Abstract
Some experts suggest that sedation of laboratory rodents with isoflurane before euthanasia with carbon dioxide (CO2) is a humane alternative to euthanasia with CO2 alone, but little research has compared aversion with these agents. Albino rats were tested in a light–dark box where they had the choice between remaining in a dark compartment filling with isoflurane or CO2, or escaping to a lit compartment. Experiment 1 validated the procedure by confirming that rats responded to agent and light intensity. In experiment 2, 9/16 and 0/16 rats remained in the dark compartment until recumbent when initially exposed to isoflurane and CO2, respectively. In experiment 3, more rats remained in the dark compartment until recumbent during initial (10/16) versus re-exposure (1/16) to isoflurane. These results indicate that initial exposure to CO2 is more aversive than isoflurane, and that re-exposure to isoflurane is more aversive than initial exposure. We conclude that sedation with isoflurane is a refinement over euthanasia with CO2 alone for rats that have not been previously exposed to inhalant anaesthetics.
Keywords: euthanasia, light/dark box, approach-avoidance
1. Introduction
Carbon dioxide (CO2) is the most commonly used euthanasia agent for rodents, but there is evidence that this gas is aversive. Rats show signs of distress when forced to remain in a chamber filling with CO2 [1] and choose to give up a food reward to escape a chamber filling with CO2 when the concentration exceeds 15 per cent [2,3] even when food-deprived for 24 h [4]. Aversion to CO2 is probably due to dyspnoea, an unpleasant sensation of breathlessness [3], which in humans begins at 8 per cent CO2 and becomes severe around 15 per cent [5,6].
Inhalant anaesthetics can be used to induce unconsciousness before euthanasia. Rats free to move between chambers spent less time in a chamber containing CO2 than one containing an inhalant anaesthetic [7,8]. Rats also show evidence of aversion to the inhalant anaesthetic isoflurane, but this aversion occurs once the animals are in a state of conscious sedation; moreover, initial exposure to isoflurane is less aversive than subsequent exposures [9].
The aim of this study was to use aversion-avoidance testing to compare rat aversion to CO2 and isoflurane against aversion to bright light. Light is known to be aversive to albino rats [10], and this aversion can be measured using a light–dark box [11]. A 25 per cent difference in light intensity is sufficient for rats to avoid the brighter side [12]. In this study, the lowest light condition (300 lx) was chosen because this is within the range of intensities used in laboratories, although rats will press a lever to turn it off [13]. The highest intensity (1600 lx) was chosen because it induces strong avoidance in rats [14,15].
2. Material and methods
(a). Subjects and housing
Rats were purchased as surplus stock from the University of British Columbia. Experiment 1 used 6 two-month old male Sprague–Dawley rats housed at a mean (± s.d.) temperature of 23.5 ± 0.2°C and relative humidity of 50 ± 6%; experiment 2 used 32 three-month old male Sprague–Dawley rats housed at 20.7 ± 0°C and 42 ± 6%; and experiment 3 used 16 three-month old male Wistar rats housed at 21.1 ± 0.3°C and 44 ± 8%. All animals were kept on a 12 L : 12 D cycle (highest light intensity: 186 lx), and all testing was conducted during the light phase at a similar time of day. Rats were pair-housed in rat cages (48 × 27 × 20 cm) containing corn cob bedding and a nest-box. Rats had free access to food (Harlan diet, Harlan Teklad, Madison, WI, USA) and autoclaved water.
(b). Experimental apparatus
The light–dark box (figure 1) was made of Plexiglas, and the light and dark compartments (30 × 30 × 14 cm) were covered with either semi-opaque white plastic or completely opaque black plastic, respectively. Thin slits were cut into the plastic sheets to allow observation into each compartment. The light compartment was illuminated with two dimmable fluorescent light bulbs placed directly above the lid of this compartment. Gas mixing between the compartments was minimized by way of a buffer compartment (7 × 30 × 14 cm), and doorways (10 × 10 cm) covered with flexible plastic overlapping strips. An exhaust duct was positioned above this buffer compartment to draw out any diffused gas. Pre-testing with an O2 analyser confirmed that isoflurane and CO2 did not diffuse into the light compartment (additional information on the experimental apparatus is available in the electronic supplementary material).
Figure 1.
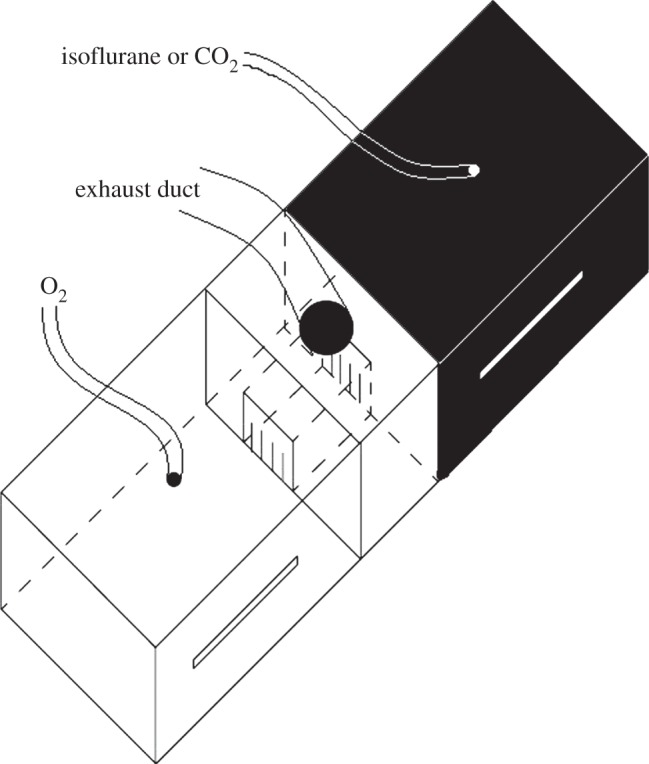
The light–dark testing apparatus.
(c). Testing procedure
Rats were habituated to the testing apparatus before testing (see the electronic supplementary material). At the beginning of each trial, a rat was placed in the dark compartment and given 30 min to explore the apparatus with the light compartment illuminated at the intensity selected for that trial, and with O2 delivered into each of the compartments at a rate of 32 per cent of the compartment volume per minute (4 l min–1). All rats settled in the dark compartment for at least 10 min by the end of the 30 min exploratory period, at which point either isoflurane (Baxter Corporation, Mississauga, Ontario, Canada) or CO2 (Praxair, Richmond, British Columbia, Canada) was turned on in the dark compartment. Isoflurane was introduced into the flow of O2 at a concentration of 5 per cent using an Isotec 4 vapourizer (Ohmeda, Steeton, West Yorkshire, England). The flow rate and concentration were the highest allowed by the anaesthetic machine and vapourizer, and were chosen to minimize the time to loss of consciousness [9]. For CO2, the O2 flow was turned off as CO2 was turned on to a flow of 24 per cent volume per minute (3 l min–1). This flow rate was chosen because it is within the range recommended by the Canadian Council on Animal Care for euthanasia [16]. Trials ended as soon as the rat became recumbent (defined as loss of muscle tone and measured as all limbs, head and tail flat on bedding) or 90 s after gas or anaesthetic flow began, whichever occurred first. Responses were scored from video using observers that were blind to treatment.
The study consisted of three experiments. The aim of experiment 1 was to validate the procedure by assessing rat responses to light intensity and agent. Six rats that had previously been exposed to isoflurane were each tested in each of six treatments (isoflurane or O2 each tested at 300, 800 or 1600 lx) using a 6 × 6 Latin square. Each square was repeated three times, and results were averaged to obtain one point per rat per treatment.
The aim of experiment 2 was to test responses to initial exposure to isoflurane and CO2. Thirty-two naive rats were randomly assigned to one of four treatments (isoflurane or CO2 at 300 or 1600 lx), with eight rats tested for each treatment and each rat tested only once.
The aim of experiment 3 was to test responses to repeat exposure to isoflurane and CO2. Sixteen naive rats were initially exposed to isoflurane (at 300 or 1600 lx), and then to CO2 (at 300 or 1600 lx). These rats were then each tested in each of four treatments (isoflurane or CO2 each tested at 300 or 1600 lx) using four identical 4 × 4 Latin squares.
In all cases where rats were retested, we provided 2 day intervals between exposures to allow recovery.
(d). Data analysis
In experiments 1 and 3, total dwelling time in the dark compartment upon re-exposure was analysed using a mixed model (SAS v. 9.2) that included rat as a random effect. In experiments 2 and 3, the effect of treatment on the number of rats staying in the dark compartment until recumbent upon initial exposure was analysed using a Fisher's exact test. In experiment 3, the difference in total dwelling time in isoflurane between initial and re-exposure was tested with a paired t-test. Initial responses to CO2 were not considered for experiment 3 as by design this agent was always tested after isoflurane.
3. Results
In experiment 1, rats spent more time in the dark compartment when it filled with O2 versus isoflurane (figure 2; F1,27 = 35.76, p < 0.0001). When exposed to isoflurane, rats spent more time in the dark compartment when the light compartment was brighter (F1,11 = 18.08, p < 0.01), validating the use of light intensity as a method for manipulating aversion.
Figure 2.
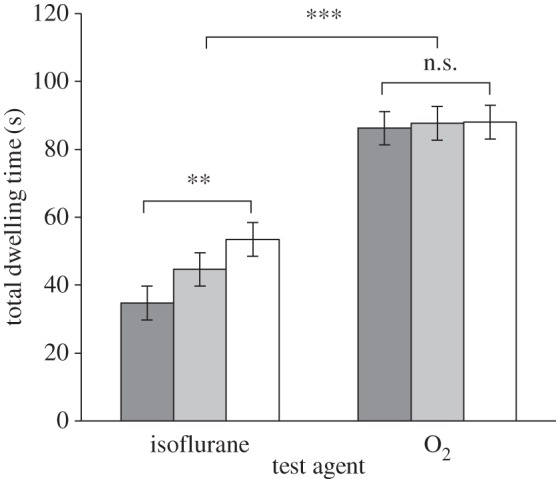
Mean (± s.e.m.) total dwelling time in the dark compartment in experiment 1 (n = 6). Dark grey bars, 300 lx; light grey bars, 800 lx; white bars, 1600 lx.
Experiment 2 examined responses of rats that had not previously been exposed to either isoflurane or CO2. These rats were more likely to stay in the dark compartment until they became recumbent when the compartment was filling with isoflurane versus CO2 (table 1; p = 0.0008). Light intensity had no effect on the number of rats becoming recumbent or total dwelling time in the dark compartment.
Table 1.
The number of rats staying in the dark compartment until recumbency during initial exposure to isoflurane (experiments 2 and 3) or CO2 (experiment 2 only), relative to the total number of rats tested. Results are shown separately for the two different light levels tested on the light side of the box and for experiments 2 (n = 32) and 3 (n = 16).
| experiment 2 |
experiment 3 |
||
|---|---|---|---|
| light (lx) | isoflurane | CO2 | isoflurane |
| 300 | 5/8 | 0/8 | 2/8 |
| 1600 | 4/8 | 0/8 | 8/8 |
| total | 9/16 | 0/16 | 10/16 |
During initial exposure to isoflurane in experiment 3, more rats became recumbent in the dark compartment when the light compartment was brighter (table 1; p = 0.007) and more rats became recumbent during initial than re-exposure to isoflurane (10/16 versus 1/16; p = 0.002). No rats stayed until recumbency during re-exposure to CO2. In re-exposure trials, there was no effect of light intensity on total dwelling time in the dark compartment for isoflurane. Unexpectedly, total dwelling time in the dark compartment was shorter when the light compartment was brighter during exposure to CO2 (24 ± 5 s versus 19 ± 5 s; F1,15 = 4.90, p = 0.04).
4. Discussion
This is the first study to show that rats will choose to remain in a chamber filling with isoflurane until recumbent. During initial exposure to isoflurane, approximately half the rats chose exposure to isoflurane until recumbent rather than escaping to a lit chamber; this preference for the dark chamber filling with isoflurane occurred when the lit chamber was bright (1600 lx) and when the light level was within the range commonly used in animal housing rooms (300 lx). In contrast, rats never tolerated exposure to CO2 until recumbent, always choosing exposure to light instead.
Re-exposure to isoflurane was more aversive than initial exposure. Only one rat tolerated re-exposure to isoflurane until the point of recumbency. This result suggests that for rats previously exposed to isoflurane; for example, during surgery, sedation with isoflurane during euthanasia may be as aversive as exposure to CO2 alone. However, other results suggest that aversion to isoflurane upon re-exposure is short lived [9]. The cause for learned aversion to isoflurane is not known, but may relate to nausea or ‘emergence delirium’ associated with recovery from inhalant anaesthesia [17]. For a discussion of the effects of light level and strain, refer to the electronic supplementary material.
We conclude that sedation with isoflurane is a refinement over exposure to CO2 alone for euthanasia of rats that have had no previous exposure to inhalant anaesthetics. For rats previously exposed to isoflurane, re-exposure may be as aversive as exposure to CO2.
Acknowledgements
The University of British Columbia Animal Care Committee approved all procedures.
We thank Matthew Leach, Jurgen Pehlke and Martin Hilmer for technical help. This research was funded by a Discovery grant to DMW from the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Niel L, Weary DM. 2006. Behavioural responses of rats to gradual-fill carbon dioxide euthanasia and reduced oxygen concentrations. Appl. Anim. Behav. Sci. 100, 295–308 10.1016/j.applanim.2005.12.001 (doi:10.1016/j.applanim.2005.12.001) [DOI] [Google Scholar]
- 2.Niel L, Weary DM. 2007. Rats avoid exposure to carbon dioxide and argon. Appl. Anim. Behav. Sci. 107, 100–109 10.1016/j.applanim.2006.08.002 (doi:10.1016/j.applanim.2006.08.002) [DOI] [Google Scholar]
- 3.Niel L, Stewart S, Weary DM. 2008. Effects of flow rate on aversion to gradual-fill carbon dioxide exposure in rats. Appl. Anim. Behav. Sci. 109, 77–84 10.1016/j.applanim.2007.02.004 (doi:10.1016/j.applanim.2007.02.004) [DOI] [Google Scholar]
- 4.Kirkden RD, Niel L, Lee G, Makowska IJ, Pfaffinger MJ, Weary DM. 2008. The validity of using an approach-avoidance test to measure the strength of aversion to carbon dioxide in rats. Appl. Anim. Behav. Sci. 114, 216–234 10.1016/j.applanim.2008.03.001 (doi:10.1016/j.applanim.2008.03.001) [DOI] [Google Scholar]
- 5.Hill L, Flack M. 1908. The effects of excess carbon dioxide and of want of oxygen upon the respiration and the circulation. J. Physiol. 37, 77–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liotti M, et al. 2001. Brain responses associated with consciousness of breathlessness (air hunger). Proc. Natl Acad. Sci. USA 98, 2035–2040 10.1073/pnas.98.4.2035 (doi:10.1073/pnas.98.4.2035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leach MC, Bowell VA, Allan TF, Morton DB. 2002. Degrees of aversion shown by rats and mice to different concentrations of inhalational anaesthetics. Vet. Rec. 250, 808–815 10.1136/vr.150.26.808 (doi:10.1136/vr.150.26.808) [DOI] [PubMed] [Google Scholar]
- 8.Leach MC, Bowell VA, Allan TF, Morton DB. 2004. Measurement of aversion to determine humane methods of anaesthesia and euthanasia. Anim. Welfare 13, S77–S86 [Google Scholar]
- 9.Makowska IJ, Weary DM. 2009. Rat aversion to induction with inhalant anaesthetics. Appl. Anim. Behav. Sci. 119, 229–235 10.1016/j.applanim.2009.04.003 (doi:10.1016/j.applanim.2009.04.003) [DOI] [Google Scholar]
- 10.Barker DJ, Sanabria F, Lasswell A, Thrailkill EA. 2010. Brief light as a practical aversive stimulus for the albino rat. Behav. Brain Res. 214, 402–408 10.1016/j.bbr.2010.06.020 (doi:10.1016/j.bbr.2010.06.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hetherington L, Benn M, Coffey PJ, Lund RD. 2000. Sensory capacity of the Royal College of Surgeons rat. Invest. Ophthalmol. Vis. Sci. 41, 3979–3983 [PubMed] [Google Scholar]
- 12.Campbell BA, Messing RB. 1969. Aversion thresholds and aversion difference limens for white light in albino and hooded rats. J. Exp. Psychol. 82, 353–359 10.1037/h0028248 (doi:10.1037/h0028248) [DOI] [Google Scholar]
- 13.Stern S, Laties VG. 1989. Comparison of 60 Hz electric fields and incandescent light as aversive stimuli controlling the behaviour of rats. Bioelectromagnetics 10, 99–109 10.1002/bem.2250100110 (doi:10.1002/bem.2250100110) [DOI] [PubMed] [Google Scholar]
- 14.Keller FS. 1941. Light-aversion in the white rat. Psychol. Rec. 4, 235–250 [Google Scholar]
- 15.Messing RB. 1972. The sensitivity of albino rats to lights of different wavelength: a behavioural assessment. Vision Res. 12, 753–761 (doi:10.1016/0042–6989(72)90001-6) [DOI] [PubMed] [Google Scholar]
- 16.Canadian Council on Animal Care 2010. CCAC guidelines on: euthanasia of animals used in science. See http://www.ccac.ca/Documents/Standards/Guidelines/Euthanasia.pdf.
- 17.Scott GM, Gold JI. 2006. Emergence delirium: a re-emerging interest. Semin. Anesth. Perioper. Med. Pain 25, 100–104 10.1053/j.sane.2006.05.013 (doi:10.1053/j.sane.2006.05.013) [DOI] [Google Scholar]


