Abstract
Late Pliocene climate changes have long been implicated in environmental changes and mammalian evolution in Africa, but high-resolution examinations of the fossil and climatic records have been hampered by poor sampling. By using fossils from the well-dated Shungura Formation (lower Omo Valley, northern Turkana Basin, southern Ethiopia), we investigate palaeodietary changes in one bovid and in one suid lineage from 3 to 2 Ma using stable isotope analysis of tooth enamel. Results show unexpectedly large increases in C4 dietary intake around 2.8 Ma in both the bovid and suid, and possibly in a previously reported hippopotamid species. Enamel δ13C values after 2.8 Ma in the bovid (Tragelaphus nakuae) are higher than recorded for any living tragelaphin, and are not expected given its conservative dental morphology. A shift towards increased C4 feeding at 2.8 Ma in the suid (Kolpochoerus limnetes) appears similarly decoupled from a well-documented record of dental evolution indicating gradual and progressive dietary change. The fact that two, perhaps three, disparate Pliocene herbivore lineages exhibit similar, and contemporaneous changes in dietary behaviour suggests a common environmental driver. Local and regional pollen, palaeosol and faunal records indicate increased aridity but no corresponding large and rapid expansion of grasslands in the Turkana Basin at 2.8 Ma. Our results provide new evidence supporting ecological change in the eastern African record around 2.8 Ma, but raise questions about the resolution at which different ecological proxies may be comparable, the correlation of vegetation and faunal change, and the interpretation of low δ13C values in the African Pliocene.
Keywords: stable isotope analysis, palaeoecology, diet, Africa, Pliocene
1. Introduction
Four decades of research have addressed the nature and magnitude of environmental change on faunal evolution in Africa in the late Pliocene and early Pleistocene. A major impediment to investigation has been a lack of well-dated fossiliferous and sedimentary sequences that continuously sample the 3–2 Ma period and that could be precisely compared with global palaeoclimate proxy records. Under the framework of the Omo Group Research Expedition [1], we here explore palaeoecological change in Plio-Pleistocene eastern Africa using dietary investigation of well-studied, phyletically evolving herbivore species lineages across the 3–2 Ma period.
The suid Kolpochoerus limnetes and the bovid Tragelaphus nakuae (with its immediate ancestor Tragelaphus rastafari) are phyletically evolving species lineages that are well represented and well studied in Plio-Pleistocene deposits of eastern Africa [2–5]. We used precisely dated fossils of these lineages from the Shungura Formation in the lower Omo Valley (northern Turkana Basin, southern Ethiopia), which provides the single most continuous and best-dated fossiliferous sequence of the 3–2 Ma time period anywhere in Africa. We measured tooth enamel stable carbon and oxygen isotope ratios in order to determine whether any significant dietary changes had occurred that might be correlated with local, regional or global palaeoenvironmental signals. We also refer to enamel isotope values for two specimens of the hippopotamid aff. Hippopotamus protamphibius reported by Souron et al. [6].
2. Material and methods
Twenty-four bovid and 60 suid specimens were sampled at the Authority for Research and Conservation of Cultural Heritage in Addis Ababa. Around 20 mg of fossil enamel from each specimen was treated with buffered acetic acid and reacted with anhydrous H3PO4, with the resulting CO2 analysed using a Finnigan MAT 252 isotopic ratio mass spectrometer. Stable carbon and oxygen isotope ratios are reported as δ-values relative to the Vienna Pee Dee Belemnite (VPDB) standard using permil (‰) notation, where δ13C = (Rsample/Rstandard − 1) × 1000; Rsample and Rstandard are the 13C/12C ratios (or 18O/16O ratios in the case of δ18O) in the sample and in the standard, respectively, and the δ13C (or δ18O) value of VPDB is defined as 0‰. Full details on materials and methods can be found in the electronic supplementary material.
3. Results
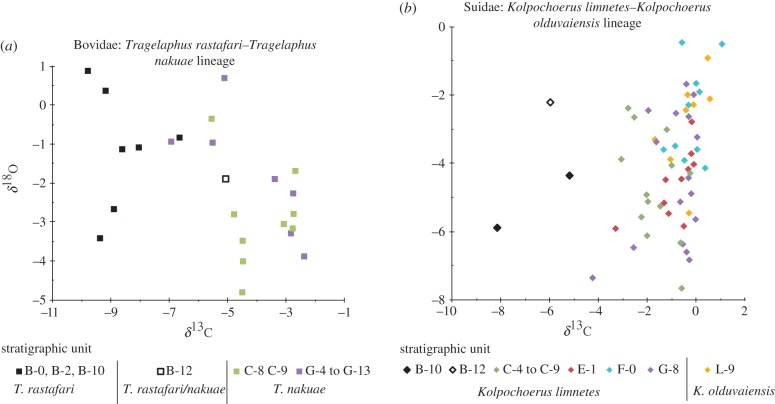
Enamel δ13C values in the Shungura bovid, suid and hippopotamid studied became more enriched by an average +4‰ between units B-10 and C-4/C-5, or sometime between 2.97 Ma and just under 2.74 Ma (figures 1 and 2) [7]. δ18O values, while showing some changes in the mean and variances among the different stratigraphic levels (especially in the suids), do not show the type of directional change observed in the δ13C data. Full results can be found in the electronic supplementary material.
Figure 1.
Enamel δ13C and δ18O values for the fossil (a) bovid and (b) suid specimens sampled. These are differentiated by stratigraphic level within the Shungura Formation. Specimens from units C-4 and younger (i.e. <∼2.8 Ma) are significantly enriched in δ13C relative to those from units B-10 and older.
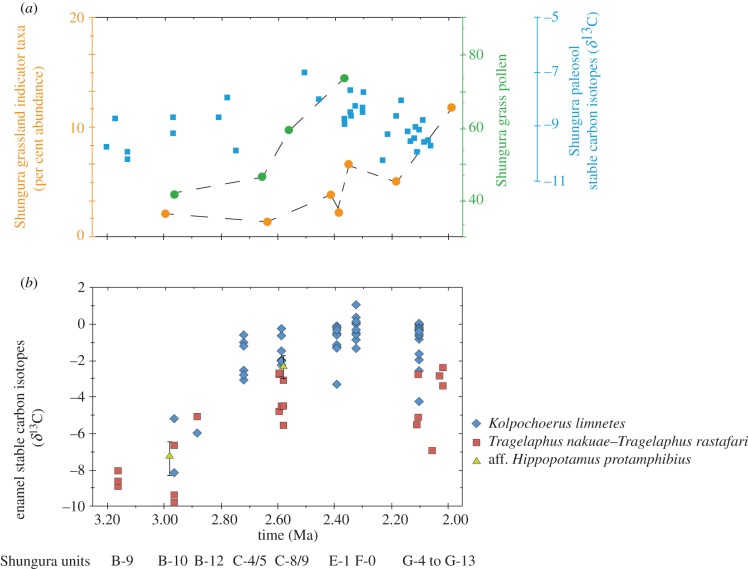
Figure 2.
Local palaeoenvironmental indicators in the Shungura Formation from 3.2 to 2.0 Ma. (a) Palaeosol stable carbon isotopes [8], grass pollen percentages [9] and proportions of mammals indicative of secondary grassland habitats [10]. (b) Fossil enamel stable carbon isotopes show a large increase in enamel δ13C around 2.8 Ma indicating palaeodietary change. The δ13C increase, however, is not correlated with the expansion of grassland habitats and loss of tree cover, or with proportional increases in grazing mammals. Different ecological proxies, however, may be sampling different spatial and temporal scales.
4. Discussion
We record a pronounced increase in enamel δ13C around 2.8 Ma in both the bovid and suid lineages studied (figure 1). Although only two Shungura hippopotamid specimens were sampled by Souron et al. [6], the timing, direction and magnitude of the change in enamel δ13C match those of the bovid and suid. Such large (greater than +4‰) increases in δ13C indicate significantly increased dietary intake of C4 plant resources in a relatively short time frame, or not more than (and perhaps much less than) 300 000 years (figure 2). In the bovid lineage, the δ13C shift is contemporaneous with a recently recognized taxonomic boundary between chronospecies T. nakuae (younger than around 2.8 Ma) and T. rastafari (older than 2.8 Ma) [3]. However, taxonomic differentiation was made mainly on the basis of braincase morphology, with no obvious indications of any dentognathic changes that might suggest a change in dietary behaviour. Enamel δ13C values in T. nakuae are also higher than recorded among living tragelaphin species [11], but the significance of this is difficult to interpret without related dental wear data (see the electronic supplementary material, section discussion).
Findings for mixed feeding or grazing behaviours in the suid and hippopotamid are not unexpected given the dietary preferences of these animals today, as well as their dental morphologies, which permit an abrasive and grass-based diet. What is surprising is the rapidity of the dietary shift implied by the δ13C data. Dental evolution in K. limnetes, such as other suid lineages of the Plio-Pleistocene, is progressive, with gradual lengthening of the third molars, premolar row reduction and increases in hypsodonty and body size indicating long-term adaptation to an increasingly abrasive diet [2,5]. Morphological changes around 2.8 Ma are not especially significant compared with those observed at other times in this lineage's evolution, and our data suggest a major dietary shift at 2.8 Ma that is decoupled from the long and gradual evolutionary progression indicated by the dentognathic morphological data. Like the suid, the aff. Hippopotamus protamphibius lineage shows gradual elongation of its third molars over time [12], and a large dietary shift in this lineage, if confirmed by further sampling, would be similarly significant.
Although similar to previously reported δ13C increases in late Miocene herbivores [13], exact dietary interpretations for our data and any resulting inferences about the balance of grasslands to woodlands are confounded by evidence that C3 grasses may have made up a larger proportion of lowland African vegetation biomass during the Pliocene than they do today [14]. In the absence of further data (e.g. dental wear, phytoliths), it is not possible to rule out the presence and exploitation of C3 grasses in the lower Omo Valley prior to 2.8 Ma. The δ13C increase may therefore represent either a significant replacement of trees by grasses (with impacts on herbivore dietary strategies), or a less remarkable replacement of pre-existing C3 grasses by C4 grasses (with limited or no change in dietary behaviour). Broader faunal isotopic sampling along with studies of dental wear should be able to further test these scenarios.
That a pronounced increase in dietary δ13C took place contemporaneously in two, possibly three, ecologically distinct herbivore lineages opens the possibility of a common environmental driver. Numerous faunal, botanical and palaeoclimate proxy records have identified 2.8–2.5 Ma as a period of drying in Africa [15–17]. In the Shungura mammalian fossil record, 2.8 Ma coincides with a period of elevated ecological and taxonomic turnover that reflects increased aridity and a reduction in humid-forested environments [10,18]. However, mammalian, pollen and palaeosol isotope records also indicate that habitats in the lower Omo Valley during this time remained relatively mesic with no indications of significant grassland habitat expansion until 2.6 Ma or later (figure 2) [8–10]. Some of these differences might be explained by factors affecting the spatial and temporal scaling of the different proxy methods. For example, the stability of the Omo watershed, which supported mesic riparian environments somewhat buffered against regional aridification that more strongly affected areas peripheral to the river itself [8,10,19], or selective feeding by herbivores that might target rare vegetation types or areas peripheral to the river axis. At face value, however, the raw data suggest that vegetation, faunal and evolutionary responses between 3 and 2.5 Ma in the lower Omo Valley may have been decoupled on the order of 100 ka or more.
Similar analyses should be extended to other African fossil faunas in order to determine whether the δ13C increase we record was part of a broader pulse of late Pliocene ecological change or was restricted to the lower Omo Valley. Regional comparisons are currently limited, but the available palaeosol and enamel stable isotope records [8,20–23] from the nearby Nachukui and Koobi Fora formations, while indicating net increases in aridity and C4 biomass through time, show no evidence for a large and step-like shift around 2.8 Ma. This suggests that the dietary changes we observe may have been restricted to the lower Omo Valley, providing another example of how these faunas, located within the same depositional basin, differed ecologically.
Neither the rate nor the magnitude of the dietary changes we record (especially in Tragelaphus) were to be expected from existing lines of contextual evidence, including dental morphology, faunal turnover, and the palaeosol and palaeobotanical proxy records. Our findings do support previous indications of net change towards increasingly arid habitats in the late Pliocene of eastern Africa, in concert with global and regional climatic records, with the additional indication that major evolutionary responses such as dietary changes, C4 grassland expansion, faunal reorganization and functional adaptation may not have been tightly coupled, even within a single depositional basin.
Acknowledgements
We thank B. Steinhilber and C. Wissing for technical assistance at the University of Tübingen; C. Blondel, A. Novello and T. White for discussions; N. Levin, J. Wynn and T. Cerling for palaeosol data, and the editor and three anonymous referees for helping improve an earlier draft of the manuscript. Funding was provided by the Agence National de la Recherche (ANR-09-BLAN-0238 to M. Brunet), the French Ministry of Foreign and European Affairs (Sous-Direction de l'Archéologie/French Embassy in Ethiopia/CFEE), the Fyssen Foundation, a National Science Foundation International Research Fellowship (grant no. 0852975 to F.B.), and a Leibniz-DAAD Research Fellowship (to F.B.). This study was authorized by the Authority for Research, Conservation and Cultural Heritage (Ethiopian Ministry of Culture and Tourism).
References
- 1.Boisserie JR, Guy F, Delagnes A, Hlusko LJ, Bibi F, Beyene Y, Guillemot C. 2008. New palaeoanthropological research in the Plio-Pleistocene Omo Group, lower Omo Valley, SNNPR, Ethiopia. C R Palevol. 7, 429–439 10.1016/j.crpv.2008.07.010 (doi:10.1016/j.crpv.2008.07.010) [DOI] [Google Scholar]
- 2.Harris JM, White TD. 1979. Evolution of the Plio-Pleistocene African Suidae, p. 128 Philadelphia, PA: American Philosophical Society [Google Scholar]
- 3.Bibi F. 2011. Tragelaphus nakuae: evolutionary change, biochronology, and turnover in the African Plio-Pleistocene. Zool. J. Linn. Soc. 162, 699–711 10.1111/j.1096-3642.2010.00691.x (doi:10.1111/j.1096-3642.2010.00691.x) [DOI] [Google Scholar]
- 4.Gentry AW. 1985. The Bovidae of the Omo Group deposits, Ethiopia (French and American collections). In Les faunes Plio-Pléistocènes de la basse Vallée de l'Omo (Ethiopie); I: Perissodactyles-Artiodactyles (Bovidae) (eds Coppens Y, Howell FC.), pp. 119–191 Paris, France: CNRS [Google Scholar]
- 5.Cooke HBS. 2007. Stratigraphic variation in Suidae from the Shungura Formation and some coeval deposits. In Hominin environments in the east African Pliocene: an assessment of the faunal evidence (eds Bobe R, Alemseged Z, Behrensmeyer AK.), pp. 107–127 Dordrecht, The Netherlands: Springer [Google Scholar]
- 6.Souron A, Balasse M, Boisserie JR. 2012. Intra-tooth isotopic profiles of canines from extant Hippopotamus amphibius and late Pliocene hippopotamids (Shungura Formation, Ethiopia): insights into the seasonality of diet and climate. Palaeogeogr. Palaeoclimatol. Palaeoecol. 342–343, 97–110 10.1016/j.palaeo.2012.05.007 (doi:10.1016/j.palaeo.2012.05.007) [DOI] [Google Scholar]
- 7.McDougall I, Brown FH, Vasconcelos PM, Cohen BE, Thiede DS, Buchanan MJ. 2012. New single crystal 40Ar/39Ar ages improve time scale for deposition of the Omo Group, Omo-Turkana Basin, East Africa. J. Geol. Soc. 169, 213–226 10.1144/0016-76492010-188 (doi:10.1144/0016-76492010-188) [DOI] [Google Scholar]
- 8.Levin NE, Brown FH, Behrensmeyer AK, Bobe R, Cerling TE. 2011. Paleosol carbonates from the Omo Group: isotopic records of local and regional environmental change in East Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 307, 75–89 10.1016/j.palaeo.2011.04.026 (doi:10.1016/j.palaeo.2011.04.026) [DOI] [Google Scholar]
- 9.Bonnefille R, Dechamps R. 1983. Data on fossil flora. In The Omo Group: Archives of the International Omo Research Expedition (ed. de Heinzelin J.), pp. 191–207 Tervuren, Belgium: Musée Royal de l'Afrique Centrale [Google Scholar]
- 10.Bobe R, Behrensmeyer AK. 2004. The expansion of grassland ecosystems in Africa in relation to mammalian evolution and the origin of the genus Homo. Palaeogeogr. Palaeoclimatol. Palaeoecol. 207, 399–420 10.1016/S0031-0182(04)00049-5 (doi:10.1016/S0031-0182(04)00049-5) [DOI] [Google Scholar]
- 11.Cerling TE, Harris JM, Passey BH. 2003. Diets of East African Bovidae based on stable isotope analysis. J. Mammal 84, 456–470 (doi:10.1644/1545-1542(2003)084<0456:DOEABB>2.0.CO;2) [DOI] [Google Scholar]
- 12.Gèze R. 1980. Les Hippopotamidae (Mammalia, Artiodactyla) du Plio-Pléistocène de l'Ethiopie. Paris, France: Université Pierre et Marie Curie [Google Scholar]
- 13.Uno KT, Cerling TE, Harris JM, Kunimatsu Y, Leakey MG, Nakatsukasa M, Nakaya H. 2011. Late Miocene to Pliocene carbon isotope record of differential diet change among East African herbivores. Proc. Natl Acad. Sci. USA 108, 6509–6514 10.1073/pnas.1018435108 (doi:10.1073/pnas.1018435108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossouw L, Scott L. 2011. Phytoliths and pollen, the microscopic plant remains in Pliocene volcanic sediments around Laetoli, Tanzania. In Paleontology and geology of Laetoli: human evolution in context volume 1: geology, geochronology, paleoecology, and paleoenvironment (ed. Harrison T.), pp. 201–215 New York, NY: Springer [Google Scholar]
- 15.deMenocal PB. 1995. Plio-Pleistocene African climate. Science 270, 53–59 10.1126/science.270.5233.53 (doi:10.1126/science.270.5233.53) [DOI] [PubMed] [Google Scholar]
- 16.Bonnefille R. 2010. Cenozoic vegetation, climate changes and hominid evolution in tropical Africa. Glob. Planet Change 72, 390–411 10.1016/j.gloplacha.2010.01.015 (doi:10.1016/j.gloplacha.2010.01.015) [DOI] [Google Scholar]
- 17.Vrba ES. 1995. The fossil record of African antelopes (Mammalia, Bovidae) in relation to human evolution and paleoclimate. In Paleoclimate and evolution, with emphasis on human origins (eds Vrba ES, Denton GH, Partridge TC, Burckle LH.), pp. 385–424 New Haven, CT: Yale University Press [Google Scholar]
- 18.Hernández Fernández M, Vrba ES. 2006. Plio-Pleistocene climatic change in the Turkana Basin (East Africa): evidence from large mammal faunas. J. Hum. Evol. 50, 595. 10.1016/j.jhevol.2005.11.004 (doi:10.1016/j.jhevol.2005.11.004) [DOI] [PubMed] [Google Scholar]
- 19.Vrba ES. 1988. Late Pliocene climatic events and hominid evolution. In The evolutionary history of the robust Australopithecines (ed. Grine FE.), pp. 2405–426 New York, NY: Aldine de Gruyter [Google Scholar]
- 20.Harris J, Cerling T. 2002. Dietary adaptations of extant and Neogene African suids. J. Zool. 256, 45–54 10.1017/S0952836902000067 (doi:10.1017/S0952836902000067) [DOI] [Google Scholar]
- 21.Harris J, Cerling T, Leakey M, Passey B. 2008. Stable isotope ecology of fossil hippopotamids from the Lake Turkana Basin of East Africa. J. Zool. 275, 323–331 10.1111/j.1469-7998.2008.00444.x (doi:10.1111/j.1469-7998.2008.00444.x) [DOI] [Google Scholar]
- 22.Cerling TE, Levin NE, Passey BH. 2011. Stable isotope ecology in the Omo-Turkana basin. Evol. Anthropol. 20, 228–237 10.1002/evan.20326 (doi:10.1002/evan.20326) [DOI] [PubMed] [Google Scholar]
- 23.Cerling TE, et al. 2011. Woody cover and hominin environments in the past 6 million years. Nature 476, 51–56 10.1038/nature10306 (doi:10.1038/nature10306) [DOI] [PubMed] [Google Scholar]