Abstract
The role of predators in food webs extends beyond their ability to kill and consume prey. Such trait-mediated effects occur when signals of the predator influence the behaviour of other animals. Because all spiders are silk-producing carnivores, we hypothesized that silk alone would signal other arthropods and enhance non-lethal effects of spiders. We quantified the herbivory inflicted by two beetle species on green bean plants (Phaseolus vulgaris) in the presence of silkworm silk and spider silk along with no silk controls. Single leaflets were treated and enclosed with herbivores in the laboratory and field. Another set of leaflets were treated and left to experience natural herbivory in the field. Entire plants in the field were treated with silk and enclosed with herbivores or left exposed to herbivory. In all cases, the lowest levels of herbivory occurred with spider silk treatments and, in general, silkworm silk produced intermediate levels of leaf damage. These results suggest that silk may be a mechanism for the trait-mediated impacts of spiders and that it might contribute to integrated pest management programmes.
Keywords: silk, spider, herbivory, trait-mediated effects, food web, non-consumptive effects
1. Introduction
Trophic cascade theory suggests that the action of top predators will be transmitted to lower trophic levels and ultimately affect primary productivity in ways that are dependent on the tightness of the interactions between various levels and their resources [1–3]. These ideas originally emerged from studies in aquatic systems [1,2], and the prevalence of trophic cascades in terrestrial systems was initially questioned [4]. However, early in the twenty-first century, two synthetic studies uncovered substantial evidence for terrestrial trophic cascades [5,6] and revealed particularly strong effects of arthropod predators in agroecosystems [6]. Nevertheless, difficulty in establishing the strength and direction of trophic linkages persists, in part, because generalist predators elicit a range of non-consumptive effects on their potential prey through shifts in morphology, life history, habitat use and behaviour [7–9].
Spiders are among the most common generalist predators in terrestrial environments and their ability to have non-consumptive effects on other arthropods has been demonstrated repeatedly [9–11]. Intriguingly, these non-lethal impacts do not strictly affect potential prey as spiders can also elicit responses from novel or improbable victims [12,13]. Spiders are famous for their use of silk, and even those species that do not build webs leave silk behind as they occupy an area [14]. Therefore, we hypothesize that silk may serve as a general warning signal to other arthropods and, if so, would serve to broaden the trophic footprint of spider assemblages. If the responders are other predators then the effects of silk could attenuate the trophic cascade, but if herbivores consume less plant biomass in the presence of silk then the top-down effects of spiders would be enhanced; this would be of particular interest to agriculturalists. We tested the hypothesis that silk alone could impact trophic linkages by reducing herbivore damage to plants in both laboratory and field experiments.
2. Material and methods
(a). Study system
We selected species in order to minimize the possibility that any observed relationships were driven by specific predator–prey coevolution. Japanese beetles (Popillia japonica) and Mexican bean beetles (Epilachna varivestis) are herbivores that have expanded their range through eastern North America within the past half-century [15,16]. Adults of both species are susceptible to predation by web spiders [12] and, conveniently, adults of both pests chew the leaves of bush-style snap beans (Phaseolus vulgaris) in ways that are easy to quantify. We tested for effects of silk on the damage caused by these herbivores using silkworm silk from cocoons of Bombyx mori, as well as dragline silk, freshly drawn from the spider Tetragnatha elongata, a native species that is common in riparian forests but is not present in our agricultural fields.
(b). Plants
Experiments were conducted on plants from a tilled field, 50 × 150 m (0.75 ha) in size, planted with P. vulgaris at Miami University's Ecology Research Center (Oxford, OH, USA; 39°31′ N, 84°43′ W). No insecticides or herbicides were used after planting. Experimental plants were selected by generating random numbers on 1 m grids imposed on the field. Leaflets for experiments were selected from those located 10–20 cm down from the crown of the plant that had damage of less than 0.1 cm2. The area of the leaflets was quantified before and after experiments, and the difference in size (before–after) was used as a measure of herbivore damage (see the electronic supplementary material).
Treated leaflets received five strands of silk evenly spaced along the longitudinal axis. Spider silk was freshly drawn from subadult and adult female T. elongata that had been held in laboratory culture for at least one week on a diet of fruit flies (Drosophila spp.) and crickets (Acheta domesticus). Silkworm silk was teased out of cocoons of degummed silk. Untreated leaflets were handled but not exposed to spiders or silk. In some cases, droplets of distilled water were used to help fibres to adhere to leaves initially.
(c). Single leaflet experiments
In laboratory trials, the petiole of each leaflet was wrapped in moist cotton, placed in a 20 cm Petri dish with a single beetle, and housed in an environmental chamber at 25°C on a 13 L : 11 D cycle. Treatments included one Japanese beetle or Mexican bean beetle crossed with no silk, spider silk or silkworm silk. After 24 h, the leaflet damage was assessed. Using the same treatments, we enclosed individual leaflets, still attached to the plant, in 20 × 20 cm bags made of 0.1 mm mesh shade cloth. After 24 h, the leaflets were collected and the herbivory quantified. We also treated single leaflets of randomly selected plants in the field with spider silk, silkworm silk or nothing and left them unenclosed to experience natural herbivory. Silk treatments were repeated after 3 days and leaflets were collected after 6 days.
(d). Whole plant experiments
In the field, we treated all leaflets on plants with silkworm or spider silk or we handled all leaflets of control plants. Six leaflets were randomly selected and marked with a plastic tie attached to the petiole at the time of treatment. Plants were sequestered with two beetles of the same species inside cylindrical enclosures (60 cm diameter, 100 cm tall) made of 0.2 cm nylon screen that was nested 4–10 cm into the soil around the plant. After 3 days, we collected the designated leaflets and determined the herbivory. Another set of plants was treated but left unenclosed to experience natural herbivory. We treated them again after 3 days and collected the designated leaflets after 6 days to determine the damage.
(e). Statistical analysis
Damage estimates for both single leaflet and whole plant enclosure experiments were compared in two-way ANOVAs with beetle species and silk treatment as factors. None of the interactions between beetle species and silk treatment was significant (all p > 0.4) so they were not included in the reported analyses. Damage estimates for plants in open field experiments where we did not know the identity of the herbivore(s) were compared in one-way ANOVAs. Tukey's post hoc tests were used to judge individual differences among treatments (overall p < 0.05). Statistical analyses were conducted using JMP (v. 9.0.2, SAS Institute, Inc.).
3. Results
(a). Single leaflet experiments
Silk reduced damage to leaflets in all experiments (figure 1 and table 1). Both spider silk and silkworm silk eliminated the damage caused by Japanese beetles and Mexican bean beetles to single leaflets in the laboratory (figure 1a and table 1). In fact, the leaflets treated with silk grew under laboratory conditions, which is why negative damage appears in figure 1a. When single leaflets were treated and enclosed with a beetle in the field, spider silk reduced damage by nearly 40 per cent over controls (figure 1b and table 1). Damage to leaflets treated with silkworm silk was intermediate to, but not significantly different from, other treatments (figure 1b and table 1). Although not significant at the 0.05 level, a similar pattern was observed when unenclosed leaflets were treated and left to experience natural herbivory (figure 1c and table 1).
Figure 1.
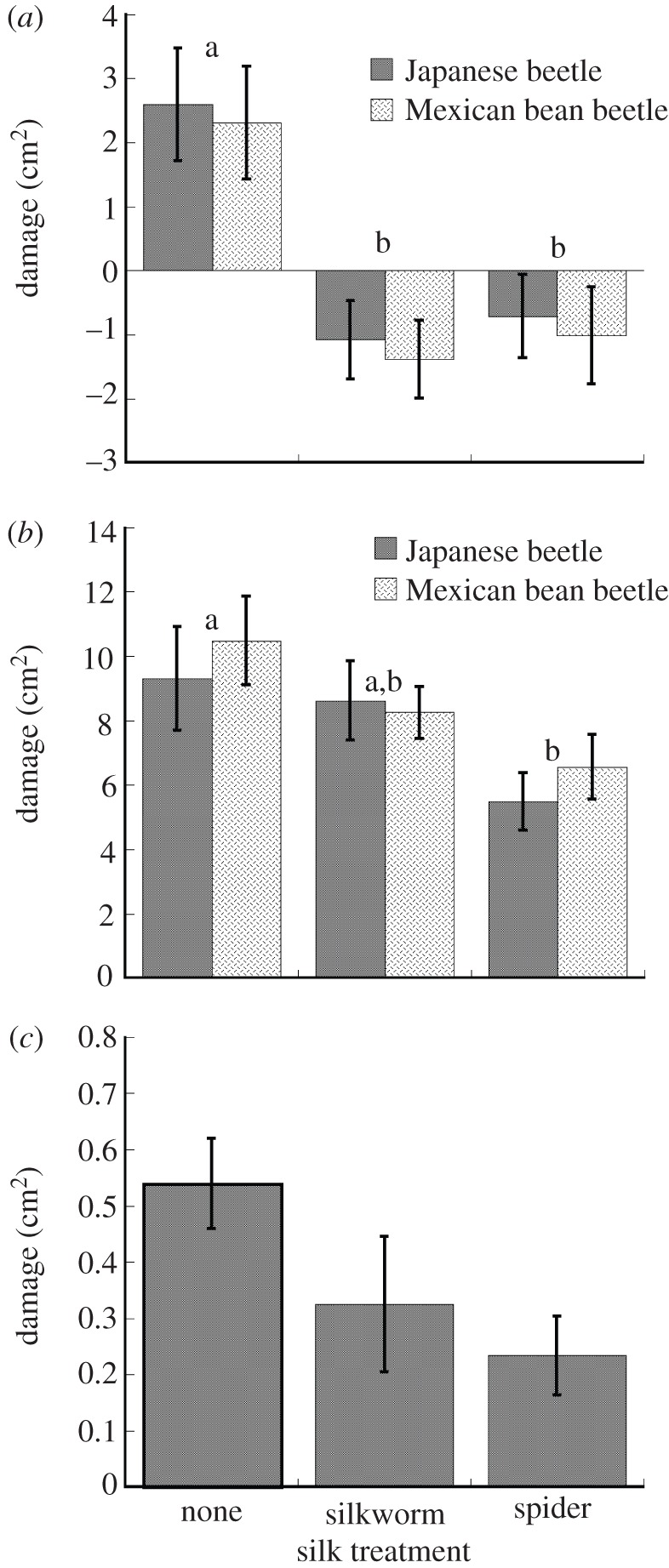
Damage (cm2) experienced by leaflets exposed to silkworm or spider silk in three experiments: (a) leaflets enclosed with a Japanese beetle or Mexican bean beetle in the laboratory; (b) leaflets on intact field plants enclosed with a Japanese beetle or Mexican bean beetle and (c) leaflets treated and left to experience natural herbivory.
Table 1.
Results of ANOVAs for damage (cm2) experienced in experiments. Silk treatments included no silk, silkworm silk and spider silk; laboratory and enclosure experiments were run with either Japanese or Mexican bean beetles.
| experiment |
d.f. | F | p | |
|---|---|---|---|---|
| leaflets in laboratory | whole model | 3,138 | 9.75 | <0.0001 |
| beetle species | 1 | 0.23 | 0.632 | |
| silk | 2 | 14.5 | <0.0001 | |
| enclosed leaflets in field | whole model | 3,86 | 3.80 | 0.0130 |
| beetle species | 1 | 0.47 | 0.4954 | |
| silk | 2 | 5.47 | 0.0058 | |
| field leaflets with natural herbivory | whole model | 2,115 | 2.78 | 0.0660 |
| enclosed plants in field | whole model | 3,56 | 3.53 | 0.0204 |
| beetle species | 1 | 0.02 | 0.8790 | |
| silk | 1 | 15.83 | 0.0003 | |
| field plants with natural herbivory | whole model | 2,27 | 2.75 | 0.0817 |
(b). Whole plant experiments
Beetles enclosed with plants treated with spider silk inflicted about half the damage as those with untreated plants (figure 2a and table 1). Damage to plants treated with silkworm silk was intermediate between controls and spider silk treatments (figure 2a). Silk-treated plants, left to experience natural herbivory, tended to receive less damage than untreated plants but the difference was not significant at the p < 0.05 level (figure 2b and table 1).
Figure 2.
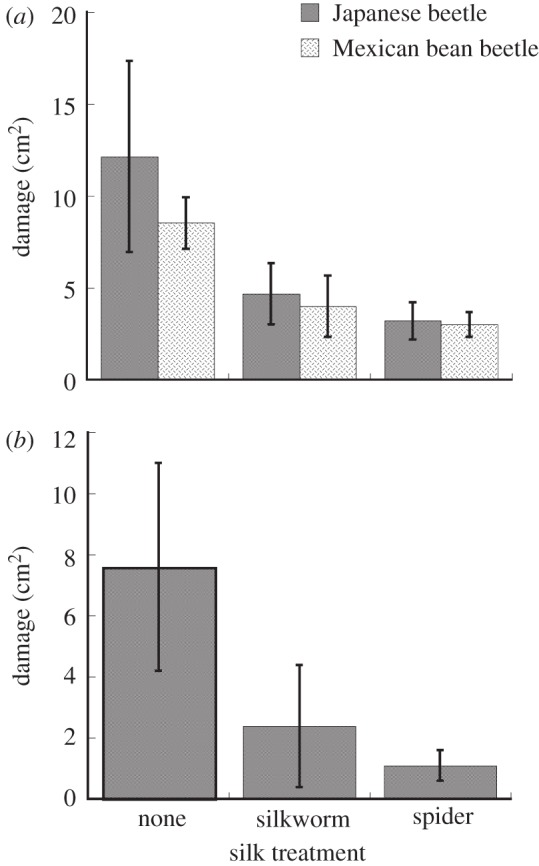
Damage (cm2) experienced by entire plants when leaves were treated with silkworm or spider silk in two experiments: (a) plants in field enclosures with either two Japanese beetles or two Mexican bean beetles, and (b) plants treated and left to experience natural herbivory.
4. Discussion
These results provide robust support for the hypothesis that the presence of silk reduces the foraging activity of pest insects leading to lower levels of herbivory. Spider silk consistently had the strongest impact on leaf damage, whereas silkworm silk tended to have more modest effects. The spider silk was freshly drawn from the spider and, as a result, undoubtedly contained more chemical information than silkworm silk [14]. Thus, it may have presented a stronger signal that was easier to detect and identify compared with silkworm silk. In addition, spiders are ubiquitous predators, whereas B. mori and other silk-producing insects have more limited ranges and more restricted possibilities for interactions with other arthropods in evolutionary time [17]. As a result, it is reasonable to conclude that beetles recognized spider silk as coming from a potential predator and that the observed reduction in herbivory was due to antipredator behaviour.
Nevertheless, commercial silkworm silk had some effects on herbivory (figures 1 and 2) and this suggests that there is a general response to silk fibre that is not tightly associated with the organism that produced it. We considered it possible that the herbivores might simply shift their herbivorous activity when confronted with vegetation with any fibre material on it and so we conducted an additional laboratory study with a broader array of treatments including natural and artificial fibres (details in the electronic supplementary material). The results of that experiment mimicked those reported here; none of the fibres tested but silk reduced Japanese beetle herbivory (see the electronic supplementary material). Hence, we believe that spider silk was responsible for altering consumption by the herbivores we studied and its effects on plant damage were uncoupled from the behaviour of the spider or any specific coevolved predator–prey association.
Our work provides a mechanism that can explain how a few spiders might simultaneously affect many potential prey species with substantial effects on resources [9]. Some of the strong non-consumptive effects of spiders may depend more on the abundance and distribution of their silk than on their density or diversity. Our short-term experiments focused only on a handful of species, and our enclosures did not allow herbivores to shift to different plant parts or disperse to different habitat patches. Nevertheless, the consistency of the pattern of responses to silk in laboratory, field enclosure and open field experiments is compelling. Further study including diverse communities and evaluating multiple temporal and spatial scales is critical to determine more precisely the role of the silk signal in specific food webs. Silk has many remarkable properties [14,17,18]; these results add plant protection to the list and suggest that strategies promoting spiders and silk production could be important to integrated pest management programmes.
Acknowledgements
We thank Miami's Ecology Research Center, A. Dorsey and K. Waisanen for assistance. The Ohio Plant Biotechnology Consortium and NSF grant no. DBI-0097393 supplied funding.
References
- 1.Carpenter SR, Kitchell JF. 1993. The trophic cascade in lakes. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Brett MT, Goldman CR. 1996. A meta-analysis of the freshwater trophic cascade. Proc. Natl Acad. Sci. USA 93, 7723–7726 10.1073/pnas.93.15.7723 (doi:10.1073/pnas.93.15.7723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shurin JB, Borer ET, Seabloom EW, Anderson K, Blanchette CA, Broitman B, Cooper SD, Halpern BS. 2002. A cross ecosystem comparison of the strength of trophic cascades. Ecol. Lett. 5, 785–791 10.1046/j.1461-0248.2002.00381.x (doi:10.1046/j.1461-0248.2002.00381.x) [DOI] [Google Scholar]
- 4.Strong DR. 1992. Are trophic cascades all wet? Differentiation and donor control in a speciose system. Ecology 73, 747–754 10.2307/1940154 (doi:10.2307/1940154) [DOI] [Google Scholar]
- 5.Schmitz OJ, Hamback PA, Beckerman AP. 2000. Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am. Nat. 155, 141–153 10.1086/303311 (doi:10.1086/303311) [DOI] [PubMed] [Google Scholar]
- 6.Halaj J, Wise DH. 2001. Terrestrial trophic cascades: how much do they trickle? Am. Nat. 157, 262–281 10.1086/319190 (doi:10.1086/319190) [DOI] [PubMed] [Google Scholar]
- 7.Abrams PA, Menge BA, Mittelbach GG, Spiller DA, Yodzis P. 1996. The role of indirect effects in food webs. In Food webs: integration of patterns and dynamics (eds Polis GA, Winemiller KO.), pp. 371–395 New York, NY: Chapman and Hall [Google Scholar]
- 8.Peacor SD, Werner EE. 2001. The contribution of trait-mediated indirect effects to the net effects of a predator. Proc. Natl Aacd. Sci. USA 98, 3904–3908 10.1073/pnas.071061998 (doi:10.1073/pnas.071061998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz OJ, Krivan V, Ovadia O. 2004. Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol. Lett. 7, 153–163 10.1111/j.1461-0248.2003.00560.x (doi:10.1111/j.1461-0248.2003.00560.x) [DOI] [Google Scholar]
- 10.Schmidt-Entling MH, Siegenthaler E. 2009. Herbivore release through cascading risk effects. Biol. Lett. 6, 773–776 10.1098/rsbl.2009.0436 (doi:10.1098/rsbl.2009.0436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawlena D, Stickland MS, Bradford MA, Schmitz OJ. 2012. Fear of predation slows plant-litter decomposition . Science 336, 1434–1438 10.1126/science.1220097 (doi:10.1126/science.1220097) [DOI] [PubMed] [Google Scholar]
- 12.Hlivko JT, Rypstra AL. 2003. Spiders reduce herbivory: nonlethal effects of spiders on the consumption of soybean leaves by beetle pests. Ann. Entomol. Soc. Am. 96, 914–919 10.1603/0013-8746(2003)096[0914:SRHNEO]2.0.CO;2 (doi:10.1603/0013-8746(2003)096[0914:SRHNEO]2.0.CO;2) [DOI] [Google Scholar]
- 13.Fill A, Long EY, Finke DL. 2012. Non-consumptive effects of a natural enemy on a non-prey herbivore population. Ecol. Entomol. 37, 43–50 10.1111/j.1365-2311.2011.01333.x (doi:10.1111/j.1365-2311.2011.01333.x) [DOI] [Google Scholar]
- 14.Craig CL. 2003. Spider webs and silks: tracing evolution from molecules to genes to phenotypes. Oxford, UK: Oxford University Press [Google Scholar]
- 15.Potter DA, Held DW. 2002. Biology and management of the Japanese beetle. Annu. Rev. Entomol. 47, 175–205 10.1146/annurev.ento.47.091201.145153 (doi:10.1146/annurev.ento.47.091201.145153) [DOI] [PubMed] [Google Scholar]
- 16.Flood B, Foster R, Hutchinson B. 1995. Mexican bean beetles. In Vegetable insect management with emphasis on the Midwest (eds Foster R, Flood B.), pp. 41–54 Willoughby, OH: Miester Publishing Co [Google Scholar]
- 17.Craig CL. 1997. Evolution of arthropod silks. Annu. Rev. Entomol. 42, 231–267 10.1146/annurev.ento.42.1.231 (doi:10.1146/annurev.ento.42.1.231) [DOI] [PubMed] [Google Scholar]
- 18.Vollrath F, Porter D. 2009. Silks as ancient models for modern polymers. Polymer 50, 5623–5632 10.1016/j.polymer.2009.09.068 (doi:10.1016/j.polymer.2009.09.068) [DOI] [Google Scholar]


