AD patients relative to controls.
Discussion
These results highlight the importance of regionally-distributed small vessel cerebrovascular disease in memory performance and AD among African American older adults. White matter microstructural changes, quantified with DTI, appear to play a lesser role in our sample.
Keywords: magnetic resonance imaging, white matter hyperintensities, diffusion tensor imaging, cognition, Alzheimer’s disease, African Americans
Introduction
In recent years, advances in acquisition and analysis of high resolution magnetic resonance imaging (MRI) data have allowed for the precise and reliable visualization and quantification of normal and abnormal cerebral white matter. Two important MRI sequences that can be used to quantify white matter include diffusion tensor imaging (DTI), and T2-weighted imaging. Both imaging modalities have been used in the context of cognitive aging and dementia research, but few studies have considered them simultaneously.
Diffusion tensor imaging provides clues about white matter microstructure by exploiting the differential diffusion of water across tissue types (Mori & Barker, 1999; Pierpaoli & Basser, 1996). Because fractional anisotropy (FA), the most common DTI metric, reflects water molecule coherence, it can be used to estimate the orientation and integrity of fiber bundles (Basser, Pajevic, Pierpaoli, Duda, & Aldroubi, 2000; Mori & Barker, 1999; Mori, Crain, Chacko, & van Zijl, 1999). Decreased FA has been shown to be related to poorer performance across several cognitive domains in healthy older individuals (Madden, Bennett, & Song, 2009; Penke & Deary, 2010; Vernooij et al., 2009) and across the adult lifespan (Grieve, Williams, Paul, Clark, & Gordon, 2007), but the exact mechanisms linking cognitive functioning with white matter microstructural integrity are not fully understood. Lowered FA could result from localized microstructural processes that affect single tracts in some individuals, but it could also be to some extent a global phenomenon that affects many tracts simultaneously (Wahl et al., 2010).
Diffusion tensor imaging has also been used to examine white matter microstructural changes associated with neurodegenerative conditions, such as Alzheimer’s disease (AD). Measures of white matter integrity have been reported to be lower among patients with AD relative to controls in several regions throughout the brain (Bozzali et al., 2002; Damoiseaux et al., 2009; Huang & Auchus, 2007; Medina et al., 2006; Naggara et al., 2006; Takahashi et al., 2002; Xie et al., 2006), and to correlate with the degree of cognitive impairment (Bozzali, et al., 2002; Duan et al., 2006). Despite observations of FA reduction among patients with AD, findings regarding the regional specificity of these observations have been less consistent. As variance in white matter integrity estimated with FA is highly collinear across tracts, some authors have suggested deriving summary measures of total white matter integrity to maximize the reliability of these measures (Penke et al., 2010). In the current study, we derive a general white matter integrity summary factor as the primary DTI measure of interest.
Whereas DTI can be used to estimate variability in the integrity of white matter due to aging, disease, or inter-individual variability, white matter hyperintensities (WMH) are a reflection of frank macrostructural white matter pathology. White matter hyperintensities most likely reflect small vessel cerebrovascular disease, as shown in pathological studies (Fazekas et al., 1993; Gouw et al., 2011; C. D. Smith, Snowdon, Wang, & Markesbery, 2000), and suggested by their strong associations with vascular risk factors, such as high or fluctuating blood pressure, diabetes, and heart disease (Brickman et al., 2010; Brickman, Schupf, et al., 2008; DeCarli et al., 1999; Liao et al., 1997; Liao et al., 1996; Manolio et al., 1994; Meyer et al., 1999; Raz, Rodrigue, Kennedy, & Acker, 2007; Strassburger et al., 1997; Viswanathan, Rocca, & Tzourio, 2009). They are more prevalent and severe among patients with AD than controls (Barber et al., 1999; Capizzano et al., 2004; Scheltens et al., 1992; Tanabe et al., 1997) and have been shown to be associated with a precipitous cognitive decline among patients with prevalent AD (Brickman, Honig, et al., 2008; Burns et al., 2005; Yoshita et al., 2006). Individuals at greatest risk for the development of AD have increased WMH volume relative to those at lesser risk (Luchsinger et al., 2009). White matter hyperintensities are distributed disproportionately in frontal (Capizzano, et al., 2004) and parietal regions (Gootjes et al., 2004), and while generally emerging as homogenous signal on MRI, the regional distribution might point to distinct underlying pathological features that might play a unique role in AD (Brickman, Muraskin, & Zimmerman, 2009).
While most previous studies have examined the relation of either WMH or FA with cognition or AD, few have examined whether one is a better predictor of cognitive function among older adults than the other in the same sample. Our overall aim was to examine the role of white matter microstructure variability (estimated with DTI) and markers of white matter pathology (appreciated through examination of WMH) in cognitive aging and dementia. First, we were interested in examining the association between FA and WMH to determine to what extent the two measures reflect the same process. Next, we examined the utility of regional WMH and DTI-derived FA to predict cognitive functioning among older adults; we were interested in testing whether FA and WMH were independently related to specific cognitive functions, or whether one marker of white matter was a better predictor of cognitive function than the other one. Finally, we compared WMH volume and DTI-derived FA across three diagnostic categories (i.e., normal control, mild cognitive impairment [MCI], and AD) that were determined independently of imaging findings. Based on findings from the extant literature (Brickman, et al., 2009; Luchsinger, et al., 2009; E. E. Smith et al., 2008), we expected higher WMH burden to be associated with a more severe degree of cognitive impairment and vary as a function of diagnostic groups. Specifically, although WMH burden is often linked to executive dysfunction (Gunning-Dixon & Raz, 2000), an emerging literature suggests a role of WMH in the pathogenesis of AD, a neurodegenerative condition marked primarily by memory impairment. Thus, we hypothesized that there would be an association between WMH and the AD “phenotype” as a quantitative trait (i.e., memory function as a continuous measure) or as a diagnostic entity. Similarly, we expected lower FA to be associated with poorer cognitive functioning, either defined continuously as diagnostic entity. As FA and WMH ostensibly reflect distinct aspects of white matter (i.e., micro- and macrostructure), we hypothesized that each to be independently associated with cognition.
This imaging substudy is part of a larger effort that seeks to elucidate unique genes involved with AD pathogenesis among African Americans. Increased incidence and prevalence rates of AD and cognitive dysfunction among African Americans have been reported in the literature (Gurland et al., 1999; Perkins & Schisterman, 2006; M.X. Tang et al., 2001; Unverzagt, Hall, Torke, & Rediger, 1996). Our belief is that one potential source of discrepancies across racial groups in diagnosis could be due to differences in cerebrovascular disease. While we do not include members of different racial groups in the current study for explicit comparison, African Americans have been severely under-represented in most large-scale neuroimaging studies of cognitive aging and dementia and we believe that it is important to establish brain-behavior relationships in this group.
Methods
The parent study is a multi-site effort that includes the participation of investigators at Columbia University, Duke University, North Carolina A&T State University, Vanderbilt University, and University of Miami. A subset of participants at the Columbia University site was recruited for participation in an MRI sub-study. The current report focuses on these individuals. This study was approved by an Institute Institutional Review Board at Columbia University Medical Center.
Subjects
Inclusion criteria for the parent study included age greater than 50 years; self-identification as Black or African American and non-Hispanic; and fluent in English. Participants in the MRI sub-study gave written informed consent for MRI scanning and did not have contraindications.
Neuropsychological evaluation
Participants were administered a neuropsychological battery, which included representative tests from a number of cognitive domains. The battery comprised the following tests: Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1975); Digit Symbol subtest of the Wechsler Adult Intelligence Scale - Revised (Wechsler, 1997); Trailmaking Test Parts A and B (Reitan, 1978); Digit Span Forward and Backward (Wechsler, 1997); California Verbal Learning Test–2 (CVLT-II; Trials 1–5 free recall, short delayed free recall, long delayed free recall, recognition hits-false positives) (Delis, 2000); Logical Memory Test (immediate recall, delayed recall) (Wechsler, 1997); Rey-Osterrieth Complex Figure Test (immediate recall, delayed recall) (Osterrieth, 1944); Controlled Oral Word Association Test (CFL; (Benton & Hamsher, 1976)); Category Fluency Test (animal and vegetable naming; (Goodglass, 1983; Morris et al., 1989); and 30-item Boston Naming Test (Kaplan, 1983; Weintraub, 2009). All neuropsychological data were analyzed in raw form.
Participants underwent a diagnostic work-up, including a medical history, assessment of functional status and memory complaints, and a neurological exam. Informants were interviewed whenever available and asked about functional status (Pfeffer, Kurosaki, Harrah, Chance, & Filos, 1982), neuropsychiatric symptoms (Cummings, Mega, Gray, Rosenberg-Thompson, & Carusi, 1994), and symptom onset (Sano, Stern, Mayeux, Hartman, & Devanand, 1987). These data, along with performance on the neuropsychological battery, were reviewed at a diagnostic consensus conference that included at least two attending neuropsychologists with expertise in this area. Neuroimaging data were not considered in the diagnostic formulation. Participants were categorized as normal controls, mild cognitive impairment (MCI) (Petersen et al., 1999), or as meeting criteria for dementia (American Psychiatric, 1994). Probable AD was diagnosed following established research criteria (McKhann et al., 1984). Some participants had evidence of some cognitive impairment which the diagnosticians did not feel that they met MCI criteria, but rather that the pattern of cognitive performance reflected lifelong functioning. For these participants, a diagnosis of “cognitive impairment not MCI” was assigned.
Participants were asked whether they had ever been diagnosed with or whether they were currently being treated for hypertension, diabetes mellitus, myocardial infarction, congestive heart failure, any other heart disease, and/or stroke. These dichotomous variables were summed to create a single peripheral vascular disease summary score, similar to what we have done in previous studies (Brickman, Schupf, et al., 2008).
Neuroimaging
Magnetic resonance imaging (MRI) was acquired with a 1.5 T Philips Intera scanner at Columbia University. The following images were acquired in the axial orientation: high-resolution 3D T1-weighted anatomical (TE/TR: 2.1/20, FOV: 240, Matrix: 256×256, flip angle: 20°, slice thickness: 1.3mm, slices: 105), fluid attenuated inverse recovery (FLAIR) T2-weighted (TE/TR: 5500/144, IR: 1900, FOV: 250, Matrix: 192×256, slice thickness: 3mm, slices: 47 no gap), 16-direction DTI (TR/TE: 10624, FOV: 224, Matrix: 112×112, slice thickness: 2mm, slices: 70 no gap). Other MRI sequences were collected as part of the study, including gradient echo and T2-weighted (without fluid attenuation), but were not used in the current analyses.
White matter hyperintensity quantification
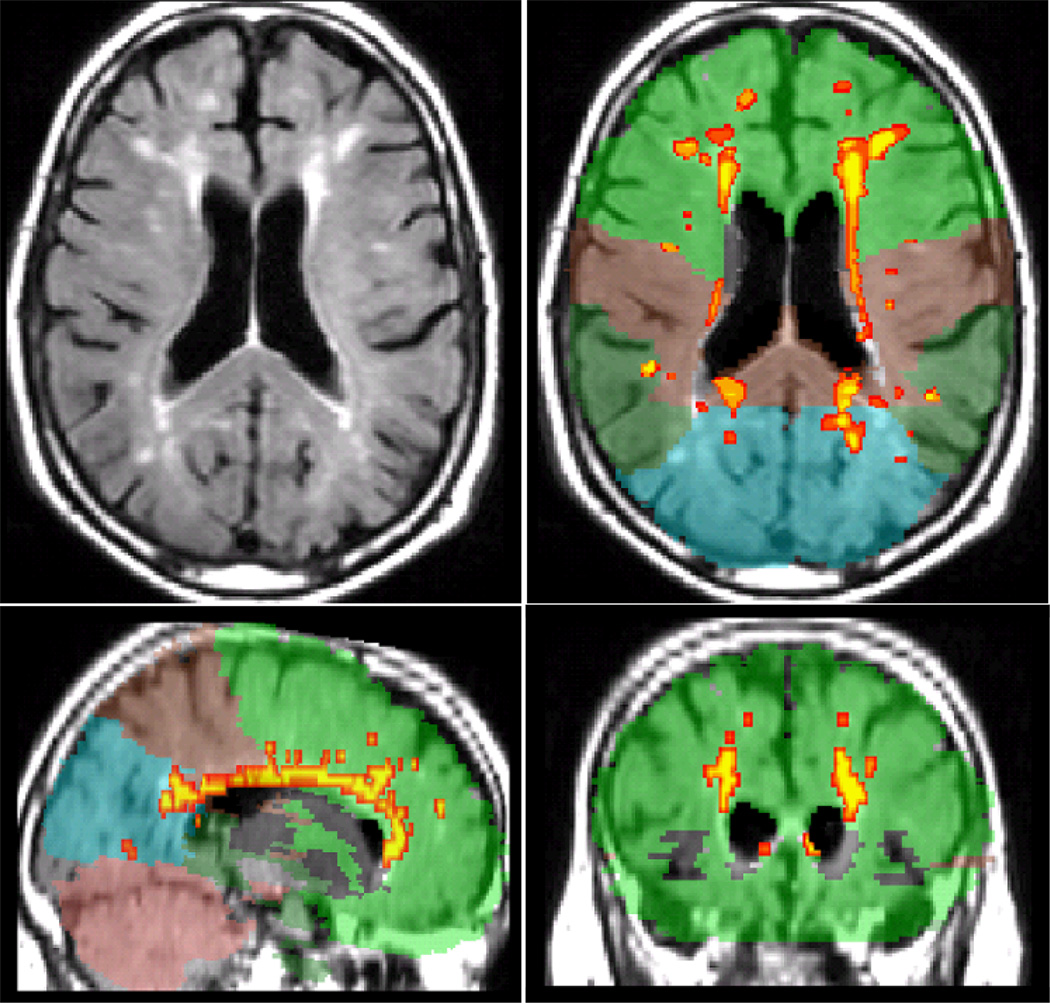
White matter hyperintensity volumes were derived on T2-weighted FLAIR images as described previously (Brickman, et al., 2009; Brickman et al., 2011; Oliveira, Brickman, Provenzano, Muraskin, & Louis, in press). Briefly, T1-weighted images were segmented into grey matter, white matter, and cerebrospinal fluid tissue classes with SPM. The normalization parameters were applied to the T2-weighted and T1-weighted images to transform them into standardized atlas space. FLAIR images were skull stripped/brain extracted using the segmented tissue classes from the T1-weighted image. On each FLAIR image, WMH seeds were defined as areas that were 2.7 SD or greater above the mean intensity value of the entire image. Once seeds were placed, each one passed through a mean intensity-based region-growing algorithm that used a 10-point connectivity scheme to label adjacent voxels that fell within 5% of the mean intensity value for the seed. The newly labeled voxels were added to the labeled voxels and a new intensity mean was calculated. This process continued iteratively until all hyperintense voxels were labeled. White matter hyperintensity volume was the sum of all labeled voxels multiplied by voxel dimensions. To calculate regional (i.e., frontal, temporal, parietal, and occipital) WMH volumes, an anatomical atlas (Admiraal-Behloul et al., 2004) was spatially normalized with the inverse transform matrix generated from the segmentation of the T1-weighted image to each participant’s labeled FLAIR image. Each region within the anatomical atlas was defined by a unique identification parameter and intersection of the labeled WMH with the unique anatomical label defined the regional WMH volume. Figure 1 displays an example of WMH labeling for a single subject.
Figure 1.
Example of regional WMH quantification for one subject. Left top: raw T2-weighted FLAIR image. Other panels: Orthogonal view of labeled image. White matter hyperintensities are labeled with red/yellow. Colors correspond to cerebral lobes (green: frontal, brown: parietal, dark green: temporal, blue: occipital, mauve: cerebellum).
Diffusion tensor imaging
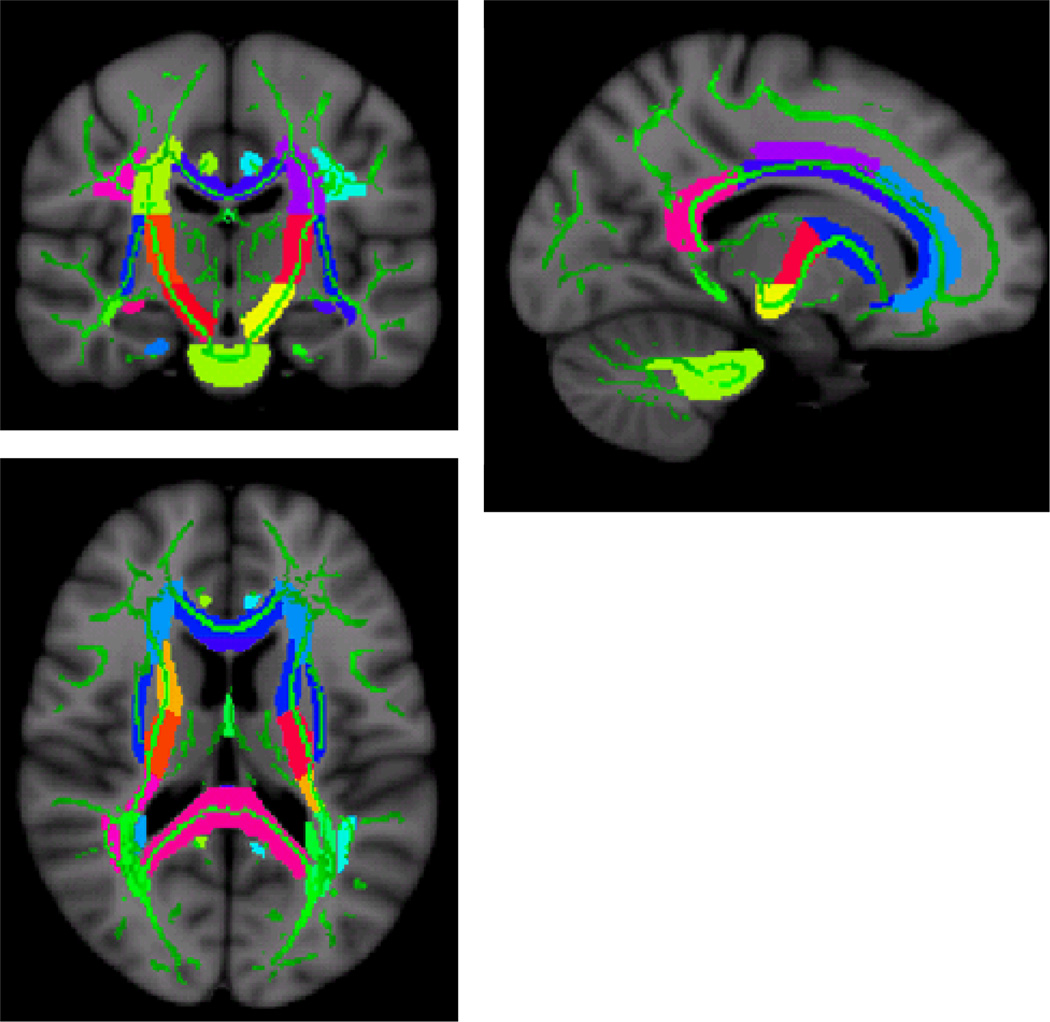
Diffusion tensor imaging data were processed with the fMRI Software Library (FSL 4.1.3. release; www.fmrib.ox.ac.uk/fsl) fMRIB Diffusion Toolbox and tract based spatial statistics (TBSS) (S. M. Smith et al., 2006; S. M. Smith et al., 2004). Briefly, after individual subjects raw DTI images were aligned to the b=0 image (Jenkinson, Bannister, Brady, & Smith, 2002), a mean FA skeleton was created by thinning the group mean FA image. This skeleton is a representation of the centers of the white matter tracts common to the group. All FA images were then nonlinearly transformed into the space of the mean image. For each subject, local FA maxima were projected onto the group template skeleton (S. M. Smith, et al., 2006). Next, we used JHU White Matter Label Map included with FSL to identify sections of the white matter skeleton that represent to the following white matter fiber tracts in both brain hemispheres (listed in Figure 2 caption). Average FA values for each WM tract were calculated from the white matter skeleton and values from the left and right hemispheres were averaged together where applicable. Figure 2 displays the regions of interest superimposed on the skeletonized map.
Figure 2.
“Skeletonized” DTI map indicating FA ROIs (colors) used to derive the summary FA score superimposed onto the mean FA skeleton (green) of the DTI subjects. The summary score comprised the following tracts: middle cerebellar peduncles, pontine crossing tracts part of the middle cerebellar peduncles, genu of the corpus callosum, body of the corpus callosum, splenium of the corpus callosum, fornix column and fornix body, corticospinal tract, medial lemniscus, inferior cerebellar peduncles, superior cerebellar peduncles, cerebral peduncles, anterior limb of the internal capsule, posterior limb of the internal capsule, retrolenticular part of the internal capsule, anterior corona radiata, superior corona radiata, posterior corona radiata, posterior thalamic radiation including optic radiation, sagittal striatum including the inferior longitudinal fasciculus, external capsule, cingulum-cingulate gyrus, cingulum hippocampus, fornix cres stria terminalis, superior longitudinal fasciculus, uncinate fasciculus, and the tapetum.
Given the collinearity among FA measurements in multiple tracts, our interest in global measures of white matter integrity, and the lack of reported regional consistency in the extant literature, we focused on a summary tract measure as a marker of global white matter integrity. Further, a derived summary measure for FA increases the reliability of measurement because it comprises several tracts and helps minimize the number of statistical comparisons. Following procedures put forth by Penke and colleagues (Penke, et al., 2010), mean FA values from each ROI were subjected to a principal component analysis and the first derived factor, which explained 54.9% of the total variance, was used in subsequent analyses. Apart from four tracts, factor loadings were all greater than 0.64. Factor loadings that were less than 0.64 came from pontine crossing-tracts part of the middle cerebellar peduncles, corticospinal tract, inferior cerebellar peduncles, and the tapetum, which ranged in factor loading from 0.284 to 0.482. In post hoc exploratory analyses we did, however, also examine individual tracts that have been implicated in AD, including inferior longitudinal fasciculus, uncinate fasciculus, fornix, and genu of the corpus callosum (Kiuchi et al., 2009; Liu et al., 2011; Pievani et al., 2010; Stricker et al., 2009).
Relative brain volume
A measure of total relative brain volume was derived with SPM5 (Wellcome Department of Cognitive Neurology, London, UK). Total relative brain volume was the sum of voxels labeled as grey matter and white matter as a ratio to the sum of grey matter, white matter, and cerebrospinal fluid, which were derived using default settings to derive. This measure was used in statistical analyses to control for individual differences in atrophy.
Statistical analysis
Descriptive demographic data, including age, sex distribution, and number of years of education, were generated for the entire imaging sample and compared across diagnostic subgroups with analysis of variance (ANOVA) and Chi-square tests. Demographic features were also compared between the MRI sub-study participants and the remaining Columbia University site participants who did not participate in the MRI sub-study (n=325, at the time of analysis). Descriptive statistics for the neuroimaging markers and performance on the neuropsychological tests were also generated and compared with ANOVA across groups. For statistical tests to address the study hypotheses, both parametric and non-parametric procedures were used. The relationship between regional WMH volumes and the FA factor score was examined with bivariate Pearson and Spearman correlations. A series of multiple linear regression analyses was run to examine associations of the white matter variables with the neuropsychological outcomes. By including the white matter measures (i.e., WMH and FA) simultaneously in the multiple regression analyses, we were able to compare explicitly the relative and independent association of each with cognition. For these analyses, all subject subgroups were combined and the lobar WMH volumes and FA measure were entered simultaneously and each of the neuropsychological outcomes was examined as dependent variables in separate analyses. Each regression model controlled for age, years of education, and relative brain volume by entering these factors as additional predictors. For all analyses, non-parametric tests were used as secondary analyses, as WMH volumes are typically not normally distributed. Given that covariate analyses are not possible with non-parametric statistical procedures, we considered the parametric tests as primary and the non-parametric tests (without covariates) as confirmatory. Although it is often possible to normalize skewed positively skewed distributions by taking the log transformation of the data, in our case there was a large proportion of individuals with zero values for WMH volumes in temporal and occipital lobe, making transformation to normality of distribution impossible. In a post hoc exploratory fashion, we re-ran the analyses with four fiber tracts previously implicated in AD entered individually or together to examine instead of the FA summary measure.
To examine group differences across controls, those with MCI, and those with AD in regional WMH and global FA we constructed two general linear models. Participants classified as “impaired not MCI” were excluded from these analyses. We chose to exclude these individuals from the group analyses because they did not fit into the pre-defined diagnostic categories. We included them in the individual-differences analyses (described above) because we were interested examining white matter correlates of cognition among older adults in general. For regional WMH analysis, Diagnostic Group (NC, MCI, and AD) was a between-groups factor and Lobe (frontal, temporal, parietal, and occipital) was a within-subjects factor. For FA, we ran a similar analysis with Diagnostic Group as the independent variable and the FA factor score as the dependent variable. This analysis was re-run with the four AD-specific FA fiber tracts instead of the FA summary measure. Both analyses controlled for age, education, and relative brain volume. Non-parametric Kruskal-Wallis tests were used to verify significant group differences.
Results
Subject characteristics
Table 1 displays demographic data for the entire sample and cognitive subgroups. Impaired not MCI subjects were younger than all other groups, normal controls and MCI subjects were similar in age, and patients with AD were older than all other groups (significant main effect of Diagnostic Group, F(3,108)=7.724, p<0.001). The groups were similar in sex distribution (χ2(3)=0.457, p=0.928) and vascular disease histories (F(3,99)=0.848, p=0.471). Patients with AD and MCI had fewer years of education than controls, whereas Impaired not MCI, MCI, and AD participants had similar number of years of education (significant main effect of Diagnostic Group, F(3,106)=4.865, p<0.001). As expected, AD patients had lower MMSE scores than MCI participants, who in turn, had lower MMSE than controls. Impaired not MCI subjects and controls had similar MMSE scores (main effect of Diagnostic Group F(3,105)=10.17, p<0.001).
Table 1.
Subject demographic data.
| NC | MCI | AD | Cognitively impaired, not MCI |
Total Sample |
Pairwise comparison (p < 0.05) |
|
|---|---|---|---|---|---|---|
| N | 61 | 30 | 9 | 12 | 112 | |
| Age, mean yrs (SD) | 70.87 (6.65) | 72.97 (7.87) | 81.00 (8.37) | 65.67 (9.87) | 71.69 (8.60) | Impaired not MCI < (NC = MCI) < AD |
| Sex, n (% women) | 49 (80) | 25 (83) | 8 (89) | 10 (83) | 92 (82) | NC = MCI = AD = Impaired not MCI |
| Education, mean yrs (SD) | 14.77 (2.62) | 12.83 (2.74) | 12.38 (1.30) | 13.67 (2.64) | 13.95 (2.73) | (MCI = AD = Impaired not MCI), (Impaired not MCI = NC) |
| Vascular summary score mean (SD) | 1.00 (0.94) | 1.26 (0.94) | 0.800 (0.83) | 0.833 (0.83) | 1.04 (0.92) | NC=MCI=AD=Impaired not MCI |
| MMSE, mean (SD) | 28.13 (1.83) | 26.83 (3.05) | 22.71 (4.35) | 26.92 (3.00) | 27.29 (2.85) | (NC = Impaired not MCI), (Impaired not MCI = MCI) < AD |
In terms of representativeness of the MRI sample in the Columbia site sample, t-tests showed no differences for education (t(434)=0.592, p=0.554) and MMSE total score (t(435)=1.552, p=0.121), but subjects receiving MRI scans were slightly older (t(435)=3.048, p=0.002). Chi-square tests showed no difference for sex of the participants (χ2(1)=0.181, p=0.671), but there was a difference in the distribution of diagnostic groups (χ2(3)=13.403, p=0.004). In the imaging sample, there was a greater representation of individuals with AD (8% versus 5%) and individuals who were classified as Impaired not MCI (10% versus 2%) and lesser representation of normal controls (54% versus 63%) compared with the remaining subjects.
Omnibus differences for the morphological measures and neuropsychological variables are reported in Table 2. The groups differed in frontal, temporal, and occipital WMH volume as well as FA and performance across all neuropsychological tests. Note that these comparisons were made for descriptive purposes and did not include relevant covariates necessary for inference.
Table 2.
Descriptive subject neuroimaging and neuropsychological data. Omnibus effects across the four diagnostic groups are reported. Note that no covariates are included in these statistical analyses.
| NC | MCI | AD | Cognitive Impaired, not MCI |
Total Sample |
Omnibus Statistical Test |
||
|---|---|---|---|---|---|---|---|
| Relative Brain volume, mean (SD) | 0.69 (0.11) |
0.68 (0.12) |
0.67 (0.13) |
0.68 (0.14) |
0.69 (0.12) |
F(3,110)=0.13, 0.943 |
|
| Regional WMH volume (cm^3), mean (SD) [median] | Frontal | 1.06 (1.90) [0.42] |
1.29 (1.33) [0.87] |
3.38 (3.44) [2.27] |
1.96 (2.79) [0.92] |
1.40 (2.11) [0.73] |
F(3,111)=3.72, p = 0.014 |
| Temporal | 0.14 (0.33) [0.01] |
0.10 (0.24) [0.02] |
0.63 (1.22) [0.09] |
0.08 (0.09) [0.05] |
0.16 (0.42) [0.02] |
F(3,111)=3.99, p = 0.010 |
|
| Parietal | 0.99 (2.15) [0.21] |
1.43 (2.84) [0.15] |
3.22 (4.34) [1.07] |
0.83 (1.02) [0.19] |
1.27 (2.55) [0.20] |
F(3,111)=2.23, p = 0.089 |
|
| Occipital | 0.09 (0.18) [0.03] |
0.04 (0.57) [0.01] |
0.31 (0.69) [0.06] |
0.12 (0.13) [0.09] |
0.10 (0.24) [0.03] |
F(3,111)=2.89, p = 0.039 |
|
| FA factor score, mean (SD) | 0.16 (0.80) |
−0.11 (0.90) |
−1.46 (1.24) |
0.68 (0.77) |
0.00 (1.00) |
F(3,88)=10.23, p < 0.001 |
|
| Neuropsychological Test Score, mean (SD) | CVLT Trials 1–5 Free Recall | 47.38 (9.30) |
34.70 (6.52) |
26.00 (6.27) |
39.08 (8.43) |
41.58 (10.81) |
F(3, 101)=23.42, p < 0.001 |
| CVLT Short Delayed Free Recall | 9.18 (2.85) |
4.96 (2.38) |
0.86 (10.7) |
6.83 (2.25) |
7.22 (3.62) |
F(3, 101)=30.29, p < 0.001 |
|
| CVLT Long Delayed Free Recall | 9.91 (2.80) |
5.30 (3.37) |
0.71 (0.95) |
6.92 (2.28) |
7.71 (3.94) |
F(3, 101)=32.18, p < 0.001 |
|
| CVLT Recognition (Hits – False Alarms) | 10.63 (3.85) |
5.26 (4.65) |
−2.43 (6.50) |
6.08 (5.23) |
7.77 (5.73) |
F(3, 101)=23.50, p < 0.001 |
|
| Digit Symbol total correct | 47.38 (23.44) |
30.50 (12.29) |
7.00 (2.83) |
39.25 (11.59) |
36.95 (15.69) |
F(3, 39)=9.74, p < 0.001 |
|
| Trailmaking Test Part A (sec) | 40.63 (11.95) |
60.25 (29.21) |
102.17 (75.20) |
47.00 (17.50) |
50.38 (29.31) |
F(3,101)=12.69, p < 0.001 |
|
| Trailmaking Test Part B (sec) | 105.55 (53.76) |
207.81 (76.97) |
280.00 (40.00) |
157.36 (77.93) |
143.92 (80.77) |
F(3,88)=20.33, p < 0.001 |
|
| Digit Span Forward | 8.60 (2.17) |
7.00 (1.82) |
6.50 (3.54) |
5.75 (1.71) |
7.46 (2.18) |
F(3,37)=3.01, p = 0.043 |
|
| Digit Span Backward | 6.93 (1.94) |
4.65 (1.22) |
2.50 (0.71) |
4.00 (0.82) |
5.37 (2.01) |
F(3,37)=9.94, p < 0.001 |
|
| Rey Figure Copy | 27.30 (4.96) |
22.98 (6.38) |
14.41 (8.31) |
25.63 (7.11) |
25.16 (6.63) |
F(3,101)=10.55, p < 0.001 |
|
| Rey Figure Immediate Recall | 11.17 (5.98) |
7.98 (4.04) |
2.90 (4.04) |
8.18 (4.25) |
9.53 (5.57) |
F(3,99)=5.60, p = 0.001 |
|
| Rey Figure Delayed Recall | 10.66 (5.50) |
7.90 (3.92) |
2.20 (2.30) |
9.00 (4.25) |
9.31 (5.23) |
F(3,97)=5.68, p = 0.001 |
|
| Verbal Fluency: CFL Total | 39.23 (12.57) |
28.04 (18.83) |
28.67 (12.77) |
30.33 (10.76) |
34.49 (15.15) |
F(3, 101)=4.56, p = 0.005 |
|
| Category Fluency: Animal Naming | 16.48 (4.06) |
12.71 (2.76) |
9.86 (3.72) |
13.83 (4.61) |
14.70 (4.31) |
F(3,102)=10.67, p < 0.001 |
|
| Logical Memory Immediate Recall | 12.14 (3.13) |
9.25 (3.32) |
6.40 (3.21) |
11.42 (2.15) |
10.97 (3.46) |
F(3,100)=9.29, p < 0.001 |
|
| Logical Memory Delayed Recall | 10.84 (3.48) |
7.39 (3.07) |
2.40 (2.19) |
8.92 (1.68) |
9.24 (3.80) |
F(3,100)=15.76, p < 0.001 |
|
| Boston Naming Test | 48.64 (7.58) |
40.39 (8.53) |
29.40 (10.92) |
42.42 (7.40) |
44.66 (9.39) |
F(3,100)=13.68, p < 0.001 |
Of the 112 subjects included in the study, DTI data were available for 89 subjects. Diffusion tensor imaging was not available for subjects either due to scan artifacts or because fatigued subjects requested to discontinue the imaging study prior to the acquisition of the DTI sequence. Individuals with and without DTI available were similar in terms of age (t(110)=0.423, p=0.673), education (t(108)=1.183, p=0.239), sex distribution (χ2(1)=0.457, p=0.499), and distribution of diagnosis (χ2(3)=1.490, p=0.685).
Relationship between regional WMH and FA
Lower FA was associated with higher WMH volume in frontal (r=−0.509, p<.001), temporal (r=0.464, p<0.00), parietal (r=−0.509, p<0.001), and occipital (r=−0.275, p=0.009) lobes. Results of the non-parametric comparisons yielded similar effect sizes, although FA comparisons with temporal and occipital WMH were reduced to non-significance.
Relationship of regional WMH and FA with cognition
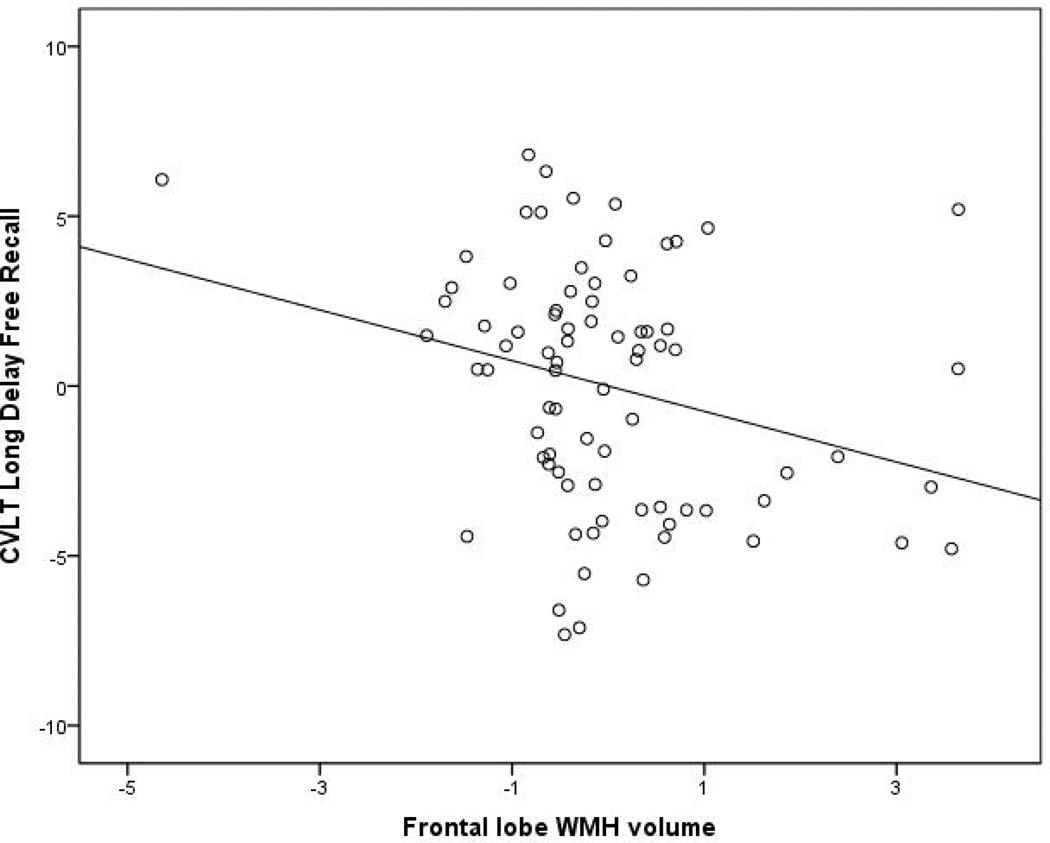
Increased WMH volume in the frontal lobes was associated with poorer performance on the CVLT long delayed free recall trial (overall model F(8, 78)= 2.632, p=0.014). For illustration, the plot of the association between frontal lobe WMH and CVLT long delayed free recall is displayed in Figure 3. This association remained significant when tested with simple bivariate correlations (Spearman’s r=−0.213, p=0.022). When the vascular disease summary score was entered into the regression analysis, the overall model was reduced to non-significance, but the relationship between frontal WMH volume and performance remained of similar magnitude (i.e., standardized beta of −0.423 vs −0.329). Similarly, increased WMH volume was associated with poorer performance on the CVLT recognition trial (overall model (F(8,78)=3.889, p=0.001), which was also verified with simple bivariate correlations (Spearman’s r = −0.195, p = 0.050). This association remained statistically significant when the vascular disease summary score was entered into the regression analysis. Increased WMH volume in the temporal lobes was associated with worse performance on the Trailmaking Test Part A and increased WMH volume in the occipital lobes was associated with better performance on the Trailmaking Test Part A (overall model F(8,78)=20.737, p<0.001). However, when examining these associations with simple bivariate correlations, neither temporal lobe WMH (Spearman’s r=0.036, p=0.717) nor occipital lobe WMH (Spearman’s r=0.013, p=0.895) was associated with performance on the Trailmaking Test Part A. Increased frontal lobe WMH was associated with poorer performance on the Digit Span forward test, although the overall model was not statistically significant (F(7,32)=1.581, p=0.187) nor was the bivariate correlation (Spearman’s r=−0.257, p=0.115). However, as the Digit Span test was only available for a subset of subjects, the lack of statistical significance may reflect insufficient power. Increased WMH in the temporal lobes was unexpectedly associated with better performance on the Logical Memory delayed trial (overall model F(8,77)=4.472, p<0.001) but this association was not confirmed with simple bivariate correlations (Spearman’s r=−0.179, p=0.074). Similarly, increased WMH in temporal lobes was associated with poorer performance on the Boston Naming Test (overall model F(8,77)=4.472, p<0.001), this association was not confirmed with simple bivariate correlations (Spearman’s r=−0.101, p=0.316). Results from all of the regression analyses are presented in Table 3. The FA summary measure was not associated with cognitive test performance in any of the analyses. When the analyses were re-run with the four AD-specific fiber tracts, none was associated reliably with cognitive test performance either when entered individually or together in a single model. In summary, only the significant relationship between higher WMH burden and lower delayed free memory recall was confirmed by non-parametric statistics.
Figure 3.
Scatterplot showing relationship between frontal lobe WMH volume and performance on the long delay recall trial of the CVLT. The plot is a partial plot displaying the results from the multiple regression analysis with WMH volumes from the other regions, the FA summary score, relative brain volume, age, and education included as additional covariates. The relationship between frontal lobe WMH volume and performance on the long delay recall trial of the CVLT remained even after removal of data from the subject who appears in the upper left side of the scatterplot.
Table 3.
Standardized beta weights and p-values (in parentheses) for neuropsychological test performance across diagnostic groups. Results are from separate multiple regression analyses run for each of the outcomes (columns). Significant effects are emphasized in bold.
| CVLT Trials 1–5 Free Recall |
CVLT Short Delayed Free Recall |
CVLT Long Delayed Free Recall |
CVLT Recognition (hits –false positives) |
Digit Symbol total correct |
Trail Making Test A |
Trail Making Test B |
Digit Span Forward |
Digit Span Backward |
Rey Figure Copy |
Rey Figure Immediate Recall |
Rey Figure Delayed Recall |
Verbal Fluency: CFL Total |
Categorial Fluency: Animals |
Logical Memory Immediate Recall |
Logical Memory Delayed Recall |
Boston Naming Test |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal WMH | −0.324 (0.073) |
−0.238 (0.100) |
−0.423 (0.020) |
−0.512 (0.003) |
0.285 (0.168) |
0.008 (0.946) |
0.117 (0.578) |
−0.576 (0.040) |
−0.389 (0.212) |
−0.110 (0.497) |
−0.174 (0.371) |
−0.041 (0.838) |
0.074 (0.700) |
−0.009 (0.960) |
0.003 (0.986) |
−0.069 (0.649) |
−0.039 (0.803) |
| Temporal WMH | 0.137 (0.427) |
0.123 (0.455) |
0.205 (0.235) |
0.840 (0.606) |
−0.034 (0.889) |
0.601 (<0.001) |
−0.174 (0.297) |
0.473 (0.150) |
0.034 (0.930) |
−0.040 (0.498) |
0.027 (0.885) |
0.015 (0.937) |
0.161 (0.385) |
0.036 (0.831) |
0.392 (0.084) |
0.514 (0.019) |
0.566 (0.013) |
| Parietal WMH | −0.069 (0.710) |
−0.022 (0.902) |
0.036 (0.844) |
0.162 (0.356) |
−0.092 (0.664) |
0.117 (0.320) |
0.280 (0.216) |
−0.332 (0.241) |
−0.136 (0.659) |
0.025 (0.881) |
−0.002 (0.992) |
−0.011 (0.960) |
−0.335 (0.097) |
0.055 (0.762) |
−0.298 (0.176) |
−0.324 (0.124) |
−0.218 (0.314) |
| Occipital WMH | 0.151 (0.279) |
0.212 (0.111) |
0.128 (0.355) |
0.100 (0.447) |
−0.211 (0.232) |
−0.270 (0.002) |
−0.167 (0.220) |
−0.037 (0.874) |
0.031 (0.907) |
0.082 (0.506) |
0.000 (0.999) |
0.039 (0.797) |
0.061 (0.677) |
−0.126 (0.360) |
−0.216 (0.161) |
−0.281 (0.058) |
−0.260 (0.088) |
| FA summary | −0.031 (0.853) |
0.052 (0.747) |
0.000 (0.999) |
0.022 (0.888) |
0.294 (0.237) |
−0.048 (0.640) |
−0.064 (0.678) |
−0.402 (0.223) |
−0.253 (0.475) |
0.122 (0.405) |
−0.011 (0.948) |
0.048 (0.787) |
−0.048 (0.783) |
0.167 (0.317) |
0.196 (0.181) |
0.160 (0.253) |
0.068 (0.638) |
| Relative brain volume | 0.045 (0.699) |
−0.033 (0.768) |
−0.153 (0.188) |
−0.038 (0.726) |
−0.115 (0.469) |
−0.076 (0.289) |
0.053 (0.641) |
0.111 (0.599) |
0.201 (0.374) |
−0.033 (0.744) |
−0.037 (0.765) |
−0.038 (0.322) |
0.047 (0.701) |
−0.095 (0.404) |
−0.034 (0.763) |
−0.483 (0.630) |
−0.124 (0.269) |
| Age | −0.238 (0.088) |
−0.378 (0.005) |
−0.225 (0.105) |
−0.246 (0.063) |
−0.292 (0.125) |
0.269 (0.002) |
0.229 (0.069) |
0.189 (0.444) |
−0.213 (0.242) |
−0.271 (0.027) |
−0.125 (0.375) |
−0.142 (0.322) |
0.008 (0.954) |
−0.211 (0.124) |
0.020 (0.881) |
−0.006 (0.965) |
−0.093 (0.476) |
| Education |
0.223 (0.045) |
0.109 (0.300) |
0.220 (0.047) |
0.237 (0.025) |
0.465 (0.002) |
−0.316 (<0.001) |
−0.422 (<0.001) |
0.117 (0.333) |
0.090 (0.646) |
0.417 (<0.001) |
0.306 (0.009) |
0.287 (0.018) |
0.336 (0.005) |
0.311 (0.005) |
0.396 (<0.001) |
0.429 (<0.001) |
0.413 (<0.001) |
Differences in regional WMH and FA between NC, MCI, and AD
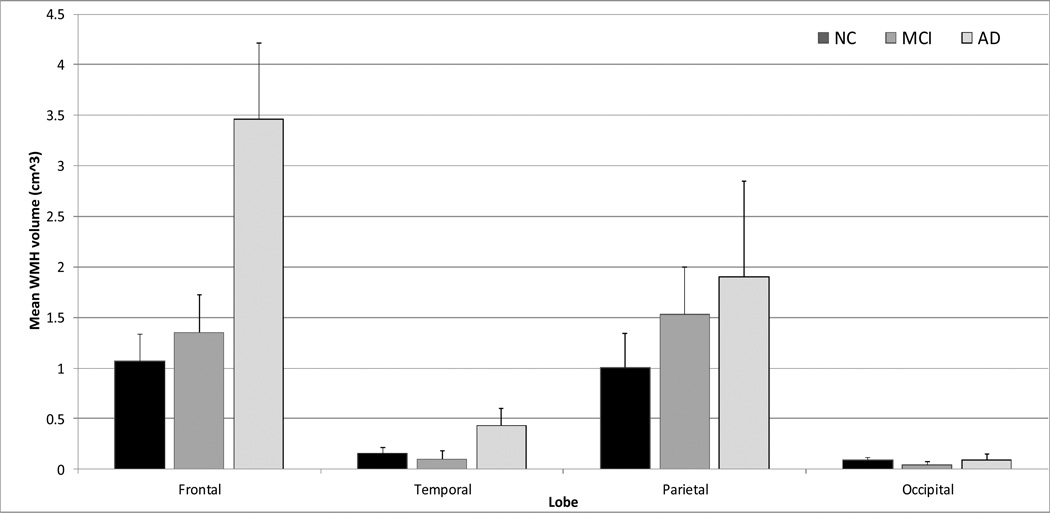
The regional distribution pattern of WMH differed across diagnostic groups (significant Diagnostic Group by Lobe interaction, F(6,273)=2.325, p=0.033). The pattern of the omnibus interaction (see Figure 4) suggests a “dose response” of increased WMH volume in frontal and parietal lobes among patients with AD, followed by subjects with MCI, and normal controls. Formal post hoc analyses were conducted with separate ANOVAs for each lobe separately. For frontal lobes, AD patients had significantly greater WMH volume than controls and subjects with MCI. In all other lobes, WMH volume was statistically similar across groups. The frontal lobe differences were confirmed with non-parametric Kruskal-Wallis Test (p=0.028 for frontal lobes). The overall model comparing FA among the three diagnostic groups showed a trend towards significance (F(2,71)=2.893, p=0.062). Post hoc examination showed that patients with AD had significantly (p<0.05) lower FA than controls and subjects with MCI, who were similar to each other. This same pattern emerged for FA in the inferior longitudinal fasciculus and genu of the corpus callosum (i.e., significant reduction among AD patients relative to MCI patients and controls), but not in the fornix or uncinate fasciculus. When the vascular disease summary score was entered as an additional covariate, the findings for both WMH and FA remained unchanged.
Figure 4.
WMH volume differences across diagnostic groups. Error bars are standard errors.
Discussion
We showed that increased WMH burden is related to decreased DTI-derived FA, suggesting that both capture aspects of white matter structure. However, the relationship of these white matter measurements to cognition or diagnostic group differed. In general, WMH severity, a marker of small vessel cerebrovascular disease, was more consistently related to cognitive function and AD than FA, a marker of white matter microstructural integrity. Specifically, we found that WMH volume in the frontal lobes was associated with poorer memory and that both frontal and parietal WMH volume is elevated among individuals with AD and at risk for AD (i.e., the MCI group). Global FA, on the other hand, in our sample was not associated with either cognitive functioning or with diagnostic group, although there was a non-significant trend for deceased FA among patients with AD and post hoc analyses did reveal statistically reliable diminution of FA among AD patients. Our findings suggest that WMH volume is a more sensitive radiological correlate of cognitive functioning among older adults than FA and, particularly when distributed in frontal and parietal lobes, may play a specific role in AD.
Although, to our knowledge, previous studies have not combined macrostructural and microstructural white matter imaging techniques to examine their differential associations with cognition and diagnosis, our finding of a relationship between the two is in line with previous investigations showing an inverse relationship in clinical samples (Engelhardt et al., 2009; Taylor et al., 2001). Despite the shared variance between the two measures, only WMH were related to cognitive function reliably in our study. As FA captures both normal variability in white matter structure as well as disease- or age-related changes (Furutani, Harada, Minato, Morita, & Nishitani, 2005; Grieve, et al., 2007; Stricker, et al., 2009), the measure may be less sensitive than frank markers of pathology, such as WMH.
It was somewhat surprising that the measures of FA did not emerge as a significant predictor of cognitive function in this study and there are several possibilities for this negative result. First, our interest was in capturing a general measure of white matter integrity. Indeed the derived FA factor accounted for 54.9% in the variance. We expected that this data reduction approach would provide the most reliable general measure of FA that would be related to cognitive function (Penke, et al., 2010) but it is possible that subtle regional differences were not captured. Second, our DTI sequences comprised 16 directions, which may have obscured more subtle relationships with cognition. Third, it is possible that among older adults with significant vascular disease histories, measurements of macrostructure that reflect cerebrovascular disease may simply be more relevant to cognitive outcomes than subtle variation in white matter microstructure.
However, we did observe a trend in the data– decreased FA among patients with AD compared to healthy controls – which has been observed in previous studies (Bozzali, et al., 2002; Takahashi, et al., 2002). Findings have not been entirely consistent, though, particularly with regard to the regional distribution of FA reduction in AD; reduced FA among AD patients has been reported in several regions, including periventricular frontal white matter (Choi, Lim, Monteiro, & Reisberg, 2005), posterior cingulate (Yoshiura et al., 2002), columns of the fornix (Ringman et al., 2007), thalamus, parietal white matter, posterior limbs of the internal capsule (Rose, Janke, & Chalk, 2008), and genu of the corpus callosum (Salat et al., 2005). Thus, while reduction in FA among patients with AD has been observed, there is a relative paucity of replication regarding specific regions; our findings suggest that when overall white matter microstructural integrity is estimated, there is little evidence of a statistically reliable general reduction among patients with AD.
Our findings are consistent with the increased regional distribution of WMH in frontal and parietal lobes, also found by Yoshita and colleagues (Yoshita, et al., 2006) as well as by Leys and colleagues (Leys et al., 1991) among patients with AD. These areas are typically associated with memory and are known to be most susceptible to degeneration in AD (Brun & Englund, 1981; Laakso et al., 1995). In the context of AD, WMH may result from perfusion abnormalities, vascular burden, and from vascular deposition of beta amyloid (Brickman, et al., 2009), or possibly from wallerian degeneration to cortical-cortical fiber connections. Frontal and parietal lobes are more vulnerable to perfusion abnormalities (Matsuda, 2001) and vascular deposition of beta amyloid (Esiri & Wilcock, 1986) than the remaining regions of the human brain. This consistent regional distribution of WMH suggests a possible pathological link of AD with vascular disease on a mechanistic level, on the one hand, and/or suggests that a cerebrovascular disease burden in addition to “primary” AD pathology can contribute to the clinical presentation of AD, on the other.
While several studies have reported associations between WMH and cognition (Dufouil, Alperovitch, & Tzourio, 2003; E. E. Smith et al., 2011), the specific relationship between WMH and memory performance we observed is not entirely consistent with previous studies. Among neurologically-healthy older adults, WMH are typically reported to be related with speeded tasks of executive function (Gunning-Dixon & Raz, 2000). However, there is an emerging literature linking WMH to MCI and AD, which are characterized primarily by an amnestic syndrome (E. E. Smith, et al., 2011). The current study is consistent with previous reports, which have shown that WMH are not only more common in AD patients, but their appearance is also elevated in people with MCI (Delano-Wood et al., 2009; Luchsinger, et al., 2009; Yoshita, et al., 2006).
Results from the current study may reflect the unique sample of exclusively African American participants. Increased prevalence and incidence rates of dementia and cognitive dysfunction among older African Americans (Gurland, et al., 1999; Perkins & Schisterman, 2006; M. X. Tang et al., 2001; Unverzagt, et al., 1996) may be due in part to increased cerebrovascular disease among this population (Brickman, Schupf, et al., 2008; Reitz et al., 2009), though previous studies have not found differential brain-behavior relationships across ethnic/racial groups (DeCarli et al., 2008). Future studies should further explicitly examine whether the mediators of cognitive function and diagnostic group vary as a function of ethnic/racial group. Relatively poor specificity of neuropsychological measures used to measure cognitive function and diagnosis patients among African Americans may be another important source of variance in our study, contributing to both relatively weak associations between the MRI-derived measures of white matter and cognition and to differences between our study and results reported in the extant literature. Mapping the longitudinal course of cognitive change would certainly contribute to a more clear understanding of the link between white matter abnormalities and progressive cognitive change.
There are weaknesses in the current study that are important to highlight. First, the number of statistical comparisons was quite high, increasing the likelihood of Type I statistical error. On the other hand, the number of AD and MCI participants was relatively low, possibly masking group differences due to diminished power and potentially increasing the Type II statistical error rate. Replication of our findings in a separate and larger cohort is clearly warranted. A group of participants did not clearly meet criteria for MCI, AD, or normal cognition and were thus labeled “cognitively impaired not MCI.” It is unclear what the nature of the cognitive impairment is and it is possible they have had cognitive difficulties for most of their adult lives. Again, longitudinal follow-up of participants will be critical to elucidate better whether this subgroup of participants have a neurodegenerative condition. Further, because we were interested in understanding the relationship between imaging markers and cognition or diagnosis and to maintain consistency with the parent study, we did not use the neuroimaging data in the diagnostic formulation of each subject. Future studies may wish to incorporate radiological data (e.g., presence of infarct, regional atrophy) into the diagnostic procedures. Finally, future studies may wish to consider other DTI metrics, which may provide complementary information or may increase sensitivity for detection of relationships between cognition and white matter microstructure.
In summary, we showed that among African American older adults, FA and WMH are associated with each other, but that only WMH were significant predictors of memory and AD diagnosis.
Acknowledgment
This work was supported by NIH grants AG028786 and AG034189, and by a grant from Gottfried und Julia Bangerter-Rhyner-Stiftung. This work was completed, in part, towards the requirements for a master’s degree at the University of Zurich (IBM).
Footnotes
The authors report no conflicts of interest.
References
- Admiraal-Behloul F, Olofesen H, Van den Heuvel DM, Schmitz N, Reiber JH, Van Buchem MA. Fully automated lobe delineation for regional white matter lesion load quantification in a large scale study. Proceedings International Society for Magnetic Resonance in medicine. 2004:138. [Google Scholar]
- American Psychiatric, A. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Barber R, Gholkar A, Scheltens P, Ballard C, McKeith IG, O'Brien JT. Medial temporal lobe atrophy on MRI in dementia with Lewy bodies. Neurology. 1999;52(6):1153–1158. doi: 10.1212/wnl.52.6.1153. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magnetic Resonance in Medicine. 2000;44(4):625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher Kd. Multilingual Aphasia Examination. Iowa City, IA: University of Iowa; 1976. [Google Scholar]
- Bozzali M, Falini A, Franceschi M, Cercignani M, Zuffi M, Scotti G, Filippi M. White matter damage in Alzheimer's disease assessed in vivo using diffusion tensor magnetic resonance imaging. Journal of Neurology, Neurosurgery & Psychiatry. 2002;72(6):742–746. doi: 10.1136/jnnp.72.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Honig LS, Scarmeas N, Tatarina O, Sanders L, Albert MS, Stern Y. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. archives of Neurology. 2008;65(9):1202–1208. doi: 10.1001/archneur.65.9.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Muraskin J, Zimmerman ME. Structural neuroimaging in Alzheimer's disease: do white matter hyperintensities matter? Dialogues in Clinical Neuroscience. 2009;11(2):181–190. doi: 10.31887/DCNS.2009.11.2/ambrickman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Archives of Neurology. 2010;67(5):564–569. doi: 10.1001/archneurol.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Brown TR. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Archives of Neurology. 2008;65(8):1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Sneed JR, Provenzano FA, Garcon E, Johnert L, Muraskin J, Roose SP. Quantitative approaches for assessment of white matter hyperintensities in elderly populations. Psychiatry Research: Neuroimaging. 2011;193(2):101–106. doi: 10.1016/j.pscychresns.2011.03.007. [pii] 10.1016/j.pscychresns.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun A, Englund E. Regional pattern of degeneration in Alzheimer's disease: neuronal loss and histopathological grading. Histopathology. 1981;5(5):549–564. doi: 10.1111/j.1365-2559.1981.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Burns JM, Church JA, Johnson DK, Xiong C, Marcus D, Fotenos AF, Buckner RL. White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Archives of Neurology. 2005;62(12):1870–1876. doi: 10.1001/archneur.62.12.1870. [DOI] [PubMed] [Google Scholar]
- Capizzano AA, Acion L, Bekinschtein T, Furman M, Gomila H, Martinez A, Starkstein SE. White matter hyperintensities are significantly associated with cortical atrophy in Alzheimer's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75(6):822–827. doi: 10.1136/jnnp.2003.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Lim KO, Monteiro I, Reisberg B. Diffusion tensor imaging of frontal white matter microstructure in early Alzheimer's disease: a preliminary study. Journal of Geriatric Psychiatry and Neurology. 2005;18(1):12–19. doi: 10.1177/0891988704271763. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA. The Neuropsychiatric Inventory:Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Smith SM, Witter MP, Sanz-Arigita EJ, Barkhof F, Scheltens P, Rombouts SA. White matter tract integrity in aging and Alzheimer's disease. Human Brain Mapping. 2009;30(4):1051–1059. doi: 10.1002/hbm.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30(3):529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Reed BR, Jagust W, Martinez O, Ortega M, Mungas D. Brain behavior relationships among African Americans, whites, and Hispanics. Alzheimer Disease and Associated Disorders. 2008;22(4):382–391. doi: 10.1097/wad.0b013e318185e7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano-Wood L, Bondi MW, Sacco J, Abeles N, Jak AJ, Libon DJ, Bozoki A. Heterogeneity in mild cognitive impairment: differences in neuropsychological profile and associated white matter lesion pathology. Journal of the International Neuropsychological Society. 2009;15(6):906–914. doi: 10.1017/S1355617709990257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JJ, Kaplan E, Ober BA. California Verbal Learning Test. 2nd ed. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Duan JH, Wang HQ, Xu J, Lin X, Chen SQ, Kang Z, Yao ZB. White matter damage of patients with Alzheimer's disease correlated with the decreased cognitive function. Surgical and Radiological Anatomy. 2006;28(2):150–156. doi: 10.1007/s00276-006-0111-2. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Alperovitch A, Tzourio C. Influence of education on the relationship between white matter lesions and cognition. Neurology. 2003;60(5):831–836. doi: 10.1212/01.wnl.0000049456.33231.96. [DOI] [PubMed] [Google Scholar]
- Engelhardt E, Moreira DM, Alves GS, Lanna ME, Alves CE, Ericeira-Valente L, Laks J. Binswanger's disease and quantitative fractional anisotropy. Arquivos de Neuropsiquiatria. 2009;67(2A):179–184. doi: 10.1590/s0004-282x2009000200002. [DOI] [PubMed] [Google Scholar]
- Esiri MM, Wilcock GK. Cerebral amyloid angiopathy in dementia and old age. Journal of Neurology, Neurosurgery & Psychiatry. 1986;49(11):1221–1226. doi: 10.1136/jnnp.49.11.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43(9):1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Furutani K, Harada M, Minato M, Morita N, Nishitani H. Regional changes of fractional anisotropy with normal aging using statistical parametric mapping (SPM) Journal of Investigative Medicine. 2005;52(3–4):186–190. doi: 10.2152/jmi.52.186. [DOI] [PubMed] [Google Scholar]
- Goodglass HK, E Assessment of aphasia and related disorders. 1983 [Google Scholar]
- Gootjes L, Teipel SJ, Zebuhr Y, Schwarz R, Leinsinger G, Scheltens P, Hampel H. Regional distribution of white matter hyperintensities in vascular dementia, Alzheimer's disease and healthy aging. Dementia and Geriatric Cognitive Disorder. 2004;18(2):180–188. doi: 10.1159/000079199. [DOI] [PubMed] [Google Scholar]
- Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJ. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. Journal of Neurology, Neurosurgery & Psychiatry. 2011;82(2):126–135. doi: 10.1136/jnnp.2009.204685. [pii] 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. American Journal of Neuroradiology. 2007;28(2):226–235. [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, Mayeux R. Rates of dementia in three ethnoracial groups. International Journal of Geriatric Psychiatry. 1999;14(6):481–493. [PubMed] [Google Scholar]
- Huang J, Auchus AP. Diffusion tensor imaging of normal appearing white matter and its correlation with cognitive functioning in mild cognitive impairment and Alzheimer's disease. Annals of the New York Academy Science. 2007;1097:259–264. doi: 10.1196/annals.1379.021. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia, PA: Lippincott Williams & Wilkins; 1983. [Google Scholar]
- Kiuchi K, Morikawa M, Taoka T, Nagashima T, Yamauchi T, Makinodan M, Kishimoto T. Abnormalities of the uncinate fasciculus and posterior cingulate fasciculus in mild cognitive impairment and early Alzheimer's disease: a diffusion tensor tractography study. Brain Research. 2009;1287:184–191. doi: 10.1016/j.brainres.2009.06.052. [pii] 10.1016/j.brainres.2009.06.052. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Soininen H, Partanen K, Helkala EL, Hartikainen P, Vainio P, Riekkinen PJ., Sr Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer's disease: correlation with memory functions. Journal of Neural Transmission: Parkinson's Disease and Dementia Section. 1995;9(1):73–86. doi: 10.1007/BF02252964. [DOI] [PubMed] [Google Scholar]
- Leys D, Pruvo JP, Parent M, Vermersch P, Soetaert G, Steinling M, et al. Could Wallerian degeneration contribute to "leuko-araiosis" in subjects free of any vascular disorder? Journal of Neurology, Neurosurgery & Psychiatry. 1991;54(1):46–50. doi: 10.1136/jnnp.54.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Cooper L, Cai J, Toole J, Bryan N, Burke G, Heiss G. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology. 1997;16(3):149–162. doi: 10.1159/000368814. [DOI] [PubMed] [Google Scholar]
- Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, Tyroler HA. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Atherosclerosis Risk in Communities Study. Stroke. 1996;27(12):2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- Liu Y, Spulber G, Lehtimaki KK, Kononen M, Hallikainen I, Grohn H, Soininen H. Diffusion tensor imaging and Tract-Based Spatial Statistics in Alzheimer's disease and mild cognitive impairment. Neurobiology of Aging. 2011;32(9):1558–1571. doi: 10.1016/j.neurobiolaging.2009.10.006. [pii] 10.1016/j.neurobiolaging.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Brickman AM, Reitz C, Cho SJ, Schupf N, Manly JJ, Brown TR. Subclinical cerebrovascular disease in mild cognitive impairment. Neurology. 2009;73(6):450–456. doi: 10.1212/WNL.0b013e3181b1636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychology Review. 2009;19(4):415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA, Kronmal RA, Burke GL, Poirier V, O'Leary DH, Gardin JM, Bryan RN. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25(2):318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- Matsuda H. Cerebral blood flow and metabolic abnormalities in Alzheimer's disease. Ann Nucl Med. 2001;15(2):85–92. doi: 10.1007/BF02988596. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Medina D, DeToledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiology of Aging. 2006;27(5):663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Rauch GM, Crawford K, Rauch RA, Konno S, Akiyama H, Haque A. Risk factors accelerating cerebral degenerative changes, cognitive decline and dementia. International Journal of Geriatric Psychiatry. 1999;14(12):1050–1061. doi: 10.1002/(sici)1099-1166(199912)14:12<1050::aid-gps56>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Mori S, Barker PB. Diffusion magnetic resonance imaging: its principle and applications. Anatomical Record. 1999;257(3):102–109. doi: 10.1002/(SICI)1097-0185(19990615)257:3<102::AID-AR7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Naggara O, Oppenheim C, Rieu D, Raoux N, Rodrigo S, Dalla Barba G, Meder JF. Diffusion tensor imaging in early Alzheimer's disease. Psychiatry Rsearch. 2006;146(3):243–249. doi: 10.1016/j.pscychresns.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Oliveira AP, Brickman AM, Provenzano FA, Muraskin J, Louis ED. White matter hyperintensity burden on magnetic resonance imaging in essential tremor: A population-based study in New York. Tremor and Other Hyperkinetic Movements. doi: 10.7916/D8K64GS0. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieth PA. Filetest de copie d'une figure complex: Contribution a l'etude de la perception et de la memoire. Archives de Psychologie. 1944;30:286–356. [Google Scholar]
- Penke L, Deary IJ. Some guidelines for structural equation modelling in cognitive neuroscience: the case of Charlton etal.'s study on white matter integrity and cognitive ageing. Neurobiology of Aging. 2010;31(9):1656–1660. doi: 10.1016/j.neurobiolaging.2009.10.019. discussion 1561–1656. [DOI] [PubMed] [Google Scholar]
- Penke L, Munoz Maniega S, Murray C, Gow AJ, Hernandez MC, Clayden JD, Deary IJ. A general factor of brain white matter integrity predicts information processing speed in healthy older people. Journal of Neuroscience. 2010;30(22):7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins NJ, Schisterman EF. The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. American Journal of Epidemiology. 2006;163(7):670–675. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki CH, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journals of Gerontology. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pievani M, Agosta F, Pagani E, Canu E, Sala S, Absinta M, Filippi M. Assessment of white matter tract damage in mild cognitive impairment and Alzheimer's disease. Human Brain Mapping. 2010;31(12):1862–1875. doi: 10.1002/hbm.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21(2):149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Reitan. Reitan Neuropsychology Laboratories. Tucson, AZ: 1978. [Google Scholar]
- Reitz C, Schupf N, Luchsinger JA, Brickman AM, Manly JJ, Andrews H, Mayeux R. Validity of self-reported stroke in elderly African Americans, Caribbean Hispanics, and Whites. Archives of Neurology. 2009;66(7):834–840. doi: 10.1001/archneurol.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, O'Neill J, Geschwind D, Medina L, Apostolova LG, Rodriguez Y, Bartzokis G. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer's disease mutations. Brain. 2007;130(Pt 7):1767–1776. doi: 10.1093/brain/awm102. [DOI] [PubMed] [Google Scholar]
- Rose SE, Janke AL, Chalk JB. Gray and white matter changes in Alzheimer's disease: a diffusion tensor imaging study. Journal of Magnetic Resonance Imaging. 2008;27(1):20–26. doi: 10.1002/jmri.21231. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26(8):1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Sano M, Stern Y, Mayeux R, Hartman S, Devanand DP. A standardized technique for establishing the onset symptoms of probable Alzheimer's disease. Journal of Clinical and Experimental Neuropsychology. 1987;9:65–65. [Google Scholar]
- Scheltens P, Barkhof F, Valk J, Algra PR, van der Hoop RG, Nauta J, Wolters EC. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer's disease. Evidence for heterogeneity. Brain. 1992;115(Pt 3):735–748. doi: 10.1093/brain/115.3.735. [DOI] [PubMed] [Google Scholar]
- Smith CD, Snowdon DA, Wang H, Markesbery WR. White matter volumes and periventricular white matter hyperintensities in aging and dementia. Neurology. 2000;54(4):838–842. doi: 10.1212/wnl.54.4.838. [DOI] [PubMed] [Google Scholar]
- Smith EE, Egorova S, Blacker D, Killiany RJ, Muzikansky A, Dickerson BC, Guttmann CR. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Archives of Neurology. 2008;65(1):94–100. doi: 10.1001/archneurol.2007.23. [DOI] [PubMed] [Google Scholar]
- Smith EE, Salat DH, Jeng J, McCreary CR, Fischl B, Schmahmann JD, Greenberg SM. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology. 2011;76(17):1492–1499. doi: 10.1212/WNL.0b013e318217e7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ, Alexander GE. Interactive effects of age and hypertension on volumes of brain structures. Stroke. 1997;28(7):1410–1417. doi: 10.1161/01.str.28.7.1410. [DOI] [PubMed] [Google Scholar]
- Stricker NH, Schweinsburg BC, Delano-Wood L, Wierenga CE, Bangen KJ, Haaland KY, Bondi MW. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer's disease supports retrogenesis. Neuroimage. 2009;45(1):10–16. doi: 10.1016/j.neuroimage.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Yonezawa H, Takahashi J, Kudo M, Inoue T, Tohgi H. Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neuroscience Letters. 2002;332(1):45–48. doi: 10.1016/s0304-3940(02)00914-x. [DOI] [PubMed] [Google Scholar]
- Tanabe JL, Amend D, Schuff N, DiSclafani V, Ezekiel F, Norman D, Weiner MW. Tissue segmentation of the brain in Alzheimer disease. American Journal of Neuroradiology. 1997;18(1):115–123. [PMC free article] [PubMed] [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Mayeux R. Incidence of Alzheimer's disease in African-Americans, Caribbean Hispanics and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Payne ME, Krishnan KR, Wagner HR, Provenzale JM, Steffens DC, MacFall JR. Evidence of white matter tract disruption in MRI hyperintensities. Biological Psychiatry. 2001;50(3):179–183. doi: 10.1016/s0006-3223(01)01160-x. [DOI] [PubMed] [Google Scholar]
- Unverzagt FW, Hall KS, Torke AM, Rediger JD. Effects of age, education and gender on CERAD neuropsychological test performance in an African American sample. Clinical Neuropsychology. 1996;10:180–190. [Google Scholar]
- Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Breteler MM. White matter microstructural integrity and cognitive function in a general elderly population. Archives of General Psychiatry. 2009;66(5):545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009;72(4):368–374. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Li YO, Ng J, Lahue SC, Cooper SR, Sherr EH, Mukherjee P. Microstructural correlations of white matter tracts in the human brain. Neuroimage. 2010;51(2):531–541. doi: 10.1016/j.neuroimage.2010.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WMS-III administration and scoring manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Discord. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Xiao JX, Gong GL, Zang YF, Wang YH, Wu HK, Jiang XX. Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology. 2006;66(12):1845–1849. doi: 10.1212/01.wnl.0000219625.77625.aa. [DOI] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, AD. Neurology. 2006;67(12):2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiura T, Mihara F, Ogomori K, Tanaka A, Kaneko K, Masuda K. Diffusion tensor in posterior cingulate gyrus: correlation with cognitive decline in Alzheimer's disease. Neuroreport. 2002;13(17):2299–2302. doi: 10.1097/00001756-200212030-00026. [DOI] [PubMed] [Google Scholar]