Abstract
Infection with parasites and pathogens is costly for hosts, causing loss of nutritional resources, reproductive potential, tissue integrity and even life. In response, animals have evolved behavioural and immunological strategies to avoid infection by pathogens and infestation by parasites. Scientists generally study these strategies in isolation from each other; however, since these defences entail costs, host individuals should benefit from balancing investment in these strategies, and understanding of infectious disease dynamics would benefit from studying the relationship between them. Here, we show that Carpodacus mexicanus (house finches) avoid sick individuals. Moreover, we show that individuals investing less in behavioural defences invest more in immune defences. Such variation has important implications for the dynamics of pathogen spread through populations, and ultimately the course of epidemics. A deeper understanding of individual- and population-level disease defence strategies will improve our ability to understand, model and predict the outcomes of pathogen spread in wildlife.
Keywords: disease avoidance, ecoimmunology, behaviour, innate immune function, house finch
1. Introduction
Infection by parasites and pathogens is costly for hosts, causing loss of nutritional resources, reproductive potential, tissue integrity and even life. As a consequence, animals have evolved behavioural and immunological strategies to avoid infection [1,2]. While immunological defences provide the primary defence against infection and disease development after pathogen exposure, behavioural defences play an important role in the avoidance of exposure to parasites and pathogens [2]. Most documented protective behaviours involve avoidance of unequivocally negative stimuli: parasites and pathogens, faeces, dead conspecifics. Targeted avoidance of diseased individuals should also be effective in minimizing the exposure to directly transmitted pathogens [3], and there is evidence that lobsters and bullfrog tadpoles use chemical cues to avoid contact with infected individuals [4,5]. However, we do not know whether birds or mammals use information about the current infection status of conspecifics to avoid pathogen exposure (but, see [6]).
Behavioural defences that minimize exposure to directly transmitted pathogens entail costs as a result of forgoing the benefits of social interactions (i.e. reproduction opportunities, improved foraging and decreased predation risk) [7]. Likewise, immune defences are costly in terms of energy expenditure, nutrient use, behavioural changes and risk of physical damage or death from an overactive or misdirected immune response [8,9].
This suggests that organisms should benefit from balancing investment in immunological and behavioural disease defences, because both entail costs but serve a common function [10]. If this is so, then individuals engaging in high-risk behaviours should compensate for increased infection risk with heightened immune function. In order for individuals to balance investment in behavioural and immunological disease defences in a social context, individuals must be able to recognize and avoid diseased conspecifics, and individuals that show heightened behavioural defences should show attenuated immune defences.
We know of no study that has shown that birds avoid individuals showing signs of disease. Therefore, we gave house finches a choice between spending time near an individual displaying sickness behaviours (the ‘sick’ partner) and an individual not displaying sickness behaviours (the ‘healthy’ partner). We show that focal individuals avoid sick partners, but that there is individual variation in avoidance behaviour. We then test whether immune function is inversely related to avoidance of sick partners; we show that individuals with weaker behavioural defences exhibit stronger immune defences and vice versa.
2. Material and methods
We conducted 26 trials to examine whether focal individuals preferred to associate with sick or healthy social partners. The experimental arena consisted of three adjacent cages, allowing individuals to interact without physical contact. In each trial, two partners (one sick and one healthy) were placed in individual cages on either side of a unique focal individual (figure 1); all individuals were male. One partner was challenged with 50 μl Complete Freund's Adjuvant (CFA, Sigma-Aldrich, St Louis, MO, USA) injected intra-abdominally (hereafter, the ‘sick’ partner), which induces a behavioural and physiological sickness response (see the electronic supplementary material, S1). The other partner was mock-challenged (handled, not injected; hereafter, the ‘healthy’ partner).
Figure 1.
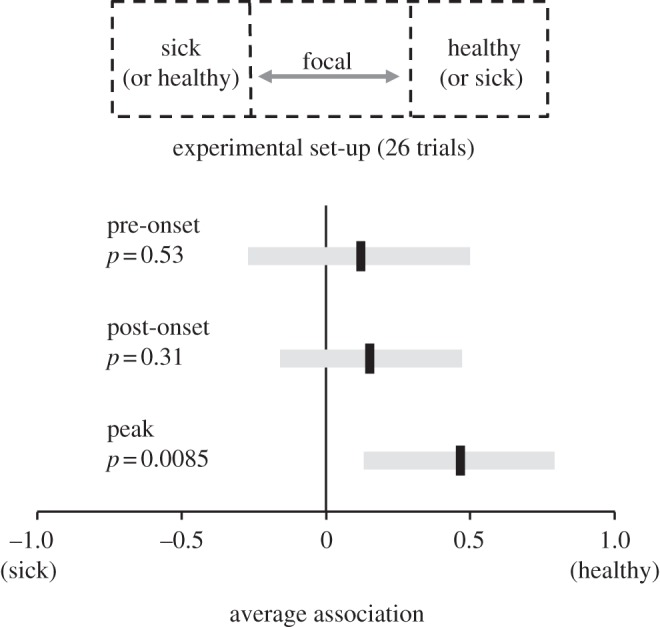
Change in focal individual social associations over time. Score of 1 indicates association with healthy partner, –1 indicates association with sick partner. Black lines indicate group means, with 95% CIs (grey bars), the horizontal grey line shows no preference. Inset shows experimental set-up.
Trials were video recorded, and the time focal individuals spent in proximity to each partner was scored. Association and avoidance (table 1) were scored for randomly chosen 2 min periods before onset, after onset and at peak sickness behaviour (0–30 min, 1–2 h, and 3–4 h post-challenge, respectively). The following day, focal individuals were CFA challenged to elicit an immune response; blood samples were collected 24 h later. Blood samples were collected from 14 individuals, using heparinized microcapillary tubes, within 20 min of entering the room where birds were housed. Plasma was isolated from blood samples by centrifugation and used to measure levels of PIT54 acute-phase protein and natural antibodies as previously described [11] (see the electronic supplementary material, S1); PIT54 binds free haemoglobin, depriving pathogens of iron and decreasing their ability to colonize host tissues [12] while natural antibodies are produced without prior pathogen exposure, serving as innate pathogen recognition receptors [13].
Table 1.
Behavioural definitions.
| behaviour | definition |
|---|---|
| association | focal individual spending more than two-thirds of a 2 min time interval near a particular social partner |
| avoider | focal individual spending two-thirds of 2 min time interval on side of cage farthest from sick social partner |
| quick avoider | focal individuals that avoided sick partners during onset of sickness behaviour |
| slow avoider | focal individuals that avoided sick social partners only at peak of sickness behaviour |
| non-avoider | focal individual that is not an avoider |
To test whether focal individuals had an innate side preference (compared with random chance), we used a two-sided χ2-test. To test whether focal individuals preferentially associated with healthy partners, we used a two-sided χ2 to compare observed preferences to those expected by chance. To test whether immune function varied with avoidance of sick individuals, we used Wilcoxon rank tests. To test whether strength of avoidance related to immune function, we used Wilcoxon rank tests to test for differences in immune function between ‘quick’ and ‘slow’ avoiders (table 1). For further details on analysis and sickness behaviour, see the electronic supplementary material, S1.
3. Results
Focal individuals did not exhibit an innate side preference (χ2 = 0.48, n = 19, d.f. = 17, p = 0.49, likelihood χ2-test). Nor did more focal individuals associate with healthy social partners before the onset of sickness behaviour (χ2 = 0.398, n = 23, d.f. = 21, p = 0.53, likelihood χ2-test) or after the initial onset of sickness behaviour (χ2 = 1.01, n = 16, d.f. = 14, p = 0.31, likelihood χ2-test) than expected by chance (figure 1). However, at peak sickness behaviour, significantly more individuals associated with healthy partners than expected by chance (χ2 = 6.92, n = 22, d.f. = 20, p = 0.0085, likelihood χ2-test); 77 per cent showing a preference associated with the healthy partner. Individuals showing no preference were excluded from the preference tests, resulting in sample size variation between time points.
To assess immunity, we compared two functional endpoints in avoiders and non-avoiders: the level of circulating natural antibody against a foreign red blood cell (indicating constitutive investment in immune surveillance [13]), and the level of a major acute-phase protein (PIT54, indicating investment in immune defence resulting from a challenge [12]) (table 2 and figure 2). There was no difference in either measure in focal individuals that had not avoided sick partners when compared with those that had prior to the onset of sickness behaviour. However, after the onset of sickness behaviour avoiders had lower natural antibody and PIT54 levels than non-avoiders. The difference was not significant for natural antibodies at the peak of avoidance; however, this may be due to small sample size (during peak sickness behaviour only two individuals in which immune function was measured were non-avoiders) rather than to lack of a biological difference (agglutination scores were 25% lower on average in non-avoiders than avoiders). Furthermore, quick avoiders exhibited a trend towards lower natural antibody levels than slow avoiders (χ2 = 3.41, n = 14, d.f. = 12, p = 0.065, Wilcoxon rank test).
Table 2.
Immune function and avoidance of sick individuals.
| time point | immune parameter | n | test statistica,b | d.f. | p-value | direction of difference |
|---|---|---|---|---|---|---|
| pre-onset | PIT54 | 14 | 1.53 | 12 | 0.13 | no difference |
| natural antibodies | 14 | 1.32 | 12 | 0.19 | no difference | |
| post-onset | PIT54 | 14 | 1.91 | 12 | 0.41 | no difference |
| natural antibodies | 14 | 4.71 | 12 | 0.030 | avoiders < non-avoiders | |
| peak | PIT54 | 14 | 4.80 | 12 | 0.029 | avoiders < non-avoiders |
| natural antibodies | 14 | 2.55 | 12 | 0.11 | avoiders < non-avoiders |
aZ reported for pre-onset time point, χ2 reported for post-onset and peak time points.
bTo avoid perceived bias, results of one-way Wilcoxon's rank test for differences ‘pre-onset’ are: χ2 = 2.55, p = 0.11 (PIT54); χ2 = 1.91, p = 0.17 (natural antibodies).
Figure 2.
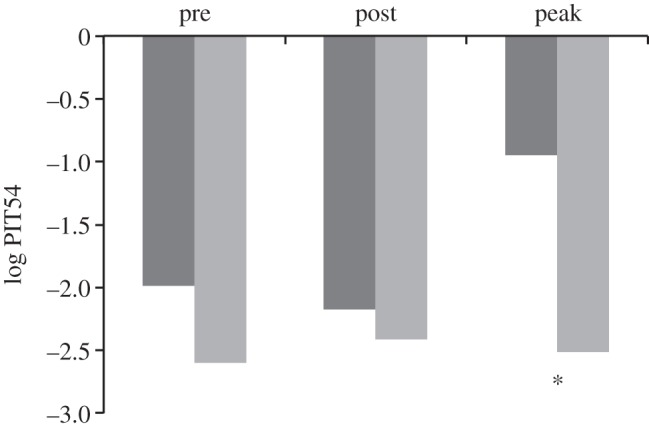
Average PIT54 levels in avoiders (light grey bars) and non-avoiders (dark grey bars); asterisk indicates p-value < 0.05.
4. Discussion
We show for the first time that an avian species avoids individuals exhibiting sickness behaviours, that there is individual variation in avoidance, and that those individuals that exhibit avoidance behaviours invest less in certain aspects of innate immune function compared with individuals that do not. Individuals avoiding sick conspecifics invested less in constitutive (natural antibodies) and inducible (PIT54) aspects of innate immune function, potentially decreasing their ability to avoid pathogen colonization upon exposure.
Sickness behaviour is likely to provide an especially salient cue for avoidance of infectious individuals, allowing susceptible individuals to avoid a wide range of pathogens. Furthermore, behaviours, including disease defence behaviours, are among the most flexible characteristics of organisms [14]. In contrast, while not inflexible, the functioning of the immune system appears intricately linked with various aspects of host ecology, including life-history strategies (i.e. the balance of investment in current reproduction versus self-maintenance) and species sociality [15–17]. The relatively plastic and broad-spectrum nature of behavioural defences suggests that they should be particularly useful during the emergence of novel pathogens. However, we expect the relative importance of disease defence strategies to be dynamic and to vary with host–pathogen coevolution. For example, the evolution of pathogen strategies to minimize behavioural changes in their host during infection (e.g. by minimizing the inflammatory response) could temporarily decrease the effectiveness of certain behavioural disease defences. While we expect a similar relationship between immunological and disease defence strategies in a range of taxa, the importance of various strategies should vary by species. For example, behavioural defence strategies in non-social species may focus on minimization of pathogen exposure during habitat exploration rather than variation in sociality.
Our work suggests that the relationship between behaviour and immune function is more complex than previously thought, and demands closer attention to understand the ramifications of individual behavioural and immunological variation for wildlife disease ecology. Future work should investigate the mechanisms underlying the relationship between pathogen defence strategies. Steroid hormones could underlie this relationship as they influence both behaviour and immune function [1] (for further discussion of potential mechanisms, see [18]). Interestingly, the relationship between steroid hormone levels and immune function is not consistent between or even within species [19]; if steroid hormones are the mechanism linking behavioural and immune disease defences, this relationship may help explain these inconsistencies. Future work should further investigate the relationship between behavioural and immunological disease defences and individual variation in the use of these strategies. We expect the relative costs and benefits of these strategies to vary with individual characteristics, such as age, sex and life-history stage. For example, male house finches preferentially forage near sick conspecifics as they initiate fewer energetically costly aggressive interactions; females, who experience no difference in aggression rates, show no such preference [6]. Thus, different segments of the population are expected to favour different balances of disease defence strategies; in general, individuals that have more to gain from social interactions should favour investment in immune defences. Under these circumstances, variation in disease defence strategies between population segments (variation that can alter transmission and recovery rates) should have important implications for which segments of the population are most affected by emerging pathogens, for the dynamics of pathogen spread through populations, and ultimately for the course of epidemics. The incorporation of this variation into models of wildlife disease dynamics will provide more accurate models of disease emergence and spread, deepening our understanding of disease dynamics in wild populations and improving our own ability to cope with threats from endemic and emerging pathogens.
Acknowledgements
Procedures approved by UC Davis IACUC, protocol 12866. Funding was provided by the American Ornithologists Union, Sigma Xi Research Society and National Science Foundation (Graduate Research Fellowship to M.Z.; grant to T.P.H (IOS-0744705)).
References
- 1.Hawley DM, Etienne RS, Ezenwa VO, Jolles AE. 2011. Does animal behavior underlie covariation between hosts’ exposure to infectious agents and susceptibility to infection? Implications for disease dynamics. Integr. Comp. Biol. 51, 528–539 10.1093/icb/icr062 (doi:10.1093/icb/icr062) [DOI] [PubMed] [Google Scholar]
- 2.Hart BL. 1990. Behavioral adaptations to pathogens and parasites: five strategies. Neurosci. Biobehav. Rev. 14, 273–294 10.1016/S0149-7634(05)80038-7 (doi:10.1016/S0149-7634(05)80038-7) [DOI] [PubMed] [Google Scholar]
- 3.Loehle C. 1995. Social barriers to pathogen transmission in wild animal populations. Ecology 76, 326–335 10.2307/1941192 (doi:10.2307/1941192) [DOI] [Google Scholar]
- 4.Kiesecker JM, Skelly DK, Beard KH, Preisser E. 1999. Behavioral reduction of infection risk. Proc. Natl Acad. Sci. USA 96, 9165–9168 10.1073/pnas.96.16.9165 (doi:10.1073/pnas.96.16.9165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behringer DC, Butler MJ, Shields JD. 2006. Avoidance of disease by social lobsters. Nature 441, 421. 10.1038/441421a (doi:10.1038/441421a) [DOI] [PubMed] [Google Scholar]
- 6.Bouwman KM, Hawley DM. 2010. Sickness behaviour acting as an evolutionary trap? Male house finches preferentially feed near diseased conspecifics. Biol. Lett. 6, 462–465 10.1098/rsbl.2010.0020 (doi:10.1098/rsbl.2010.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander RD. 1974. The evolution of social behavior. Annu. Rev. Ecol. Syst. 5, 325–383 10.1146/annurev.es.05.110174.001545 (doi:10.1146/annurev.es.05.110174.001545) [DOI] [Google Scholar]
- 8.Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G. 2003. Assessing the cost of mounting an immune response. Am. Nat. 161, 367–379 10.1086/346134 (doi:10.1086/346134) [DOI] [PubMed] [Google Scholar]
- 9.Klasing KC, Leshchinsky TV. 1999. Functions, costs, and benefits of the immune system during development and growth. Ostrich 69, 2817–2832 [Google Scholar]
- 10.Zylberberg M. 2011. Disease defense strategies: linking behavior, immune function and disease ecology in Galapagos finches and house finches. Davis, CA: University of California [Google Scholar]
- 11.Zylberberg M, Lee KA, Klasing KC, Wikelski M. 2012. Increasing avian pox prevalence varies by species, and with immune function, in Galápagos finches. Biol. Conserv. 153, 72–79 10.1016/j.biocon.2012.04.022 (doi:10.1016/j.biocon.2012.04.022) [DOI] [PubMed] [Google Scholar]
- 12.Millet S, Bennett J, Lee KA, Hau M, Klasing KC. 2007. Quantifying and comparing constitutive immunity across avian species. Dev. Comp. Immunol. 31, 188–201 10.1016/j.dci.2006.05.013 (doi:10.1016/j.dci.2006.05.013) [DOI] [PubMed] [Google Scholar]
- 13.Matson KD, Ricklefs RE, Klasing KC. 2005. A hemolysis–hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev. Comp. Immunol. 29, 275. 10.1016/j.dci.2004.07.006 (doi:10.1016/j.dci.2004.07.006) [DOI] [PubMed] [Google Scholar]
- 14.West-Eberhard MJ. 1989. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20, 249–278 10.1146/annurev.es.20.110189.001341 (doi:10.1146/annurev.es.20.110189.001341) [DOI] [Google Scholar]
- 15.Lee KA, Wikelski M, Robinson WD, Robinson TR, Klasing KC. 2008. Constitutive immune defences correlate with life-history variables in tropical birds. J. Anim. Ecol. 77, 356–363 10.1111/j.1365-2656.2007.01347.x (doi:10.1111/j.1365-2656.2007.01347.x) [DOI] [PubMed] [Google Scholar]
- 16.Ricklefs RE. 1992. Embryonic development period and the prevalence of avian blood parasites. Proc. Natl Acad. Sci. USA 89, 4722–4725 10.1073/pnas.89.10.4722 (doi:10.1073/pnas.89.10.4722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kundu S, Faulkes CG. 2004. Patterns of MHC selection in African mole-rats, family Bathyergidae: the effects of sociality and habitat. Proc. R. Soc. Lond. B 271, 273–278 10.1098/rspb.2003.2584 (doi:10.1098/rspb.2003.2584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin LB, Liebl AL, Trotter JH, Richards CL, McCoy K, McCoy MW. 2011. Integrator networks: illuminating the black box linking genotype and phenotype. Integr. Comp. Biol. 51, 514–552 10.1093/icb/icr049 (doi:10.1093/icb/icr049) [DOI] [PubMed] [Google Scholar]
- 19.Roberts ML, Buchanan KL, Evans MR. 2004. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim. Behav. 68, 227–239 10.1016/j.anbehav.2004.05.001 (doi:10.1016/j.anbehav.2004.05.001) [DOI] [Google Scholar]


