Sialidase inhibitors preserve an inhibitory circuit that reduces the severity of sepsis
Bacterial sepsis is a major cause of mortality of hospitalized patients, accounting for over 200,000 deaths per year in the United states alone1. A major factor in the morbidity of this condition stems from the over-reaction of the immune system to the bacteria, causing massive edema and harm to vital tissues. Using a model of intestinal sepsis, Chen et al.2 show in this issue that bacteria secrete an enzyme, sialidase, which exacerbates the inflammatory response of the immune system by disrupting a dendritic cell (DC) inhibitory circuit designed to keep immune responses in check when ‘self’ cells are damaged. The bacterial sialidase cleaves sialic acid from the glycoprotein ligand of a sialic acid binding immunoglobulin lectin (Siglec) inhibitory receptor, Siglec-G/10, abrogating its ability to dampen an immune response. Blocking the action of the sialidase with small molecule inhibitors preserves Siglec-G/10 ligands, resulting in a reduction in the inflammatory response and resulting morbidity. The results suggest that sialidase inhibitors have the potential for treatment of severe bacterial sepsis.
Although the sequelae of bacterial sepsis and septic shock are complex, the excessive mortality of this condition has lead to intense investigations into the virulence factors of the bacterial pathogens. Virulence factors identified to date include bacterial components, collectively called pathogen associated molecular patterns (PAMPs), which directly activate inflammatory responses through toll-like receptors (TLRs)3. A hallmark of the activation of TLRs is the production of inflammatory cytokines such as IL-6 and TNF, which act locally, but are released systemically producing a cascade of inflammatory responses, damaging normal tissues. Accumulating evidence suggests that danger-associated molecular patterns (DAMP)s released from damaged host cells also activate TLRs and contribute to the magnitude of the inflammatory insult and severity of septic disease3.
An important aspect of immune homeostasis is the discrimination of self and non-self, allowing activation of immune cells to combat pathogens while preventing inadvertent activation against self. In a previous report4, the authors demonstrated the existence of an inhibitory circuit that mediated suppression of TLR signaling by ‘self’ DAMPs such as high mobility box 1 (HMGB1), an intracellular DNA binding protein released from necrotic cells. HMGB1 was shown to bind to CD24, a membrane glycoprotein on dendritic cells (DCs), which in turn is bound by the inhibitory receptor Siglec-G/10 cell on the same cell. This ternary complex was shown to dampen TLR signaling induced by HMGB1. The importance of this inhibitory circuit in sepsis is documented by Chen et al. in this issue2. Indeed, mice deficient in either Siglec-G/10 or CD24 exhibit dramatically increased mortality and production of inflammatory cytokines.
The inhibitory dendritic cell receptor Siglec-10 and its murine ortholog Siglec-G are members of the siglec family, which recognize sialic acid containing glycans as ligands. Of the 14 human siglecs identified to date, 12 are primarily expressed on white blood cells that constitute the immune system5. They are increasingly recognized for their roles in aiding the immune system from distinguishing self and non-self through the recognition of self-glycans as ligands5–7. Many of the siglecs, like Siglec-G/10, are inhibitory co-receptors that contain cell activation via immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic tail, and dampen signaling from activating receptors such as the B cell receptor and TLRs4, 5, 8, 9.
Siglec-G/10 is expressed primarily on B cells, where it has been implicated in tolerizing B cells to self-antigens5, 7, 8, but is also expressed on macrophages and DCs2, 4. Chen et al. provide evidence that the induced inhibitory circuit mediated by Siglec-G on DCs involves recognition of sialylated glycans on CD24 (Fig. 1). To confirm that the inhibitory effects of Siglec-G in sepsis were mediated by DCs, Chen et al. produced a transgenic mouse expressing CD24 under a DC specific promoter. Relative to the CD24 null mice, the transgenic mice with CD24 expressed only in DCs produced lower levels of cytokines and exhibited reduced mortality in the intestinal sepsis model. Still an open question is how the inhibitory signal created by DAMP engagement of CD24/Siglec-G can suppress DAMP mediated signaling from TLRs.
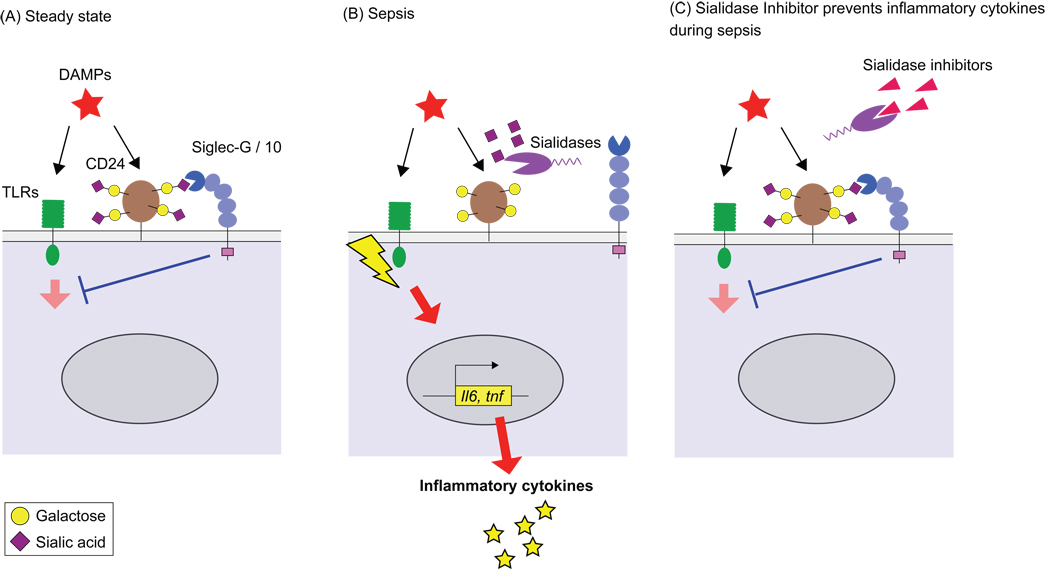
Figure 1.
Sialidase disrupts the Siglec-G inhibitory circuit that suppresses TLR signaling by DAMPs. (A). DAMPs induce a negative inhibition of TLR signaling by binding to a CD24 bound to Siglec-G/10 via recognition of sialic acids on its glycan chains. (B) Bacterial sialidases cleave sialic acids on CD24 disrupting the CD24/Siglec-G/10 inhibitory circuit, leading to enhanced cytokine production. (C) Sialidase inhibitors block the desialylation of CD24, preserving the CD24/Siglec-G/10 inhibitory circuit, and dampening the inflammatory response.
The importance of this inhibitory circuit in intestinal sepsis suggested the possibility that sialidases produced by bacteria may be exacerbating the inflammatory response in wild type mice by disrupting the ternary complex of HMGB1/CD24/Sigec-G/10 by cleaving sialic acids from CD24 required for recognition by Siglec-G/10 (Fig. 1). Indeed, bacterial sialidases were found in blood of mice following intestinal puncture, resulting in loss of cell surface sialic acids from DCs. In vitro sialidase treatment of wild type DCs exhibited amplified cytokine production in response to HMGB1, but had no effect on the elevated levels of cytokines produced by DCs from CD24 or Siglce-G null mice. Moreover sepsis induced by administration of the sialidase producing S. pneumoniae produced a more profound pathogenicity than a mutant missing it’s two neuraminidase genes (NanA/NanB).
These findings motivated Liu and coworkers to assess the potential of sialidase inhibitors as a way to preserve the CD24/Siglec-G/10 inhibitory circuit, and reduce the exacerbated response produced by sialidase. Two sialidase inhibitors, 2,3-dehydro-2-deoxy-N-acetylneuraminic acid and 2,3-dehydro-2-deoxy-N-glycolylneuraminic acid, protected the wild type mice from the experimental sepsis by cecal ligation and puncture, but not either Cd24−/− nor siglecg−/− mice. The results suggest that sialidase inhibitors should be further investigated as a tool to reduce the virulence and morbidity of sepsis involving bacterial pathogens that produce sialidases, such as Streptoccocus pneumoniae, or polybacterial infections such as produced from punctured intestine.
In this regard, it is noteworthy that Grewel PK et. al. have studied the contribution of another glycan receptor that is protective in sepsis with S. pneumoniae10. They found that platelets are especially prone to loss of sialic acid by sialidase, resulting in clearance of the asialo-platelets by the asialo-glycoprotein receptor (ASGR) of liver hepatocytes. ASGR null mice exhibit more profound mortality as a result of increased levels of asialo-platelets and increased coagulopathy. In contrast to the results of Chen et al., they found that a sialidase deficient strain of S. pneumoniae produced increased mortality in wild type mice. The apparently contradictory results may reflect different levels of sialidase that impact the activities of two different receptor systems, one that clears asialo platelets and reduces coagulation, and the other that is required for dampening the inflammatory response, but is destroyed by sialidase.
Sialidase inhibitors are not new to the pharmaceutical industry, since the influenza virus medicines, Tamiflu™ and Relenza™ are well known to act by inhibiting the action of the influenza virus sialidase, required for spreading of the virus from infected cells. The results of Chen et al. may stimulate further investigations to broaden the utility of this class of enzyme inhibitors by expanding the toolbox for treatment of bacterial sepsis.
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Guo-Yun Chen XC, King Samantha, Cavassani KarenA, Cheng Jiansong, Zheng Xincheng, Cao Hongzhi, Yu Hai, Qu Jingyao, Fang Dexing, Wu Wei, Bai Xue-Feng, Liu Jin-Qing, Woodiga ShireenA, Chen Chong, Sun Lei, Hogaboam CoryM, Kunkel StevenL, Zheng Pan, Liu Yang. Disruption of Sialic acid-dependent CD24-Siglec G Interaction Exacerbates polybacterial Sepsis. Nature Biotechnology. 2011 doi: 10.1038/nbt.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 6.Carlin AF, et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duong BH, et al. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J Exp Med. 2010;207:173–187. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jellusova J, Wellmann U, Amann K, Winkler TH, Nitschke L. CD22 x Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity. J Immunol. 2010;184:3618–3627. doi: 10.4049/jimmunol.0902711. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki N, Rademacher C, Paulson JC. CD22 Regulates Adaptive and Innate Immune Responses of B Cells. J Innate Immun. doi: 10.1159/000322375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grewal PK, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14:648–655. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]