Abstract
Male homosexual behaviour—although found in most extant clades across the Animal Kingdom—remains a conundrum, as same-sex mating should decrease male reproductive fitness. In most species, however, males that engage in same-sex sexual behaviour also mate with females, and in theory, same-sex mating could even increase male reproductive fitness if males improve their chances of future heterosexual mating. Females regularly use social information to choose a mate; e.g. male attractiveness increases after a male has interacted sexually with a female (mate choice copying). Here, we demonstrate that males of the tropical freshwater fish Poecilia mexicana increase their attractiveness to females not only by opposite-sex, but likewise, through same-sex interactions. Hence, direct benefits for males of exhibiting homosexual behaviour may help explain its occurrence and persistence in species in which females rely on mate choice copying as one component of mate quality assessment.
Keywords: homosexuality, mate choice copying, non-independent mate choice, same-sex mating
1. Introduction
Woody Allen once said ‘bisexuality immediately doubles your chances for a date on Saturday night’ and, even if he meant something else, he could nonetheless be right if homosexual behaviour were also to increase the chances of future heterosexual interactions. Male homosexual behaviour can be found in most extant classes across the Animal Kingdom [1,2], but represents a Darwinian puzzle as same-sex mating should decrease male reproductive fitness [3,4]. Several adaptive hypotheses have been forwarded to explain the persistence and frequency of homosexual behaviour (thoroughly reviewed in [4]). However, males that engage in homosexual behaviour often also mate with females, thus exclusive homosexuality is not the rule [1,4].
In gregarious animals, female mate choice often integrates social information gathered while observing potential mates interacting with other individuals [5–7]. This helps females to evaluate male reproductive quality and suitability as a potential mating partner, and it also reduces the costs associated with mate searching [8]. For example, female fish (Poecilia latipinna) prefer not only males whom they observed consorting with conspecific females, but also with females of the related unisexual Poecilia formosa [7], a phenomenon termed mate choice copying [8,9]. So, if females have a predilection to associate with males they saw mating—even with heterospecific females—mating behaviour per se seems to have gained signal value. Indeed, displaying mating behaviour conveys information not only about a male's readiness to mate, but also mate quality, as its performance is associated with costs [10,11]. However, female choice and male dominance hierarchies often prevent subordinate males from accessing females [12]. In this context, an as yet unexplored hypothesis is that less attractive and/or subordinate males could exploit female mate choice copying, rendering themselves more attractive through the performance of homosexual mating behaviour and thus increasing their chances of reproducing.
We tested this idea in the internally fertilizing, livebearing fish Poecilia mexicana, a species with a promiscuous mating system and female mate choice copying [13]. Homosexual behaviour is regularly seen in subordinate (typically cryptically coloured and small-bodied) P. mexicana males [14] (see the electronic supplementary material), while colourful and large-bodied males aggressively defend shoals of females, thereby preventing subordinate males from accessing them. Females show a mating preference for large, typically dominant males [15], which could lead subordinate (small to medium-sized) males—residing at the periphery of female shoals and being largely unable to approach females directly—to exploit homosexual behaviour as a strategy to solicit consensual mating. Using binary association preference tests, we asked if males' attractiveness increases after females saw them interacting sexually with either a female or another male. We further evaluated focal females’ ability to discriminate between male and female animations to ensure that females in our mate choice experiment were able to distinguish between hetero- and homosexual situations.
2. Material and methods
(a). Study organism
Test animals were laboratory-reared descendants of fish collected near Tampico in northeastern Mexico and from the southern Mexican Riío Oxolotán [16]. During homosexual interactions, poeciliid males typically make oral contact with another male's copulatory organ (a pre-mating behaviour called ‘nipping’)—just like they regularly nip at the female genital opening prior to copulating—or males try to copulate with other males [17]. As genital nipping is the most frequent sexual behaviour in P. mexicana [17], we used this behaviour for animations showing either hetero- or homosexual interactions (for detailed description of P. mexicana mating behaviour, see the electronic supplementary material).
(b). Experiment 1: female mate choice
Using a dichotomous choice design, we first evaluated female preferences (standard length (SL): 45.6 ± 0.7 mm; n = 92) for two virtual stimulus males (sized on screen to 30.0 mm SL each) by showing each focal female video animations of a male with either 50 per cent reduced or 50 per cent increased colour intensity and measured association preferences for 10 min (with switched side assignment after 5 min, see the electronic supplementary material). As predicted, most females preferred to associate with the more colourful male type (92 out of 116 trials, see the electronic supplementary material), and only those trials were further considered. During the subsequent second part of the tests, females were randomly assigned to one of three different treatments and for a period of 10 min (with switched side assignment after 5 min, see the electronic supplementary material) were presented with two animations showing either: (i) the drab and the colourful male swimming alongside another, slightly larger (40.0 mm SL) and immediately colourful model male (control); (ii) the drabber male performing heterosexual behaviour with a model female (40.0 mm SL), whereas the more colourful male was swimming alongside the model female; or (iii) the drabber male performing homosexual behaviour (i.e. pre-copulatory nipping) with a model male, whereas the more colourful male was swimming alongside the model male. In the third part of the tests we re-measured female preferences for the same two animations shown in the first part (measurement of 10 min with changing of side assignments after 5 min). We used 12 different video animations per treatment (see the electronic supplementary material).
(c). Experiment 2: discrimination between female and male video animations
Our results prompted the question as to whether focal females discriminate between observed hetero- and homosexual interactions, i.e. between the video-animated model males and females. In a dichotomous choice test (experiment 2), we thus measured association preferences of P. mexicana females from the Rio Oxolotán population (43.3 ± 0.8 mm SL; n = 10) for animated shoals showing either five males or five females swimming side-by-side over a period of 10 min (with switched side assignments after 5 min). We used the same model animals as in experiment 1 to create the animations, and SL of all model individuals were sized on screen to 40 mm.
(d). Statistical analyses
To compare focal females’ change in preference for the drabber stimulus male in experiment 1, we calculated a copying score (fraction of time spent near drab male during 2nd preference test—fraction of time spent near drab male during 1st preference test [13]) and used a general linear model (GLM) with arcsine (square root)-transformed copying scores as the dependent variable and ‘treatment’ (three levels) as a fixed factor. We included ‘population’ (two levels) as another factor, which had no significant effect. Furthermore, we initially included ‘focal female body size’ as a covariate, but removed it from the final model as it had no significant effect. To control for an effect of the different stimulus animations, we included ‘video ID’ as a random factor in population-wise GLM's, but again did not detect a significant effect (see the electronic supplementary material).
Times spent near each animation in experiment 2 were compared using paired-sample t-test. See the electronic supplementary material for a full description of the methods and the complete results.
3. Results
(a). Experiment 1: female mate choice
We compared the increase in strength of females’ preferences for the drabber male (copying score) and found a significant difference among treatments (GLM; mean square = 0.811, F2,88 = 8.308, p < 0.001). Post hoc Fisher's LSD tests uncovered a significant increase in the drabber male's attractiveness not only in the heterosexual interaction treatment compared with the control treatment (p < 0.001), but also in the homosexual interaction treatment (p = 0.002; figure 1). No statistically significant difference was detected between homosexual and heterosexual treatments (p = 0.678).
Figure 1.
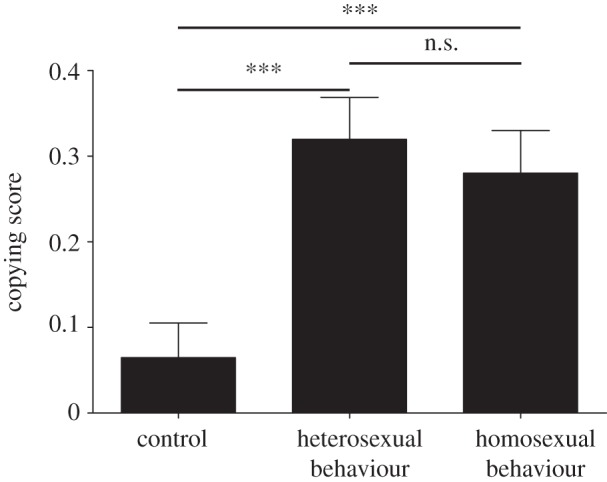
Changes in mating preferences of Atlantic molly females that had observed male hetero- and homosexual interactions. Female preferences for either of two video-animated stimulus males were re-evaluated after focal females could observe one of the following three situations: the initially non-preferred (drabber) stimulus male was (i) swimming alongside a model male (control; n = 31 trials), (ii) performing pre-copulatory nipping behaviour with a model female (heterosexual behaviour; n = 30), or (iii) performing pre-copulatory nipping behaviour with a model male (homosexual behaviour; n = 31). Positive copying scores indicate that focal females increased their preferences for previously non-preferred males. ***p < 0.001. Data are mean ± s.e.m.
(b). Experiment 2: discrimination between female and male video animations
Females in experiment 2 spent 266.1 ± 29.7 s near the animated female shoal and 151.8 ± 23.6 s near the shoal consisting of males; this difference was statistically significant (t-test: t16 = 2.622, p = 0.019), indicating females’ ability to discriminate between male and female animated images.
4. Discussion
The results from our first experiment show that P. mexicana females increase their preference for initially non-preferred males not only after observing those males interacting sexually with females, but also when having observed them initiating homosexual behaviour. Our second experiment corroborates the tendency of P. mexicana females to form same-sex shoals and confirms that focal females in our first experiment were able to distinguish between male and female video animations, i.e. between hetero- and homosexual situations.
Female mate choice copying—defined as ‘a female … mating with the same male as [an] observed female did before’ [18]—is found in a wide range of species [9], so mating with initiators of male homosexual interactions could also be widespread. As homosexual behaviour is regularly seen in small P. mexicana males [14], we speculate that it might represent an alternative mating tactic used by subordinate, and thus, less attractive males to overcome reproductive constraints owing to the intrinsic female mate preference for large, colourful and dominant males [15]. One could argue that small males initiating homosexual behaviour would face harsh aggressive responses by the male they approach, especially when approaching a larger male. However, the intensity of male aggressive interactions in P. mexicana is highest in combats between two large (dominant) males and is considerably lower in small males. Also fight intensity decreases with increasing body size difference of the opponents [19]. An alternative explanation of our results could be that females exhibit a general preference for increased male activity levels even in a non-sexual context. However, because all males moved with roughly comparable swimming velocity in all treatments and differed only in whether or not they would approach a male or a female, this explanation seems rather unlikely.
Exclusive male homosexuality is thus far only found in humans, sheep [20] and some bird species [3,21], but rates of bisexual male mating behaviour are moderate to high in numerous group-living species, including humans [4], and we suggest that increased female preferences for males showing homosexual interactions may possibly be an intriguing mechanism explaining the adaptive significance of male bisexual behaviour in at least some of those species.
Acknowledgements
The experiments reported here comply with the current laws of Germany and were approved by Regierungspräsidium Darmstadt (V-54-19c-20/15-F104/Anz.18).
H. Geupel kindly helped with animal care. R. Riesch and Y. Moodley kindly commented on a previous version; two anonymous reviewers provided very helpful suggestions. Financial support came from the German Research Foundation (DFG; PL 470/3–1).
References
- 1.Bailey NW, Zuk M. 2009. Same-sex sexual behavior and evolution. Trends Ecol. Evol. 24, 439–446 10.1016/j.tree.2009.03.014 (doi:10.1016/j.tree.2009.03.014) [DOI] [PubMed] [Google Scholar]
- 2.Bagemihl B. 1999. Biological exuberance: animal homosexuality and natural diversity. London, UK: Profile Books [Google Scholar]
- 3.Sommer V, Vasey PL. 2006. Homosexual behaviour in animals, an evolutionary perspective. Cambridge, UK: Cambridge University Press [Google Scholar]
- 4.Poiani A. 2010. Animal homosexuality: a biosocial perspective. Cambridge, UK: Cambridge University Press [Google Scholar]
- 5.Westneat DF, Walters A, McCarthy TM, Hatch MI, Hein W. 2000. Alternative mechanisms of nonindependent mate choice. Anim. Behav. 59, 467–476 10.1006/anbe.1999.1341 (doi:10.1006/anbe.1999.1341) [DOI] [PubMed] [Google Scholar]
- 6.Danchin É, Giraldeau L-A, Valone TJ, Wagner RH. 2004. Public information: from nosy neighbors to cultural evolution. Science 305, 487–491 10.1126/science.1098254 (doi:10.1126/science.1098254) [DOI] [PubMed] [Google Scholar]
- 7.Schlupp I, Marler C, Ryan M. 1994. Benefit to male sailfin mollies of mating with heterospecific females. Science 263, 373–374 10.1126/science.8278809 (doi:10.1126/science.8278809) [DOI] [PubMed] [Google Scholar]
- 8.Gibson RM, Höglund J. 1992. Copying and sexual selection. Trends Ecol. Evol. 7, 229–232 10.1016/0169-5347(92)90050-L (doi:10.1016/0169-5347(92)90050-L) [DOI] [PubMed] [Google Scholar]
- 9.Dugatkin LA. 1996. Copying and mate choice. In Social learning in animals: the roots of culture (eds Heyes CM, Galef BGJ.), pp. 85–105 New York, NY: Academic Press [Google Scholar]
- 10.Watson PJ, Arnqvist G, Stallmann RR. 1998. Sexual conflict and the energetic costs of mating and mate choice in water striders. Am. Nat. 151, 46–58 10.1086/286101 (doi:10.1086/286101) [DOI] [PubMed] [Google Scholar]
- 11.Tortosa FS, Redondo T. 1992. Frequent copulations despite low sperm competition in white storks (Ciconia ciconia). Behaviour 121, 288–314 10.1163/156853992X00408 (doi:10.1163/156853992X00408) [DOI] [Google Scholar]
- 12.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 13.Heubel KU, Hornhardt K, Ollmann T, Parzefall J, Ryan MJ, Schlupp I. 2008. Geographic variation in female mate-copying in the species complex of a unisexual fish, Poecilia formosa. Behaviour 145, 1041. 10.1163/156853908784474533 (doi:10.1163/156853908784474533) [DOI] [Google Scholar]
- 14.Tobler M, Wiedemann K, Plath M. 2005. Homosexual behaviour in a cavernicolous fish, Poecilia mexicana (Poeciliidae, Teleostei). Zeitschrift für Fischkunde 7, 95–99 [Google Scholar]
- 15.Bierbach D, et al. 2011. Predator-induced changes of female mating preferences: innate and experiential effects. BMC Evol. Biol. 11, 190. 10.1186/1471-2148-11-190 (doi:10.1186/1471-2148-11-190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tobler M, Palacios M, Chapman LJ, Mitrofanov I, Bierbach D, Plath M, Arias-Rodriguez L, de Leon FJ, Mateos M. 2011. Evolution in extreme environments: replicated phenotypic differentiation in livebearing fish inhabiting sulfidic springs. Evolution 65, 2213–2228 10.1111/j.1558-5646.2011.01298.x (doi:10.1111/j.1558-5646.2011.01298.x) [DOI] [PubMed] [Google Scholar]
- 17.Parzefall J. 1969. Zur vergleichenden Ethologie verschiedener Mollienesia-Arten einschließlich einer Höhlenform von Mollienesia sphenops. Behaviour 33, 1–38 10.1163/156853969X00297 (doi:10.1163/156853969X00297) [DOI] [PubMed] [Google Scholar]
- 18.Witte K, Massmann R. 2003. Female sailfin mollies, Poecilia latipinna, remember males and copy the choice of others after 1 day. Anim. Behav. 65, 1151–1159 10.1006/anbe.2003.2160 (doi:10.1006/anbe.2003.2160) [DOI] [Google Scholar]
- 19.Bierbach D, Klein M, Sassmannshausen V, Schlupp I, Riesch R, Parzefall J, Plath M. 2012. Divergent evolution of male aggressive behaviour: another reproductive isolation mechanism in extremophile poeciliid fishes. Int. J. Evol. Biol. 2012 (ID 148745). 10.1155/2012/148745 (doi:10.1155/2012/148745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price EO, Katz LS, Wallach SJR, Zenchak JJ. 1988. The relationship of male-male mounting to the sexual preferences of young rams. Appl. Anim. Behav. Sci. 21, 347–352 10.1016/0168-1591(88)90069-X (doi:10.1016/0168-1591(88)90069-X) [DOI] [Google Scholar]
- 21.Elie J, Mathevon N, Vignal C. 2011. Same-sex pair-bonds are equivalent to male–female bonds in a life-long socially monogamous songbird. Behav. Ecol. Sociobiol. 65, 2197–2208 10.1007/s00265-011-1228-9 (doi:10.1007/s00265-011-1228-9) [DOI] [Google Scholar]


