Abstract
Births are highly synchronized among females in many mammal populations in temperate areas. Although laying date for a given female is also repeatable within populations of birds, limited evidence suggests low repeatability of parturition date for individual females in mammals, and between-population variability in repeatability has never, to our knowledge, been assessed. We quantified the repeatability of parturition date for individual females in five populations of roe deer, which we found to vary between 0.54 and 0.93. Each year, some females gave birth consistently earlier in the year, whereas others gave birth consistently later. In addition, all females followed the same lifetime trajectory for parturition date, giving birth progressively earlier as they aged. Giving birth early should allow mothers to increase offspring survival, although few females managed to do so. The marked repeatability of parturition date in roe deer females is the highest ever reported for a mammal, suggesting low phenotypic plasticity in this trait.
Keywords: repeatability, Capreolus capreolus, trajectory, birth date
1. Introduction
Most large herbivores in the holarctic zone are characterized by highly seasonal and synchronous birth periods [1]. Optimal synchronized timing of parturition should match the vegetation flush [2] and should minimize offspring predation [3]. Birth dates strongly influence reproductive success in vertebrates [4], as they can markedly affect early growth and survival of newborns as reported in different species [5,6]. Variation in environmental conditions such as those linked to global change influences the timing of reproduction by affecting plant phenology [7]. Parturition date often occurs earlier in older and heavier females than in younger and lighter females [8]. In many bird species, some females are consistently early layers, whereas others are consistently late [9], suggesting an influence of maternal attributes on parturition date [5], independent of environmental conditions. Estimated repeatability of laying date varies among bird species [9]. However, estimation of both within- and among-population repeatability of parturition date has been overlooked in mammals (but see [10]).
Using datasets from five populations of roe deer, Capreolus capreolus, experiencing different climate, density and predation pressure, we quantified the repeatability of parturition date for individual females. In addition, we took advantage of the detailed monitoring in one population to assess individual trajectories of parturition date in relation to female age. Because parturition date, like laying date, is known to be heritable [4], we expected (i) a given female to give birth at approximately the same date each year over its lifetime, leading it to (ii) follow a consistent age-related trajectory of parturition dates during its lifetime.
2. Material and methods
(a). Data collection
Parturition dates were collected in five different areas: Bogesund (2600 ha, 59°40′ N, Sweden) with a continental climate, relatively harsh snowy winters and mild and dry summers [11]; and Grimsö (13 000 ha, 59°23′ N, Sweden) with more severe winters and longer snow cover [12]—both are coniferous forests; Storfosna (1050 ha, 63°40′ N, Norway), a mosaic landscape, with mild winters and cool summers [13]; Aurignac (7500 ha, 43°13′ N, France), a mixed landscape of open fields and small woodland patches and an oceanic climate [14]; and Trois Fontaines (1360 ha, 48°43′ N, France), an oak-beech forest with a continental climate and relatively cold winters [15]. Roe deer females were individually marked with collars (very high frequency, global positioning system or numbered), and parturition dates were estimated either by daily observation of visually large females, or by back-calculating from fawn age at capture. Fawns were aged using umbilicus characteristics and behaviour at marking, or using the relationship between an individual's growth rate and mean birth weight [13,15,16] (see the electronic supplementary material for data).
(b). Statistical analyses
We fitted a linear mixed model to measure repeatability, with mother identity as a random intercept, and year as a discrete fixed factor to control for sampling variation among females and interannual variation in environmental conditions [17]. We excluded offspring sex from our analyses because this factor has no influence on parturition date [13,15]. We calculated repeatability as the ratio of the variance associated with the random effect (i.e. mother identity) over the total variance in parturition date [17]. Confidence intervals for repeatability were estimated using permutation tests. For each population, we replicated our analyses, using all available females or only using females with at least two parturitions to assess the robustness of our results to the occurrence of single measurements in a mixed modelling framework.
To assess individual trajectories of parturition date over female lifespan, we fitted a mixed model explaining variation in parturition date in relation to age, with female identity as a random factor on the intercept. We measured a female's deviation from the average population trajectory over the course of her lifetime by adding a random effect of female identity on the slope (age) using a likelihood-ratio test (LRT). This latter analysis was performed only in Trois Fontaines, the only site where the exact age of females was known.
3. Results
Parturition dates were normally distributed (all Kolmogorov–Smirnov tests, p > 0.195), yielding similar mean and median dates of parturition (table 1 and figure 1). Median parturition date was earliest at Aurignac (12 May) and Trois Fontaines (16 May) and latest at Bogesund (4 June). The coefficient of variation in parturition date was low in all populations (0.057–0.082). Around 80 per cent (from 79% at Aurignac to 92% at Trois Fontaines) of parturition dates occurred within a period of one month. The negative relationship between synchrony and repeatability was marginally non-significant (Spearman's ρ = −0.9, p = 0.083), but latitude was not related to either of these variables (see figure 1 and the electronic supplementary material).
Table 1.
Repeatability (R) measured in five populations of roe deer. (Median parturition date (median PD), mean parturition date (mean PD) and the coefficient of variation (s.d./mean, CV) are given in Julian date for each population. The number of females in each population (Nm) and the mean number of parturition dates per female (Nb) are also provided. For each population, two models are presented: one using all available females (AM) and the other using only females with at least two recorded parturitions (RM).)
| population | R | CI 2.5% | CI 97.5% | median PD | mean PD | CV | Nm | Nb |
|---|---|---|---|---|---|---|---|---|
| Trois Fontaines | ||||||||
| AM | 0.538 | 0.383 | 0.655 | 136 | 135.50 | 0.064 | 145 | 1.93 |
| RM | 0.479 | 0.302 | 0.623 | 136 | 135.16 | 0.060 | 65 | 3.08 |
| Aurignac | ||||||||
| AM | 0.708 | 0.500 | 0.845 | 132 | 133.53 | 0.082 | 87 | 1.39 |
| RM | 0.684 | 0.385 | 0.844 | 133 | 134.58 | 0.077 | 19 | 2.78 |
| Bogesund | ||||||||
| AM | 0.563 | 0.164 | 0.788 | 155.5 | 154.60 | 0.057 | 43 | 1.53 |
| RM | 0.607 | 0.191 | 0.817 | 155 | 154.40 | 0.060 | 20 | 2.15 |
| Grimsö | ||||||||
| AM | 0.930 | 0.726 | 0.991 | 150 | 149.40 | 0.060 | 26 | 1.38 |
| RM | 0.935 | 0.649 | 0.993 | 146.5 | 148.17 | 0.058 | 8 | 2.25 |
| Storfosna | ||||||||
| AM | 0.585 | 0.277 | 0.770 | 143 | 143.45 | 0.066 | 46 | 1.73 |
| RM | 0.506 | 0.148 | 0.728 | 143.5 | 143.87 | 0.063 | 22 | 2.55 |
Figure 1.
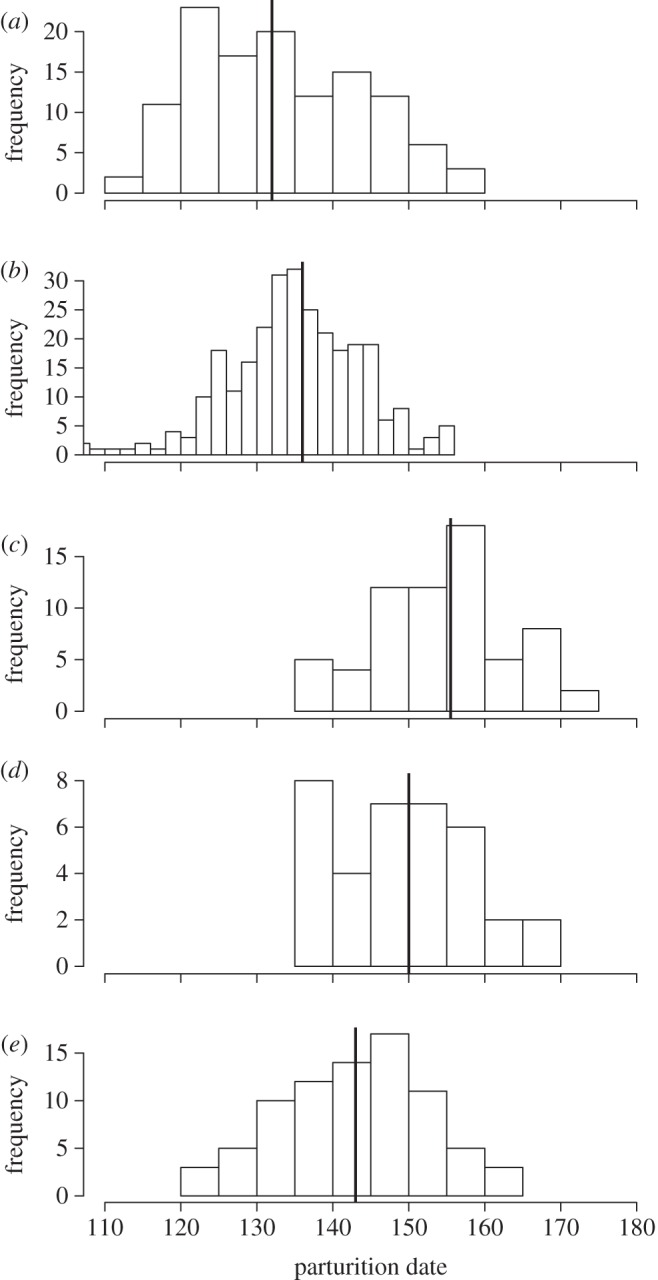
Distribution of parturition dates (Julian date) in the roe deer populations of (a) Aurignac (2007–2012), (b) Trois Fontaines (1985–2010), (c) Bogesund (2001–2006), (d) Grimsö (2000–2009) and (e) Storfosna (1991–1994) from the lowest to the highest latitude. The median parturition date is represented by a black vertical line.
Individual females tended to give birth each year at approximately the same date (table 1). The repeatability of parturition date was consistently high within all populations—Aurignac: 0.708 (0.500–0.845), Grimsö: 0.930 (0.726–0.991), Storfosna: 0.585 (0.277–0.770), Bogesund: 0.563 (0.164–0.788) and Trois Fontaines: 0.538 (0.383–0.655). When removing females with only one recorded parturition date, we found qualitatively similar results (table 1).
At Trois Fontaines, females gave birth earlier as they aged (β = −0.62, s.e. = 0.22, t = −3.04, p = 0.003). Adding an interaction between age and the random effect of female identity did not improve the fit (LRT, χ2 = 3.258, p = 0.196), indicating that, on average, females followed a similar trajectory of parturition date as they aged.
4. Discussion
We have provided compelling evidence that, within five populations, the date at which a given roe deer female gives birth is highly repeatable over its lifetime: some females consistently gave birth early in the birthing season, whereas others consistently gave birth late. Moreover, a detailed analysis in one population showed that all females follow similar age-related trajectories in parturition date, giving birth earlier as they age.
In accordance with previous studies on ungulates in temperate areas [1], parturition dates were synchronous in all populations. However, high birth synchrony at the population level does not preclude marked interindividual variation in parturition date. Indeed, we found that among-female variation in parturition date was higher than within-female variation. The within-individual repeatability of parturition date ranged from 0.54 to 0.93 among populations. In birds, repeatability of laying date range between 0.10 and 0.61 [18,19], whereas the only available value for a mammal was 0.10 (in red deer, derived from Nussey et al. [10]), suggesting that repeatability in roe deer is particularly high. This high repeatability suggests a low level of phenotypic plasticity for this trait and, therefore, little potential for a rapid response to drastic changes. Furthermore, even though the high among-female variation in parturition dates suggests the possibility for roe deer to respond to a selective pressure, weak among-year variation indicates that the birth period of roe deer has not yet been modified in response to climate change, contrary to the situation in other mammals such as red deer [20].
Variation in parturition date was related to interindividual heterogeneity in all populations. Yearly variation in annual mean parturition dates was low. We found that the among-population variation in repeatability was substantial (from 0.54 to 0.93), but no environmental variable could account for it (e.g. timing of vegetation flush or latitude, see the electronic supplementary material). The high value for repeatability at Grimsö and Aurignac could be owing to low sample size (Grimsö) or the low number of parturitions recorded per female (at Grimsö and Aurignac). At Aurignac, the low value (compared with other populations) of birth synchrony could be linked to the high spatial heterogeneity of the habitat, which in turn influences female quality. The high variation of roe deer density in Storfosna probably did not influence repeatability, which was of a similar level to that at Bogesund where the density remained constant over the study period [11].
Our results on roe deer females indicate that repeatability for parturition date in mammals can be as high as, or even higher, than that for laying date in birds [9,21]. Because giving birth early is rewarding in terms of fitness [8,22], females that give birth consistently late must be suffering constraints which prevent them from giving birth early, even in good years. Low among-year variation in parturition date suggests that roe deer may be unable to track current change in plant phenology using environmental cues [23]. The unique existence of delayed implantation in roe deer among large herbivores might be involved. Implantation occurs between late December and early January, when day length begins to increase, and is likely to be photoperiod-dependent [24]. Roe deer parturition date is probably related to implantation date rather than to the date of mating, which could explain the high degree of birth synchrony we observed.
Acknowledgements
All necessary permits were obtained for the described field studies. The protocol of capture of roe deer under the authority of the ONCFS have been approved by the Director of Food, Agriculture and Forest (prefectoral order 2009-14 from Paris). The land manager of both sites, the Office National des Forets (ONF) permitted the study of the populations (Partnership Convention ONCFS-ONF dated 2005-12-23).
We are grateful to the Office National de la Chasse et de la Faune Sauvage and to the many volunteers and colleagues who participated in the roe deer captures in winter and fawn searches in spring in all the study sites.
References
- 1.Bronson FH. 1989. Mammalian reproductive biology. Chicago, IL: University of Chicago Press [Google Scholar]
- 2.Rutberg AT. 1987. Adaptive hypotheses of birth synchrony in ruminants: an interspecific test. Am. Nat. 130, 692–710 10.1086/284739 (doi:10.1086/284739) [DOI] [Google Scholar]
- 3.Ims RA. 1990. On the adaptive value of reproductive synchrony as a predator-swamping strategy. Am. Nat. 136, 486–498 10.1086/285109 (doi:10.1086/285109) [DOI] [Google Scholar]
- 4.Price T, Kirkpatrick M, Arnold SJ. 1988. Directional selection and the evolution of breeding date in birds. Science 240, 798–799 10.1126/science.3363360 (doi:10.1126/science.3363360) [DOI] [PubMed] [Google Scholar]
- 5.Sydeman W, Eddy J. 1995. Repeatability in laying date and its relationship to individual quality for common murres. Condor 97, 1048–1052 10.2307/1369543 (doi:10.2307/1369543) [DOI] [Google Scholar]
- 6.Clutton-Brock TH, Guinness FE, Albon SD. 1982. Red deer: behavior and ecology of two sexes. Chicago, IL: University of Chicago Press [Google Scholar]
- 7.Both C, et al. 2004. Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc. R. Soc. B 271, 1657–1662 10.1098/rspb.2004.2770 (doi:10.1098/rspb.2004.2770) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feder C, Martin JGA, Festa-Bianchet M, Bérubé C, Jorgenson J. 2008. Never too late? Consequences of late birth date for mass and survival of bighorn lambs. Oecologia 156, 773–781 10.1007/s00442-008-1035-9 (doi:10.1007/s00442-008-1035-9) [DOI] [PubMed] [Google Scholar]
- 9.Korpimäki E. 1990. Low repeatability of laying date and clutch size in Tengmalm's owl: an adaptation to fluctuating food conditions. Ornis Scand. 21, 282–286 10.2307/3676393 (doi:10.2307/3676393) [DOI] [Google Scholar]
- 10.Nussey DH, Kruuk LE, Donald A, Fowlie M, Clutton-Brock TH. 2006. The rate of senescence in maternal performance increases with early-life fecundity in red deer. Ecol. Lett. 9, 1342–1350 10.1111/j.1461-0248.2006.00989.x (doi:10.1111/j.1461-0248.2006.00989.x) [DOI] [PubMed] [Google Scholar]
- 11.Kjellander P, Gaillard JM, Hewison A. 2006. Density-dependent responses of fawn cohort body mass in two contrasting roe deer populations. Oecologia 46, 521–530 10.1007/s00442-005-0188-z (doi:10.1007/s00442-005-0188-z) [DOI] [PubMed] [Google Scholar]
- 12.Swenson J, Angelstam P. 1993. Habitat separation by sympatric forest grouse in Fennoscandia in relation to boreal forest succession. Can. J. Zool. 71, 1303–1310 10.1139/z93-180 (doi:10.1139/z93-180) [DOI] [Google Scholar]
- 13.Linnell JDC, Andersen R. 1998. Timing and synchrony of birth in a hider species, the roe deer Capreolus capreolus. J. Zool. 244, 497–504 10.1111/j.1469-7998.1998.tb00055.x (doi:10.1111/j.1469-7998.1998.tb00055.x) [DOI] [Google Scholar]
- 14.Hewison A, Angibault JM, Cargnelutti B, Coulon A, Rames JL, Serrano E, Verheyden H, Morellet N. 2007. Using radio-tracking and direct observation to estimate roe deer Capreolus capreolus density in a fragmented landscape: a pilot study. Wildl. Biol. 13, 313–320 10.2981/0909-6396(2007)13[313:URADOT]2.0.CO;2 (doi:10.2981/0909-6396(2007)13[313:URADOT]2.0.CO;2) [DOI] [Google Scholar]
- 15.Gaillard JM, Delorme D, Jullien JM, Tatin D. 1993. Timing and synchrony of birth in roe deer. J. Mamm. 74, 738–744 10.2307/1382296 (doi:10.2307/1382296) [DOI] [Google Scholar]
- 16.Jullien J, Delorme D, Gaillard JM. 1992. Détermination de l’âge du faon de chevreuil (Capreolus capreolus) dans son premier mois de vie. Mammalia 56, 307–311 [Google Scholar]
- 17.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956 10.1111/j.1469-185X.2010.00141.x (doi:10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 18.Potti J. 1999. From mating to laying: genetic and environmental variation in mating dates and prelaying periods of female pied flycatchers Ficedula hypoleuca. Ann. Zool. Fennici 36, 187–194 [Google Scholar]
- 19.Wiggins D. 1991. Natural-selection on body size and laying date in the tree swallows. Evolution 45, 1169–1174 10.2307/2409724 (doi:10.2307/2409724) [DOI] [PubMed] [Google Scholar]
- 20.Moyes K, Nussey DH, Clements MN, Guinness FE, Morris A, Morris S, Pemberton JM, Kruuk LEB, Clutton-Brock TH. 2011. Advancing breeding phenology in response to environmental change in a wild red deer population. Glob. Change Biol. 17, 2455–2469 10.1111/j.1365-2486.2010.02382.x (doi:10.1111/j.1365-2486.2010.02382.x) [DOI] [Google Scholar]
- 21.Lessells C, Boag P. 1987. Unrepeatable repeatabilities a common mistake. Auk 104, 116–121 10.2307/4087240 (doi:10.2307/4087240) [DOI] [Google Scholar]
- 22.Lewis S, Wanless S, Elston DA, Schultz MD, Mackley E, Du Toit M, Underhill JG, Harris MP. 2006. Determinants of quality in a long-lived colonial species. J. Anim. Ecol. 75, 1304–1312 10.1111/j.1365-2656.2006.01152.x (doi:10.1111/j.1365-2656.2006.01152.x) [DOI] [PubMed] [Google Scholar]
- 23.Visser ME, Both C. 2005. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B. 272, 2561–2569 10.1098/rspb.2005.3356 (doi:10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sempéré A, Blanvillain C, Mauget R, Chemineau P. 1993. The role of the photoperiod in the sexual cycle in female roe deer (Capreolus capreolus). In Deer in China: biology and management (eds Ohtaishi N, Sheng HI.), pp. 364–371 Amsterdam, The Netherlands: Elsevier [Google Scholar]


