Abstract
The extreme body size of blue whales requires a high energy intake and therefore demands efficient foraging strategies. As an obligate lunge feeder on aggregations of small zooplankton, blue whales engulf a large volume of prey-laden water in a single, rapid gulp. The efficiency of this feeding mechanism is strongly dependent on the amount of prey that can be captured during each lunge, yet food resources tend to be patchily distributed in both space and time. Here, we measured the three-dimensional kinematics and foraging behaviour of blue whales feeding on krill, using suction-cup attached multi-sensor tags. Our analyses revealed 360° rolling lunge-feeding manoeuvres that reorient the body and position the lower jaws so that a krill patch can be engulfed with the whale's body inverted. We also recorded these rolling behaviours when whales were in a searching mode in between lunges, suggesting that this behaviour also enables the whale to visually process the prey field and maximize foraging efficiency by surveying for the densest prey aggregations. These results reveal the complex manoeuvrability that is required for large rorqual whales to exploit prey patches and highlight the need to fully understand the three-dimensional interactions between predator and prey in the natural environment.
Keywords: Mysticeti, manoeuvrability, foraging
1. Introduction
Among the great whales, the very largest animals are represented by several species of rorquals (Balaenopteridae), a family of baleen whales that are characterized by their extreme lunge-feeding strategy. This feeding behaviour, which involves the engulfment of a large volume of prey-laden water at high speed and wide gape angles, is enabled by an integrated set of biomechanical and physiological adaptations [1]. Such engulfment capacity enables balaenopterids to forage in bulk on dense patches of small fishes or zooplankton, thereby reaping the energetic benefits of abundant resources [2]. Because larger rorquals possess relatively larger mouths, and thus a greater mass-specific engulfment capacity, the efficiency of lunge-feeding is expected to increase with body size [3]. However, physical principles dictate that large body size will decrease specific aspects of locomotor performance, such as the ability to accelerate and manoeuvre, and so larger balaenopterids must lunge at higher absolute speeds to sufficiently capture prey (i.e. krill) during foraging bouts [4].
In addition to the locomotor challenges associated with extreme body size, the largest rorqual species, the blue whale (Balaenoptera musculus), exhibits relatively small flippers and flukes that also limit manoeuvring performance [5]. Such limited manoeuvrability has been implicated in the blue whale's nearly-complete dependence on krill [4], which is relatively less agile than other prey types (i.e. fishes) that are often targeted by smaller rorqual species. Nevertheless, krill exhibits well-documented escape responses [6] and thus blue whales must implement high-performance predatory strategies to efficiently exploit these abundant marine resources. Here, we used digital tags to reveal a behaviour that consists of complete 360° rolling manoeuvres during foraging. We interpreted these behaviours as serving dual functions to visualize the prey field and also to reorient the body and jaws to capture prey. These data underscore the need to understand foraging mechanics at a fine scale, and add to a diverse and growing repertoire for how large whales meet their energetic demands.
2. Material and methods
We used high-resolution digital acoustic recording tags (DTAGs) to investigate the kinematics of foraging blue whales. Blue whales (n = 22) were tagged in the southern California Bight, during the summer months of 2010. We used a 6 m carbon-fibre pole to deploy the suction-cup tags from a rigid-hulled inflatable boat. The DTAGs are equipped with stereo-hydrophones (sampling rate: 64 kHz), and an auxiliary sensor suite that includes a pressure transducer and a triaxial accelerometer and magnetometer [7]. All auxiliary sensor channels were sampled at 50 Hz, but were decimated to 5 Hz in post-processing. The orientation of the whale was determined from accelerometer and magnetometer data [7,8]. Swimming strokes and acceleration rate of change (i.e. ‘jerk’) were calculated from the raw accelerometer signals (50 Hz), whereas speed was estimated from the level of flow-noise [2,9]. As a complementary dataset, we used video data from a deployment of Crittercam, an animal-borne imaging device, to provide the whale's perspective during lunge-feeding events [10], deployed in August of 2008.
3. Results and discussion
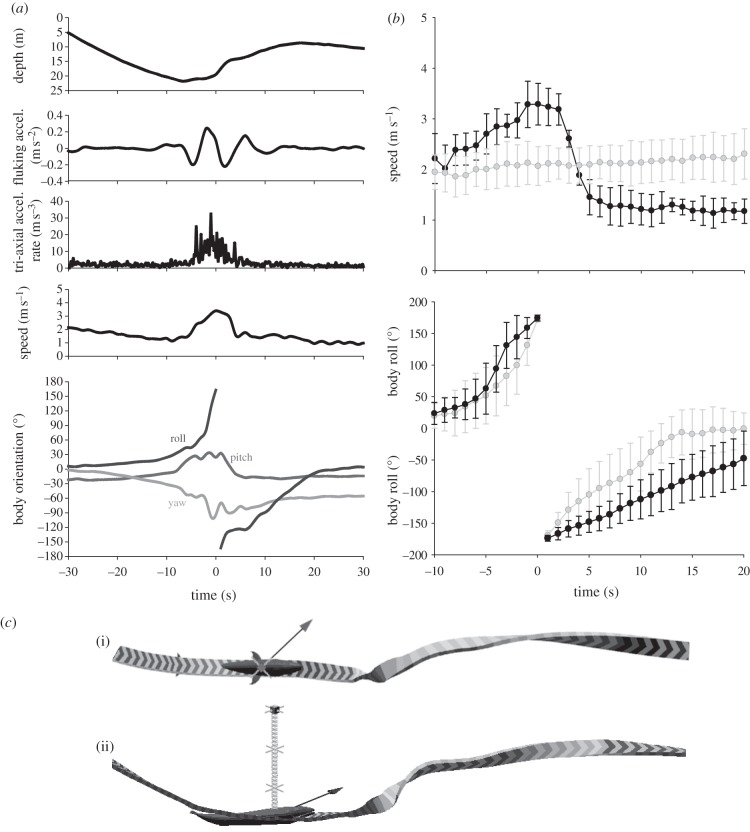
Our kinematic analyses revealed 44 instances of 360° rolling manoeuvres in 11 out of 22 tagged blue whales (see the electronic supplementary material, table S1). Average tag attachment durations lasted 6.7 h. Thus, whales with no evidence of this behaviour may not have been tagged during the appropriate conditions to have performed the manoeuvre. Tagged whales performed 360° rolls during lunges and also at other times within foraging dives such as during the ascent or descent phases of the dive. However, we did not detect 360° rolls when the whales were not foraging (i.e. resting at the sea surface). During lunges, the whale's body was upside down having rolled 180° at maximum speed (t = 0 s), which appears to follow mouth opening (t = −1 s). The maximum rotation rate after the apex of the manoeuvre (t > 0 s, roll = 180°) was significantly lower (n = 11, t-statistic = −5.588, p < 0.001) during lunges (12 ± 3° s−1) compared with non-feeding lunges (33±8° s−1). We attributed this difference to the increased added mass of the engulfed water in the throat pouch of feeding whales, given that the maximum rotation rate before the apex of the manoeuvre was not significantly different (n = 11, t-statistic = 1.012, p = 0.336) between feeding manoeuvres (48 ± 22° s−1) and non-feeding manoeuvres (37 ± 13° s−1; figure 1).
Figure 1.
Kinematics of 360° rolling manoeuvres. (a) Example of one lunge. Increasing roll values represent clockwise rotation about the animal's long axis (from the whale's perspective) and negative values indicate a counter-clockwise rotation. (b) Variation in speed among all individuals during lunges (black) and not during lunges (grey). Error bars represent 1 s.d. among individuals. (c) Overhead (i) and lateral view (ii) of the whale's trajectory during a surface feeding 360° leftward rolling lunge.
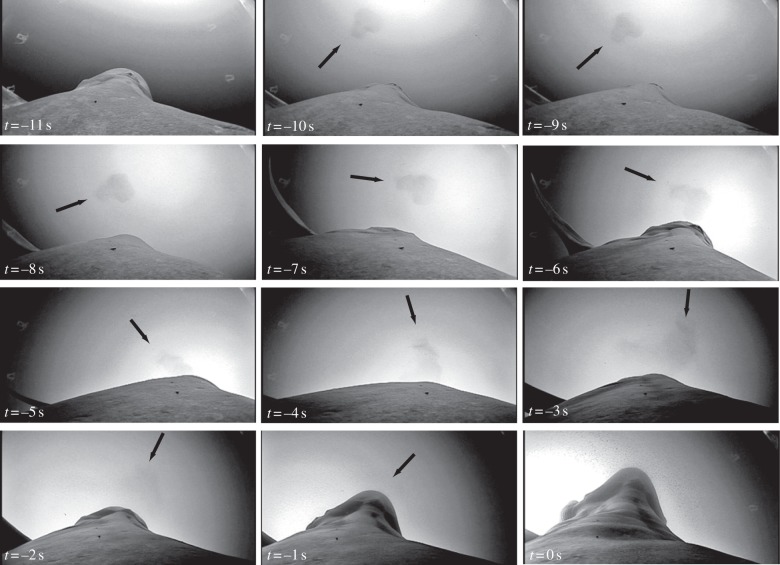
Crittercam video footage provided video data of a 360° rolling lunge from the perspective of a blue whale (figure 2). As the whale began the ascent phase of this foraging dive, the target krill patch was visible, and the whale's rolling behaviour was clearly evident as the asymmetrically shaped krill patch in view rotates prior to engulfment. These video data, which directly show the animal seeking and targeting this isolated krill patch from below, suggest that these behaviours play an important role in repositioning the jaws to engulf the densest proportion of the prey patch [2,10]. Rolling behaviours have been recorded during lunges in other rorqual species, including fin and humpback whales, although the magnitude of the roll is typically up to 90° and does not exceed 150° [11,12]. Theoretical models of rorqual–krill interactions indicate that this rolling behaviour is aimed at anticipating the prey's escape trajectory [13], such that the jaws are repositioned to where krill will be at the time of mouth opening, and thus maximizes prey capture. Within this context, and given the fact that only 10 per cent of lunges in this study involved a 360° roll, this behaviour may represent a honed strategy that is specific for a certain prey patch shape or size. Given that some krill patches can be extremely dense, a blue whale may be able to meet its daily energetic demands in only one foraging dive [2]. Thus, for a high-quality krill patch that is difficult to attack, blue whales may be motivated to perform these extraordinary acrobatic manoeuvres in order to maximize foraging efficiency. Without the manoeuvre, blue whales could miss the krill patch completely, resulting in the mismanagement of limited dive time and a decrease in foraging efficiency.
Figure 2.
Crittercam footage during the pre-engulfment phase of a rolling lunge near the sea surface. Following a right-hand turn (t =−11s), a rolling manoeuvre results in the rotation of the visual field indicated by the asymmetrically shaped krill patch (arrows, −10 s < t < −1 s). The outward rotation of the left mandible is evident as engulfment begins (−1 s < t < 0 s).
We also observed 360° rolls when blue whales were in transit in between lunges, just prior to a lunge, and during the ascent and descent phases of a dive. These results suggest that this behaviour may also be important for visually processing the near-field prey distribution. As in all cetaceans, the eyes are positioned laterally, and thus rolling the body should enhance panoramic vision in multiple dimensions. An analogous phenomenon may be present in sperm whales, which also exhibit substantial rolling behaviour during feeding, except that information on prey distribution is instead provided by echolocation signals [14]. Unlike changes in pitch or yaw, which are typically used for directional changes, rolling has been associated with a wide variety of unique functions and behaviours [15]. Detailed investigations of rolling manoeuvres in other systems include remora removal in spinner dolphins [15], air-righting in geckos [16] and alligator death-rolls [17]. The discovery of blue whale rolling lunges adds to the number of specific uses for this biomechanical event, and also increases our understanding of animal manoeuvrability during predator–prey interactions.
Although blue whale manoeuvrability is constrained by large size and morphological design [4,5], this type of manoeuvre should maximize foraging efficiency at different temporal and spatial scales. This behaviour will increase the probability of finding the highest quality prey patch by optimizing the whale's field of view and, during the lunge itself, the roll repositions the jaws to engulf the densest nearby portion of the aggregation. The ability to engulf high-density prey patches is a fundamental component of the filter feeding strategy of blue whales, thereby increasing the energetic efficiency of foraging and facilitating the maintenance of extreme body size [2]. These results highlight the challenges of foraging in a complex three-dimensional environment with patchily abundant, ephemeral food resources. In contrast to the possible echo-ranging behaviour observed during night-time feeding in humpback whales [11], this study suggests that vision plays an important role in finding prey. Considering that night-time feeding events have been reported for blue whales in some geographical regions [18], unique sensory systems with tactile vibrissae integrated into the engulfment apparatus [1] may facilitate prey capture in low light conditions [11,19]. Although it appears that rorquals use a variety of sensory modalities to find prey under different conditions, more studies are needed that combine the three-dimensional kinematics of foraging predators with time-resolved prey distribution data to fully understand the complex interplay between predator and prey [19,20].
Acknowledgements
This study was conducted in accordance with the US NMFS Permitting authority (permit no. 14534, issued to N. Cyr with B. Southall as chief scientist), the Channel Islands National Marine Sanctuary (permit nos 2010-004, issued to B. Southall), and a consistency determination from the California Coastal Commission.
References
- 1.Pyenson ND, Goldbogen JA, Vogl AW, Szathmary G, Drake RL, Shadwick RE. 2012. Discovery of a sensory organ that coordinates lunge feeding in rorqual whales. Nature 485, 498–501 10.1038/nature11135 (doi:10.1038/nature11135) [DOI] [PubMed] [Google Scholar]
- 2.Goldbogen JA, Calambokidis J, Oleson E, Potvin J, Pyenson ND, Schorr G, Shadwick RE. 2011. Mechanics, hydrodynamics and energetics of blue whale lunge feeding: efficiency dependence on krill density. J. Exp. Biol. 214, 131–146 10.1242/jeb.048157 (doi:10.1242/jeb.048157) [DOI] [PubMed] [Google Scholar]
- 3.Goldbogen JA, Potvin J, Shadwick RE. 2010. Skull and buccal cavity allometry increase mass-specific engulfment capacity in fin whales. Proc. R. Soc. B 277, 861–868 10.1098/rspb.2009.1680 (doi:10.1098/rspb.2009.1680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldbogen JA, Calambokidis J, Croll D, McKenna MF, Potvin J, Pyenson ND, Schorr G, Shadwick RE, Tershy BR. 2012. Scaling of lunge feeding performance in rorqual whales: mass-specific energy expenditure increases with body size and progressively limits diving capacity. Funct. Ecol. 26, 216–226 10.1111/j.1365-2435.2011.01905.x (doi:10.1111/j.1365-2435.2011.01905.x) [DOI] [Google Scholar]
- 5.Woodward BL, Winn JP, Fish FE. 2006. Morphological specializations of baleen whales associated with hydrodynamic performance and ecological niche. J. Morphol. 267, 1284–1294 10.1002/jmor.10474 (doi:10.1002/jmor.10474) [DOI] [PubMed] [Google Scholar]
- 6.O'Brien DP. 1987. Description of escape responses of krill (Crustacea, Euphausiacea), with particular reference to swarming behavior and the size and proximity of the predator. J. Crustacean Biol. 7, 449–457 10.2307/1548294 (doi:10.2307/1548294) [DOI] [Google Scholar]
- 7.Johnson M, Tyack PL. 2003. A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J. Ocean. Eng. 28, 3–12 10.1109/JOE.2002.808212 (doi:10.1109/JOE.2002.808212) [DOI] [Google Scholar]
- 8.Ware C, Friedlaender AS, Nowacek DP. 2011. Shallow and deep lunge feeding of humpback whales in fjords of the West Antarctic Peninsula. Mar. Mamm. Sci. 27, 587–605 10.1111/j.1748-7692.2010.00427.x (doi:10.1111/j.1748-7692.2010.00427.x) [DOI] [Google Scholar]
- 9.Simon M, Johnson M, Madsen PT. 2012. Keeping momentum with a mouthful of water: behavior and kinematics of humpback whale lunge feeding. J. Exp. Biol. 215, 3786–3798 10.1242/jeb.071092 (doi:10.1242/jeb.071092) [DOI] [PubMed] [Google Scholar]
- 10.Calambokidis J, Schorr GS, Steiger GH, Francis J, Bakhtiari M, Marshal G, Oleson EM, Gendron D, Robertson K. 2007. Insights into the underwater diving, feeding, and calling behavior of blue whales from a suction-cup-attached video-imaging tag (Crittercam). Mar. Technol. Soc. J. 41, 19–29 10.4031/002533207787441980 (doi:10.4031/002533207787441980) [DOI] [Google Scholar]
- 11.Stimpert AK, Wiley DN, Au WWL, Johnson MP, Arsenault R. 2007. ‘Megapclicks’: acoustic click trains and buzzes produced during night-time foraging of humpback whales (Megaptera novaeangliae). Biol. Lett. 3, 467–470 10.1098/rsbl.2007.0281 (doi:10.1098/rsbl.2007.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldbogen JA, Calambokidis J, Shadwick RE, Oleson EM, McDonald MA, Hildebrand JA. 2006. Kinematics of foraging dives and lunge-feeding in fin whales. J. Exp. Biol. 209, 1231–1244 10.1242/jeb.02135 (doi:10.1242/jeb.02135) [DOI] [PubMed] [Google Scholar]
- 13.Potvin J, Goldbogen JA, Shadwick RE. 2010. Scaling of lunge feeding in rorqual whales: an integrated model of engulfment duration. J. Theor. Biol. 267, 437–453 10.1016/j.jtbi.2010.08.026 (doi:10.1016/j.jtbi.2010.08.026) [DOI] [PubMed] [Google Scholar]
- 14.Miller PJO, Johnson MP, Tyack PL. 2004. Sperm whale behaviour indicates the use of echolocation click buzzes ‘creaks’ in prey capture. Proc. R. Soc. Lond. B 271, 2239–2247 10.1098/rspb.2004.2863 (doi:10.1098/rspb.2004.2863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fish FE, Nicastro AJ, Weihs D. 2006. Dynamics of the aerial maneuvers of spinner dolphins. J. Exp. Biol. 209, 590–598 10.1242/jeb.02034 (doi:10.1242/jeb.02034) [DOI] [PubMed] [Google Scholar]
- 16.Jusufi A, Kawano DT, Libby T, Full RJ. 2010. Righting and turning in mid-air using appendage inertia: reptile tails, analytical models and bio-inspired robots. Bioinspir. Biomim. 5, 045001. 10.1088/1748-3182/5/4/045001 (doi:10.1088/1748-3182/5/4/045001) [DOI] [PubMed] [Google Scholar]
- 17.Fish FE, Bostic SA, Nicastro AJ, Beneski JT. 2007. Death roll of the alligator: mechanics of twist feeding in water. J. Exp. Biol. 210, 2811–2818 10.1242/jeb.004267 (doi:10.1242/jeb.004267) [DOI] [PubMed] [Google Scholar]
- 18.Doniol-Valcroze T, Lesage V, Giard J, Michaud R. 2011. Optimal foraging theory predicts diving and feeding strategies of the largest marine predator. Behav. Ecol. 22, 880–888 10.1093/beheco/arr038 (doi:10.1093/beheco/arr038) [DOI] [Google Scholar]
- 19.Friedlaender AS, Hazen EL, Nowacek DP, Halpin PN, Ware C, Weinrich MT, Hurst T, Wiley D. 2009. Diel changes in humpback whale Megaptera novaeangliae feeding behavior in response to sand lance Ammodytes spp. behavior and distribution. Mar. Ecol. Prog. Ser. 395, 91–100 10.3354/meps08003 (doi:10.3354/meps08003) [DOI] [Google Scholar]
- 20.Hazen EL, Friedlaender AS, Thompson MA, Ware CR, Weinrich MT, Halpin PN, Wiley DN. 2009. Fine-scale prey aggregations and foraging ecology of humpback whales Megaptera novaeangliae. Mar. Ecol. Prog. Ser. 395, 75–89 10.3354/meps08108 (doi:10.3354/meps08108). [DOI] [Google Scholar]