Abstract
The refugial speciation model, or ‘species pump’, is widely accepted in the context of tropical biogeography and has been advocated as an explanation for present species distributions in tropical Western and Central Africa. In order to test this hypothesis, a phylogeny of the cryptic arachnid order Ricinulei, based on four nuclear and mitochondrial DNA markers, was inferred. This ancient clade of litter-dwelling arthropods, endemic to the primary forests of Western and Central Africa and the Neotropics, might provide insights into the mode and tempo of evolution in Africa. Twenty-six African ricinuleid specimens were sampled from eight countries spanning the distribution of Ricinulei on the continent, and analysed together with Neotropical samples plus other arachnid outgroups. The phylogenetic and molecular dating results suggest that Ricinulei diversified in association with the fragmentation of Gondwana. The early diversification of Ricinoides in Western and Central Africa around 88 (±33) Ma fits old palaeogeographical events better than recent climatic fluctuations. Unlike most recent molecular studies, these results agree with fossil evidence, suggesting that refugia may have acted as ‘museums’ conserving ancient diversity rather than as engines generating diversity during successive episodes of climatic fluctuation in Africa.
Keywords: Africa, refugia, marine incursion, Arachnida, Ricinulei, biogeography
1. Introduction
The existence of forest refugia is broadly accepted in tropical biogeography, because there is ample evidence for forest fragmentation linked to past climatic change in temperate and tropical regions [1,2]. Two different hypotheses have been advanced regarding the role of refugia in shaping the current biodiversity of tropical Africa. The refugial speciation model [3], or ‘species pump’, invokes genetic differentiation between allopatric populations, fragmented and trapped in refugia by the expansion of savannah during Quaternary glacial maxima [4]. This hypothesis predicts the origin of most rainforest species to be relatively recent [5]. On the other hand, past climatic changes could have depleted rather than augmented biodiversity, suggesting that refugia might have acted as ‘museums’ for ancient lineages, and divergence between sister species might predate climatic fluctuations [6]. Whereas fossil evidence for plants has long suggested an ancient diversity followed by cycles of extinction owing to climatic fluctuation [7], most molecular studies (see the electronic supplementary material, figure S1) portray climatic fluctuation as an agent of speciation and diversification.
Because of their great age [8], extreme endemism [9,10] and low vagility, Ricinulei Thorell, 1876 represent an excellent model for studying the biogeography of Western and Central Africa. Commonly known as ‘hooded tick spiders’ or ‘tick beetles’, these small (less than 11 mm) predatory arthropods are among the most obscure and cryptic of the arachnid orders [11]. A mere 72 extant ricinuleid species are currently described [12], and grouped in three genera: Ricinoides Ewing, 1929 from tropical Western and Central Africa, and the Neotropical Cryptocellus Westwood, 1874 and Pseudocellus Platnick, 1980. African ricinuleids are restricted to the moist soil and litter habitats of rainforests [13], whereas Neotropical ricinuleids have also been collected in caves [12].
The first molecular phylogeny of Ricinulei is presented here, with the aims of providing a temporal framework for the diversification of Ricinoides in Western and Central Africa, and investigating the effects of forest refugia on the generation and maintenance of tropical biodiversity.
2. Material and methods
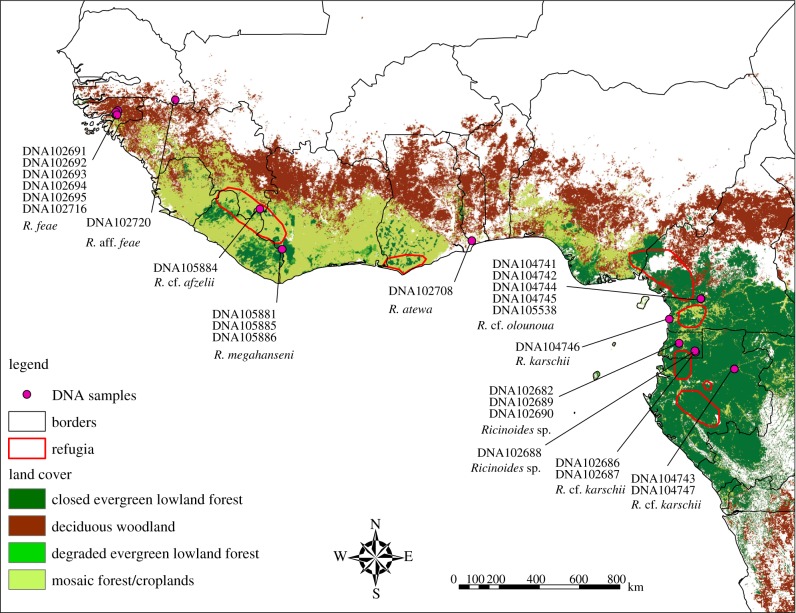
Eleven Ricinoides species, recorded from 14 countries (including first record of Ricinulei from Senegal, reported here), are currently recognized in Africa [13,14]. Specimens were collected by sifting leaf litter, Winkler extraction and actively searching in appropriate habitats (especially under logs in forested areas) throughout their known distributional range. Voucher specimens used for DNA isolation have been deposited in the following institutions: American Museum of Natural History (AMNH), New York, NY, USA; Musée Royal de l'Afrique Central (MRAC), Tervuren, Belgium; Museum of Comparative Zoology (MCZ), Harvard University, Cambridge, MA, USA (table 1). The ingroup taxon sample comprises 26 specimens, representing at least seven ricinuleid species, from eight African countries (figure 1). Ten Neotropical ricinuleids and 20 representatives of seven other chelicerate orders were included as outgroup taxa (table 1).
Table 1.
Tissue sample numbers, provenance data, GenBank accession numbers and voucher repositories for Ricinulei and outgroup taxa from which DNA sequence data were generated for the present study. COI, cytochrome c oxidase subunit I.
| MCZ voucher | repository | latitude | longitude | ID | country | 18S rRNA | 28S rRNA | 12S rRNA | COI |
|---|---|---|---|---|---|---|---|---|---|
| DNA102704 | MCZ | −3.68333 | −70.25000 | Cryptocellus peckorum Platnick and Shadab, 1977 | Colombia | JX951342 | JX951355 | — | — |
| DNA102711 | MCZ | −4.12000 | −69.92222 | C. peckorum Platnick and Shadab, 1977 | Colombia | JX951320 | JX951357 | JX951380 | — |
| DNA102712 | MCZ | −4.04472 | −69.98972 | C. peckorum Platnick and Shadab, 1977 | Colombia | JX951321 | JX951358 | JX951381 | — |
| DNA102713 | MCZ | −4.12028 | −69.97528 | C. peckorum Platnick and Shadab, 1977 | Colombia | JX951322 | JX951359 | JX951382 | JX951406 |
| DNA102701 | MCZ | 9.67167 | −83.02500 | Cryptocellus sp. | Costa Rica | JX951317 | JX951354 | JX951377 | JX951404 |
| DNA102710 | MCZ | −4.37889 | −69.99028 | Cryptocellus sp. | Colombia | JX951319 | JX951356 | JX951379 | — |
| DNA103735 | MCZ-IZ-80067 | 8.40667 | −83.32833 | Cryptocellus sp. | Costa Rica | JX951327 | JX951364 | JX951387 | JX951410 |
| DNA103733 | MCZ | 15.11440 | −89.68047 | Pseudocellus sp. | Guatemala | JX951325 | JX951362 | JX951385 | — |
| DNA103734 | MCZ | 15.58333 | −86.66833 | Pseudocellus sp. | Honduras | JX951326 | JX951363 | JX951386 | JX951409 |
| DNA103736 | MCZ-IZ-79799 | 15.71566 | −92.93817 | Pseudocellus sp. | Mexico | JX951328 | JX951365 | JX951388 | JX951411 |
| DNA102708 | AMNH | 6.25039 | 1.04039 | Ricinoides atewa Naskrecki, 2008 | Ghana | JX951318 | — | JX951378 | JX951405 |
| DNA105884 | MRAC 225977 | 7.66667 | −8.43333 | Ricinoides cf. afzelii | Guinea | JX951338–9 | — | — | — |
| DNA104741 | MCZ | 3.64538 | 11.29033 | Ricinoides cf. olounoua | Cameroon | JX951329 | — | JX951389 | JX951412 |
| DNA104742 | MCZ | 3.64447 | 11.29107 | Ricinoides cf. olounoua | Cameroon | JX951330 | — | JX951390 | JX951413 |
| DNA104744 | MCZ | 3.66153 | 11.30262 | Ricinoides cf. olounoua | Cameroon | JX951332 | JX951367 | JX951391 | JX951415 |
| DNA104745 | MCZ | 3.66195 | 11.30025 | Ricinoides cf. olounoua | Cameroon | JX951333 | — | JX951392 | JX951416 |
| DNA105538 | MCZ | 3.64513 | 11.29078 | Ricinoides cf. olounoua | Cameroon | JX951336 | — | JX951395 | JX951419 |
| DNA102691 | AMNH LP4658 | 12.08156 | −14.80103 | Ricinoides feae (Hansen, 1921) | Guinea-Bissau | JX951312 | JX951349 | JX951373 | JX951399 |
| DNA102692 | AMNH LP4660 | 12.08156 | −14.80103 | R. feae (Hansen, 1921) | Guinea-Bissau | JX951313 | JX951350 | JX951374 | JX951400 |
| DNA102693 | AMNH LP4661 | 12.08156 | −14.80103 | R. feae (Hansen, 1921) | Guinea-Bissau | JX951314 | JX951351 | JX951375 | JX951401 |
| DNA102694 | AMNH LP4662 | 11.88442 | −14.83569 | R. feae (Hansen, 1921) | Guinea-Bissau | JX951315 | JX951352 | — | JX951402 |
| DNA102695 | AMNH LP4664 | 12.00250 | −14.89053 | R. feae (Hansen, 1921) | Guinea-Bissau | JX951316 | JX951353 | JX951376 | JX951403 |
| DNA102716 | AMNH LP4663 | 11.88442 | −14.83569 | R. feae (Hansen, 1921) | Guinea-Bissau | JX951323 | JX951360 | JX951383 | JX951407 |
| DNA102720 | AMNH LP4659 | 12.55294 | −12.22761 | Ricinoides aff. feae | Senegal | JX951324 | JX951361 | JX951384 | JX951408 |
| DNA104746 | MCZ | 2.74108 | 9.88180 | Ricinoides karschii (Hansen & Sørensen, 1904) | Cameroon | JX951334 | — | JX951393 | JX951417 |
| DNA102686 | MCZ | 1.25278 | 11.05278 | Ricinoides cf. karschii | Equatorial Guinea | JX951306 | JX951344 | JX951370 | JX951397 |
| DNA102687 | MCZ | 1.25278 | 11.05278 | Ricinoides cf. karschii | Equatorial Guinea | JX951307 | JX951345 | JX951371 | JX951398 |
| DNA104743 | MCZ | 0.50639 | 12.79422 | Ricinoides cf. karschii | Gabon | JX951331 | JX951366 | — | JX951414 |
| DNA104747 | MCZ | 0.50448 | 12.79525 | Ricinoides cf. karschii | Gabon | JX951335 | JX951368 | JX951394 | JX951418 |
| DNA105881 | MRAC 230596 | 5.86000 | −7.45000 | Ricinoides megahanseni Legg, 1982 | Ivory Coast | JX951337 | — | — | — |
| DNA105885 | MRAC 230597 | 5.86000 | −7.45000 | R. megahanseni Legg, 1982 | Ivory Coast | JX951340 | — | — | — |
| DNA105886 | MRAC 230598 | 5.86000 | −7.45000 | R. megahanseni Legg, 1982 | Ivory Coast | JX951341 | — | — | — |
| DNA102682 | MCZ | 1.65806 | 10.31139 | Ricinoides sp. | Equatorial Guinea | JX951305 | JX951343 | JX951369 | JX951396 |
| DNA102688 | MCZ | 1.31583 | 11.02944 | Ricinoides sp. | Equatorial Guinea | JX951308 | JX951346 | — | — |
| DNA102689 | MCZ | 1.65806 | 10.31139 | Ricinoides sp. | Equatorial Guinea | JX951309 | JX951347 | — | — |
| DNA102690 | MCZ | 1.65833 | 10.31556 | Ricinoides sp. | Equatorial Guinea | JX951310–1 | JX951348 | JX951372 | — |
| Outgroups | |||||||||
| Acari | Ornithodoros moubata (Murray, 1877) | L76355 | — | NC_004357 | NC_004357 | ||||
| Acari | Haemaphysalis flava Neumann, 1897 | — | — | NC_005292 | NC_005292 | ||||
| Acari | Ixodes hexagonus Leach, 1815 | — | — | NC_002010 | NC_002010 | ||||
| Acari | Rhipicephalus sanguineus (Latreille, 1806) | L76342 | — | NC_002074 | NC_002074 | ||||
| Acari | Carios capensis (Neumann, 1901) | — | — | NC_005291 | NC_005291 | ||||
| Amblypygi | Phrynus sp. | — | — | NC_010775 | NC_010775 | ||||
| Araneae | Calisoga longitarsis (Simon, 1891) | — | — | NC_010780 | NC_010780 | ||||
| Araneae | Haplopelma schmidti von Wirth, 1991 | AY425722.1 | — | NC_005925 | NC_005925 | ||||
| Araneae | Habronattus oregonensis (Peckham & Peckham, 1888) | — | — | NC_005942 | NC_005942 | ||||
| Araneae | Nephila clavata L. Koch, 1878 | — | — | NC_008063 | NC_008063 | ||||
| Araneae | Hypochilus thorelli Marx, 1888 | — | AF303505 | NC_010777 | NC_010777 | ||||
| Araneae | Heptathela hangzhouensis Chen, Zhang & Zhu 1981 | AY425719.1 | — | NC_005924 | NC_005924 | ||||
| Scorpiones | Buthus occitanus (Amoreux, 1789) | — | — | NC_010765 | NC_010765 | ||||
| Scorpiones | Centruroides limpidus (Karsch, 1879) | — | — | NC_006896 | NC_006896 | ||||
| Scorpiones | Mesobuthus martensii (Karsch, 1879) | FJ948787.1 | FJ948787.1 | NC_009738 | NC_009738 | ||||
| Scorpiones | Uroctonus mordax Thorell, 1876 | — | — | NC_010782 | NC_010782 | ||||
| Solifugae | Eremobates palpisetulosus (Fichter, 1941) | — | — | NC_010779 | NC_010779 | ||||
| Solifugae | Nothopuga sp. | — | — | EU024482 | EU024482 | ||||
| Uropygi | Mastigoproctus giganteus (Lucas, 1835) | AF005446 | AY859587.1 | NC_010430 | NC_010430 | ||||
| Opiliones | Phalangium opilio Linnaeus, 1758 | AF124937 | — | NC_010766 | NC_010766 | ||||
| Xiphosura | Limulus polyphemus (Linnaeus, 1758) | L81949 | AF212167 | NC_003057 | NC_003057 | ||||
Figure 1.
Map of Western and Central Africa showing localities of the African Ricinulei samples from which DNA was sequenced for the present study. Land cover map after Mayaux et al. [15]. Hypothetical refugia at the Last Glacial Maximum after Maley [1].
DNA extraction and sequencing were conducted using protocols optimized for other arachnids [16,17] for the following genes: 12S rRNA (12S), cytochrome c oxidase subunit I, 18S rRNA (18S) and 28S rRNA (28S). Sequences were aligned using Muscle v. 3.7 [18]. Divergence time estimation was performed in a Bayesian framework using Beast v. 1.5.4 [19], with an uncorrelated lognormal model of rate evolution [20]. This approach integrates the uncertainty of calibration points and topology, considered important because the phylogenetic placement of Ricinulei within Arachnida remains uncertain [21].
Beast does not use a coupled Markov chain Monte Carlo (MCMC), potentially making it more prone to becoming trapped in local optima. A maximum-likelihood analysis was, therefore, performed using RAxML HPC v. 7.2.7 alpha [22] with a GTR + gamma model applied to each gene. The optimal tree was then used as an initial starting tree for Beast. Arachnid diversification was constrained based on the oldest known fossils [23], using an exponential distribution prior and setting the standard deviation to obtain a 95 per cent range between 428 (oldest known fossil arachnid, a scorpion) and 445 Ma (oldest known fossil chelicerate, a horse-shoe crab). The Beast analysis was run for 60 million generations with sampling every 1000 generations.
3. Results and discussion
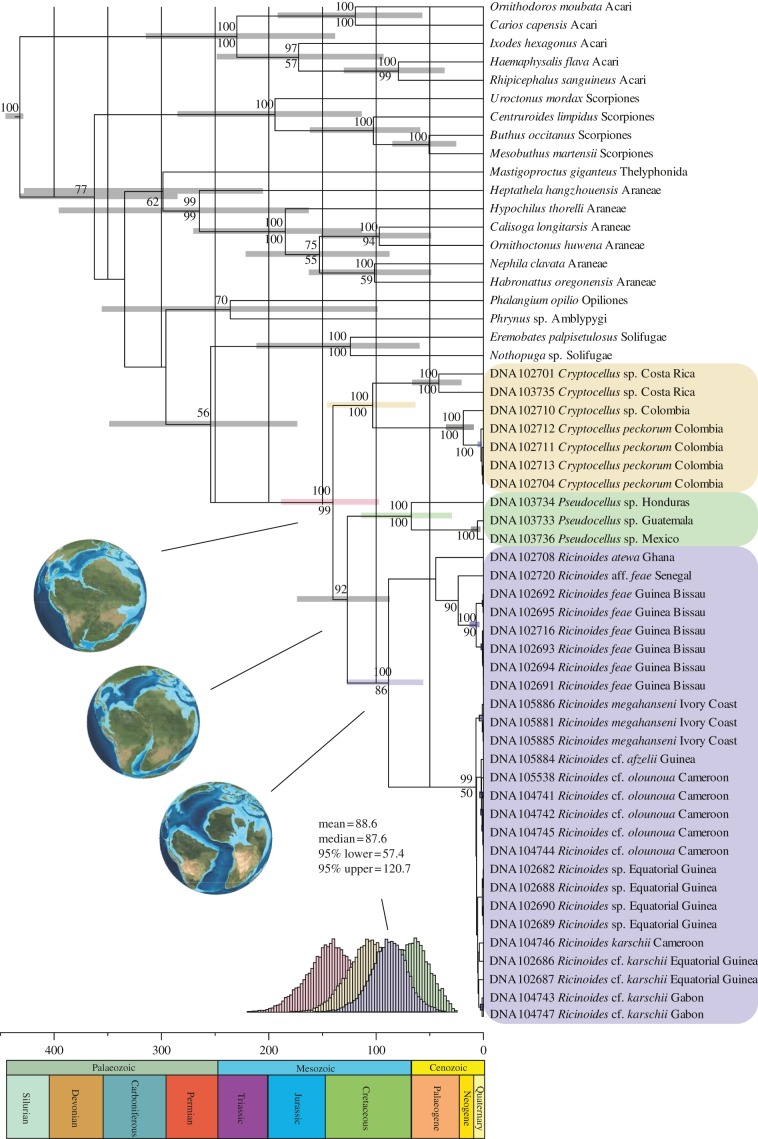
The results recover the monophyly of Ricinulei and its component genera, Cryptocellus, Pseudocellus and Ricinoides, with high support (figure 2). The origin of the group (the divergence from its sister group) is dated to around 250 Ma. The oldest known fossil ricinuleid (not considered part of the Neoricinulei crown group), dated to 319 Ma [24], is concordant with the 95% confidence interval on the tree, and independently corroborates the analytical results and biogeographic conclusions. Ricinoides is sister to one of the two Neotropical genera, Pseudocellus, suggesting that the entire diversification of Ricinulei predates the fragmentation of Gondwana. This biogeographic interpretation, previously proposed based on morphological evidence [25], is corroborated by the molecular dating. Based on the results presented here, the diversification of Ricinoides in Western and Central Africa occurred in the Late Cretaceous, around 88 ± 33 Ma. Specimens from Guinea and Ivory Coast group with those from Cameroon, Equatorial Guinea and Gabon, probably an artefact of missing data (lack of mitochondrial gene sequences for the former; table 1). Samples from the Ivory Coast group with the remaining West African samples in the maximum-likelihood analysis, as expected based on their similar morphology and geographical proximity. Among the West African samples, the divergence between Ricinoides atewa Naskrecki, 2008 and other species from Senegal and Guinea-Bissau is dated to around 44 Ma.
Figure 2.
Maximum credibility tree for African Ricinulei and outgroup taxa, obtained by Bayesian inference in Beast [19]. 95% Confidence intervals for ages are represented by bars at nodes. Clade posterior probabilities above 50% are indicated above nodes and maximum-likelihood bootstrap frequencies below nodes. Posterior distributions for nodes of interest are also depicted below the tree. Palaeogeographic maps from Ron Blakey, NAU Geology at http://jan.ucc.nau.edu/~rcb7/.
Tropical forest biodiversity is lower in Africa than in South America and Southeast Asia [26]. This difference has been attributed to extinctions caused by forest fragmentation and potentially even the complete disappearance of the forest during past periods of severe aridification [5]. This is exemplified by the low diversity of another ancient group of soil animals, velvet worms (Onychophora Grube, 1853), with a single species of Peripatidae Evans, 1901 in tropical Africa (Gabon and Cameroon), when compared with four species in Southeast Asia and 68 in the Neotropics [27]. The relatively low diversity of the tropical African forests is consistent with the majority of molecular evidence, which suggests that rainforest endemics are mostly recent (Late Miocene–Pleistocene) and diversified according to the refugial speciation model [4].
The results presented provide molecular evidence for an endemic African rainforest taxon, Ricinoides, the origin of which can be traced to the fragmentation of Gondwana, and confirms evidence from other ground-dwelling arthropods [28] for an early diversification in the Late Cretaceous, around 90 Ma, in Western and Central Africa. This period corresponds to a time of diversification among angiosperms and associated reduction in gymnosperm diversity, documented in the Cenomanian stage of the Late Cretaceous [29]. Even if the Mesozoic rainforests were structurally and compositionally different from those in present-day tropical Africa, stratified forests may have been present since the Late Cretaceous, based on the presence of large seeds and fruits in the fossil record [30]. The major divergence observed among species in Western and Central Africa around 90 Ma could be the result of vicariance caused by successive marine incursions (figure 2) that commenced in the Late Cretaceous [31]. Refugia may also have played a role in allopatric speciation on a smaller scale for more recent species in the area of present-day Cameroon and Gabon.
The results presented suggest further that ancient diversifications exist in Western and Central Africa and the biodiversity of this region may have been greater during Late Cretaceous to Palaeocene times. Climate change may have depleted diversity after the separation of Africa and South America, with subsequent stable refugia acting as ‘museums’ for ancient lineages. In this respect, the data are largely congruent with the fossil record, which suggests that entire lineages of Neotropical palms were present in Africa until at least the Late Oligocene (27–28 Ma) [7]. Such models [5] may have received little support from molecular studies until now (but see [6]), because few phylogeographic studies have been conducted on old lineages with high endemism and low population density (see electronic supplementary material). Furthermore, extinction rates are difficult to infer from molecular studies [32] in the absence of fossils from outside the putative refugia. Ricinoides thus represents one of the oldest endemic African genera for which the origin, early diversification and subsequent survival in Miocene forest refugia has been studied and tested phylogenetically.
Acknowledgements
J.M. was supported by ‘Investissement d'Avenir’ grants managed by Agence Nationale de la Recherche (CEBA, ref. ANR-10-LABX-0025; TULIP, ref. ANR-10-LABX-41). Fieldwork was supported by Putnam Expedition grants from the Museum of Comparative Zoology and by the American Museum of Natural History. This work was supported by U.S. National Science Foundation grant nos DEB-0328644, DEB-1144417, DEB-1144492 and EAR-0228699 and from a Selective Excellence grant from The George Washington University. Several samples were made available by J. Longino's LLAMA project (NSF DEB-064015). G. Chassan-Jouolieu, J. Huff, N. Legrand, J. Mavoungou, C. Prieto and V. Vignoli assisted with fieldwork. P. Naskrecki and R. Jocqué provided additional specimens. IRET Gabon provided logistical support. D. Dimitrov and V. Nicolas commented on the manuscript.
References
- 1.Maley J. 1989. Late Quaternary climatic changes in the African rain forest: forest refugia and the major role of sea surface temperature variations. In Paleoclimatology and paleometeorology: modern and past patterns of global atmospheric transport, vol. 282 (eds Leinen M, Sarnthein M.), pp. 585–616 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 2.Hamilton AC, Taylor D. 1991. History of climate and forests in tropical Africa during the last 8 million years. Clim. Change 19, 65–78 10.1007/BF00142215 (doi:10.1007/BF00142215) [DOI] [Google Scholar]
- 3.Haffer J. 1969. Speciation in Amazonian forest birds. Science 165, 131–137 10.1126/science.165.3889.131 (doi:10.1126/science.165.3889.131) [DOI] [PubMed] [Google Scholar]
- 4.Plana V. 2004. Mechanisms and tempo of evolution in the African Guineo–Congolian rainforest. Phil. Trans. R. Soc. Lond. B 359, 1585–1594 10.1098/rstb.2004.1535 (doi:10.1098/rstb.2004.1535). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton AC. 1976. The significance of patterns of distribution shown by forest plants and animals in tropical Africa for the reconstruction of upper Pleistocene palaeoenvironments: a review. In Palaeoecology of Africa & of the surrounding islands & Antarctica, vol. 9 (ed. Van Zinderen Bakker EM.), pp. 63–97 Cape Town, South Africa: A.A. Balkema [Google Scholar]
- 6.Evans BJ, Kelley DC, Tinsley RC, Melnick DJ, Cannatella DC. 2004. A mitochondrial DNA phylogeny of African clawed frogs: phylogeography and implications for polyploid evolution. Mol. Phylogenet. Evol. 33, 197–213 10.1016/j.ympev.2004.04.018 (doi:10.1016/j.ympev.2004.04.018) [DOI] [PubMed] [Google Scholar]
- 7.Pan AD, Jacobs BF, Dransfield J, Baker WJ. 2006. The fossil history of palms (Arecaceae) in Africa and new records from the Late Oligocene (28–27 Mya) of north-western Ethiopia. Bot. J. Linn. Soc. 151, 69–81 10.1111/j.1095-8339.2006.00523.x (doi:10.1111/j.1095-8339.2006.00523.x) [DOI] [Google Scholar]
- 8.Selden PA. 1992. Revision of the fossil ricinuleids. Trans. R. Soc. Edinb. Earth Sci. 83, 595–634 10.1017/S0263593300003333 (doi:10.1017/S0263593300003333) [DOI] [Google Scholar]
- 9.Pollock J. 1967. Notes on the biology of Ricinulei (Arachnida). J. West Afr. Sci. Assoc. 12, 19–22 [Google Scholar]
- 10.Harvey MS. 2002. The neglected cousins: what do we know about the smaller arachnid orders? J. Arachnol. 30, 357–372 10.1636/0161-8202(2002)030[0357:TNCWDW]2.0.CO;2 (doi:10.1636/0161-8202(2002)030[0357:TNCWDW]2.0.CO;2) [DOI] [Google Scholar]
- 11.Cooke JAL. 1967. Observations on the biology of Ricinulei (Arachnida) with descriptions of two new species of Cryptocellus. J. Zool. Lond. 151, 31–42 10.1111/j.1469-7998.1967.tb02864.x (doi:10.1111/j.1469-7998.1967.tb02864.x) [DOI] [Google Scholar]
- 12.Valdez-Mondragón A, Francke OF. 2011. Four new species of the genus Pseudocellus (Arachnida: Ricinulei: Ricinoididae) from Mexico. J. Arachnol. 39, 365–377 10.1636/Ha11-02.1 (doi:10.1636/Ha11-02.1) [DOI] [PubMed] [Google Scholar]
- 13.Naskrecki P. 2008. A new ricinuleid of the genus Ricinoides Ewing (Arachnida, Ricinulei) from Ghana. Zootaxa 1698, 57–64 [Google Scholar]
- 14.Penney D, Marusik Y, Wheater CP, Langan AM. 2009. First Gambian Ricinulei (Arachnida: Ricinoididae): northernmost African record for the order. Zootaxa 2021, 66–68 [Google Scholar]
- 15.Mayaux P, Bartholomé E, Fritz S, Belward A. 2004. A new land-cover map of Africa for the year 2000. J. Biogeogr. 31, 861–877 10.1111/j.1365-2699.2004.01073.x (doi:10.1111/j.1365-2699.2004.01073.x) [DOI] [Google Scholar]
- 16.Murienne J, Harvey MS, Giribet G. 2008. First molecular phylogeny of the major clades of Pseudoscorpiones (Arthropoda: Chelicerata). Mol. Phylogenet. Evol. 49, 170–184 10.1016/j.ympev.2008.06.002 (doi:10.1016/j.ympev.2008.06.002) [DOI] [PubMed] [Google Scholar]
- 17.Murienne J, Karaman I, Giribet G. 2010. Explosive evolution of an ancient group of Cyphophthalmi (Arachnida: Opiliones) in the Balkan Peninsula. J. Biogeogr. 37, 90–102 10.1111/j.1365-2699.2009.02180.x (doi:10.1111/j.1365-2699.2009.02180.x) [DOI] [Google Scholar]
- 18.Edgar RC. 2004. MUSCLE: a multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 10.1093/nar/gkh340 (doi:10.1093/nar/gkh340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond AJ, Rambaut A. 2007. Beast: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214. 10.1186/1471-2148-7-214 (doi:10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. 2006. Relaxed phylogenetics and dating with confidence. PLOS Biol. 4, e88. 10.1371/journal.pbio.0040088 (doi:10.1371/journal.pbio.0040088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunlop JA, Kamenz C, Talarico G. 2009. A fossil trigonotarbid arachnid with a ricinuleid-like pedipalpal claw. Zoomorphology 128, 305–313 10.1007/s00435-009-0090-z (doi:10.1007/s00435-009-0090-z) [DOI] [Google Scholar]
- 22.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 10.1093/bioinformatics/btl446 (doi:10.1093/bioinformatics/btl446) [DOI] [PubMed] [Google Scholar]
- 23.Dunlop JA. 2010. Geological history and phylogeny of Chelicerata. Arthropod Struct. Dev. 39, 124–142 10.1016/j.asd.2010.01.003 (doi:10.1016/j.asd.2010.01.003) [DOI] [PubMed] [Google Scholar]
- 24.Brauckmann C. 1987. Neue Arachniden (Ricinuleida, Trigonotarbida) aus dem Namurium B vin Hagen-Vorhalle (Ober-Karbon; West-Deutschland). Dort. Beit. Land. 21, 97–109 [Google Scholar]
- 25.Platnick NI. 1980. On the phylogeny of Ricinulei. In 8 Internationaler Arachnologen-Kongreß, Abgehalten an der Universitat fur Bodenkultur Wien 7–21 Juli (ed. Gruber J.), pp. 349–353 Vienna, Austria: H. Egermann [Google Scholar]
- 26.Richards PW. 1973. Africa, the odd man out. In Tropical forest ecosystems in Africa and South America: a comparative review (eds Meggers BJ, Ayensu ES, Duckworth WD.) pp. 21–26 Washington, DC: Smithsonian Institution Press [Google Scholar]
- 27.de Sena Oliveira I, Read VM, Mayer G. 2012. A world checklist of Onychophora (velvet worms), with notes on nomenclature and status of names. ZooKeys 211, 1–70 10.3897/zookeys.211.3463 (doi:10.3897/zookeys.211.3463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giribet G, Vogt L, Pérez-González A, Sharma P, Kury AB. 2010. A multilocus approach to harvestman (Arachnida: Opiliones) phylogeny with emphasis on biogeography and the systematics of Laniatores. Cladistics 26, 408–437 10.1111/j.1096-0031.2009.00296.x (doi:10.1111/j.1096-0031.2009.00296.x) [DOI] [PubMed] [Google Scholar]
- 29.Jacobs BF. 2004. Palaeobotanical studies from tropical Africa: relevance to the evolution of forest, woodland and savannah biomes. Phil. Trans. R. Soc. Lond. B 359, 1573–1583 10.1098/rstb.2004.1533 (doi:10.1098/rstb.2004.1533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morley RJ. 2000. Origin and evolution of tropical rain forests. Chichester, UK: John Wiley & Sons [Google Scholar]
- 31.Burke K, Gunnell Y. 2008. The African erosion surface: a continental-scale synthesis of geomorphology, tectonics, and environmental change over the past 180 million years. (Memoir 201) Boulder, CO: The Geological Society of America [Google Scholar]
- 32.Rabosky DL. 2010. Extinction rates should not be estimated from molecular phylogenies. Evolution 64, 1816–1824 (doi:10.1111/j.1558–5646.2009.00926.x) [DOI] [PubMed] [Google Scholar]