Abstract
Objective
Stroke often produces marked physical and cognitive impairments leading to functional dependence, caregiver burden, and poor quality of life. We examined the course of disability during a 1-year follow-up period after stroke among patients who were administered antidepressants for 3 months compared to patients given placebo for 3 months.
Methods
A total of 83 patients entered a double-blind randomized study of the efficacy of antidepressants to treat depressive disorders and reduce disability after stroke. Patients were assigned to either fluoxetine (N = 32), nortriptyline (N = 22) or placebo (N = 29). Psychiatric assessment included administration of the Present State Examination modified to identify DSM-IV symptoms of depression. The severity of depression was measured using the 17-item Hamilton Depression Rating Scale. The modified Rankin Scale was used to evaluate the disability of patients at initial evaluation and at quarterly follow-up visits for 1 year. Impairment in activities of daily living was assessed by Functional Independence Measure at the same time.
Results
During the 1-year follow-up period, and after adjusting for critical confounders including age, intensity of rehabilitation therapy, baseline stroke severity, and baseline Hamilton Depression Rating Scale, patients who received fluoxetine or nortriptyline had significantly greater improvement in modified Rankin Scale scores compared to patients who received placebo (t [156] = − 3.17, p = 0.002).
Conclusions
Patients treated with antidepressants had better recovery from disability by 1-year post stroke (i.e., 9 months after antidepressants were stopped) than patients who did not receive antidepressant therapy. This effect was independent of depression suggesting that antidepressants may facilitate the neural mechanisms of recovery in patients with stroke.
Keywords: antidepressants, disability, recovery, stroke
Stroke often produces marked physical impairment leading to dependence in activities of daily living (ADL), caregiver burden, and decreased quality of life. The effect of antidepressants on recovery in ADL after stroke has been controversial.1–3 Palomaki et al.4 performed a randomized controlled study in 100 stroke patients given mianserin (60 mg/d) or placebo for 1 year. Mianserin treatment did not influence functional outcome as measured by Rankin Scale or Barthel Index. When we compared 10 patients with poststroke depression in remission following antidepressant treatment with 10 patients matched for severity of initial impairment and poststroke depression who failed to respond to antidepressants, we found that remission of poststroke depression was associated with greater ADL recovery than nonremission.5 Acler et al.6 reported that 10 nondepressed patients after stroke treated with 10 mg/d of citalopram showed significantly greater recovery over 1 month as measured by the National Institute of Health Stroke Scale compared with similar patients given placebo.
Because of the potential benefit of a 3-month course of antidepressant medication on poststroke depression and recovery in ADLs, we assessed in a group of 56 depressed and 48 nondepressed patients for severity of disability using the modified Rankin Scale (mRS) during 1 year, in a randomized, three-arm, double-blind trial over 3 months, utilizing fluoxetine, nortriptyline, and placebo. We included nondepressed patients to assess whether antidepressants would augment stroke recovery independent of depression. We have described the effect of treatment on depression in a prior publication.7 In the present analysis, we hypothesized that recovery from disability would be greater at 1-year follow-up among patients administered either fluoxetine or nortriptyline independent of depression compared to patients given placebo. Physical recovery is influenced by many variables, including severity of and type of stroke, specialized acute care, amount of rehabilitation therapy, and depression. We tried to assess all of these factors and determine whether there was an independent effect of antidepressant medications on stroke recovery.
METHODS
Patient Selection
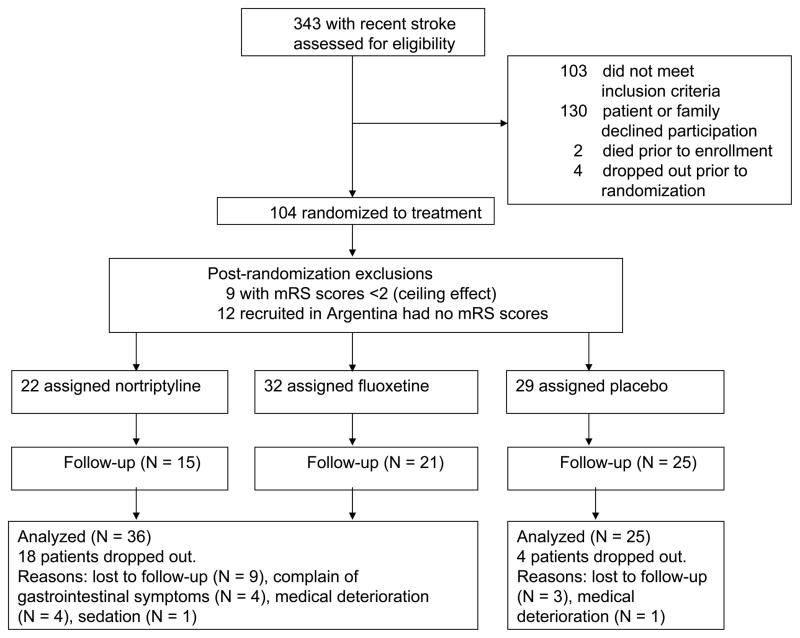
A total of 343 patients between 18 and 85 years, who had a stroke in the previous 6 months, were screened for participation in a study of antidepressant therapy. The protocols were approved by the institutional review boards and written informed consent was obtained from each subject. In addition, we required that the subject’s immediate family and treating physician also agreed to the subject’s participation. Exclusion criteria included: (1) any other significant medical illness that would threaten the patient’s life or recovery from stroke; (2) severe comprehension deficit that precluded a verbal interview (defined as making an error on part 1 of the Token Test);8 (3) prior history of other brain diseases, with the exception of prior stroke. The flow chart of enrollment is shown in Figure 1. Of these patients, 56 depressed and 48 nondepressed enrollees were recruited. The sites of enrollment were Younkers Rehabilitation of Iowa Methodist Medical Center in Des Moines, Iowa (N = 89), the Raul Carrea Institute of Argentina (N = 12), the neurologic service at the University of Iowa Hospitals and Clinics in Iowa City (N = 1) and the VA Medical Center in Iowa City (N = 2). Among the 104 patients who were randomized to treatment, nine patients were excluded from this analysis because of the “ceiling effect” of having an initial score of zero or one and the 12 patients from Argentina were excluded because they had no mRS assessments. Thus, the data from 83 subjects are included in this analysis (Figure 1).
FIGURE 1.
A Schematic Display of the Flow of Patients included in This Study
Treatment Protocol
Random assignment to active treatment with either fluoxetine or nortriptyline or placebo was made independent of whether they were depressed or nondepressed. A small number of patients had a specific contraindication to one of the drugs. For example, fluoxetine was contraindicated in patients who had an intracerebral hemorrhage. Among the 54 patients randomized to active treatment, six had a contraindication to fluoxetine.
Forty-six subjects had baseline evidence of depression; 19 subjects were assigned to treatment with fluoxetine (mean HDRS score = 20.4 ± 4.7), 10 to treatment with nortriptyline (mean HDRS score = 22.5 ± 8.5) and 17 to placebo (mean HDRS score = 17.5 ± 6.2). Thirty-seven nondepressed subjects were also randomized, 13 were assigned to fluoxetine (mean HDRS score = 6.7 ± 3.7), 12 to nortriptyline (mean HDRS score = 5.3 ± 3.2), and 12 to placebo (mean HDRS score = 6.0 ± 3.2). All patients were seen at enrollment and at 3, 6, 9, and 12 weeks after beginning the medication and at 6, 9, and 12 months follow-up. The doses of nortriptyline were 25 mg/d for the first week, 50 mg/d for weeks 2–3, 75 mg/d for weeks 3–6, and 100 mg/d for the final 6 weeks. Doses of fluoxetine were 10 mg/d for the first 3 weeks, 20 mg/d for weeks 4–6, 30 mg/d for weeks 7–9, and 40 mg/d for the final 3 weeks. Blood levels of nortriptyline but not fluoxetine were obtained at the 9 week follow-up (dose of nortriptyline 100mg/d). To maintain the blind, blood was drawn at week 9 for all subjects. Doses were decreased if severe side effects developed or blood levels were above the therapeutic range. This occurred in nine subjects. Of these, five were treated with nortriptyline (two subjects had blood levels above the therapeutic range, two experienced sedation, and one experienced gastrointestinal symptoms) and four were treated with fluoxetine (one subject had severe anxiety, two had severe gastrointestinal symptoms, and one had insomnia). To maintain the double-blind, doses were decreased for equal numbers of subjects receiving placebo by the investigator who was not blind to assignment.
In the 9-month follow-up phase (i.e., after completing 3 months of treatment), some patients were given antidepressants by treating physicians. The type, dose, and duration of all prescribed medications as well as medical events were carefully recorded.
Assessment
Subjects were asked about background characteristics and coexistent medical conditions at the initial evaluation. They were also weighed at the beginning and end of the 12-week treatment trial, and their blood pressure and pulse were taken at each 3-week follow-up visit. The follow-up visits were conducted in the treating hospital or, most often, in the subject’s home or long-term care facility. Depression was defined as meeting DSM-IV criteria for major depressive disorder or minor depressive disorder following the structured mental status examination of the Present State Exam (PSE)9 with HDRS score of 12 or greater. We used these criteria in our prior studies.10 The severity of depression was measured using the 17-item HDRS.11 The use of these instruments in stroke patients has been validated in other studies.12–14
The mRS15,16 was used to evaluate the disability of patients at the initial evaluation and at 3 month follow-up visits throughout the 1 year. The mRS is a seven level scale with 0 = no symptoms, 1 = symptoms but no disability for daily function, 2 = slight disability unable to carry out previous activities, but can function without assistance, 3 = moderate disability, requires some help but can walk without assistance, 4 = moderately severe, unable to walk without assistance and needs assistance for bodily care, 5 = severe disability, bedridden requiring constant nursing care, 6 = dead. Impairment in ADL was assessed using Functional Independence Measure (FIM),17 an 18-item, 72-point scale with higher numbers indicating less impairment. A neurologist assessed the severity of stroke using the National Institute of Health Stroke Scale (NIHSS).18 The intensity of rehabilitation care was evaluated using total hours of physical rehabilitation per week, summed over initial, 3, 6, 9, and 12-month visits when the mRS and FIM were measured. This included only physical therapy performed by specialists. It did not include rehabilitation performed by subjects or their caregivers. All assessments were conducted by examiners who were blind to the subject’s group allocation.
Imaging
Computerized tomography or magnetic resonance scans were obtained from the treating acute hospital for each of the patients in the study to assess location, type, and mechanism of stroke.
Statistical Analysis
Continuous variables were presented as means and standard deviations, or median and interquartile range. Categorical variables were compared using Fisher’s exact test, and continuous variables were compared using the Mann–Whitney U test. To evaluate the treatment effect over time while adjusting for other covariates, a mixed model analysis with an unstructured correlation for the repeated measures was used. MRS scores and FIM scores were assumed to follow a normal distribution. Group indicator (treatment versus control), time points (0, 3, 6, 9, 12-months; treated as a continuous measure), and the interaction between group and time were included in the model. Time variable was considered as continuous variable. Covariates included age, total hours of physical rehabilitation, baseline NIHSS score, and baseline HDRS score.
Although parametric approaches, such as mixed models are commonly used to assess change in psychiatric symptoms with repeated measures over time, some measurements of psychiatric symptoms such as mRS do not fit standard parametric methods because the scale values do not represent equal intervals. As an alternative statistical approach, Arndt et al.19 suggested a nonparametric approach using Kendall’s tau-b (τb) which performs well as a measure of the patient’s symptom course during a longitudinal study. The Kendall’s tau-b correlation coefficients between mRS scores and time (0, 3, 6, 9, 12-months) for active and placebo-treated patients were calculated. An ANCOVA using ranks of Kendall’s τb coefficients were compared between active and placebo as a sensitivity analysis. Covariates included age, total hours of physical rehabilitation, baseline NIHSS score, and baseline HDRS score. P values less than 0.05 were considered statistically significant. All statistical analyses were performed using SAS 9.2 for Windows (SAS Institute Inc, Cary, NC).
RESULTS
Participants
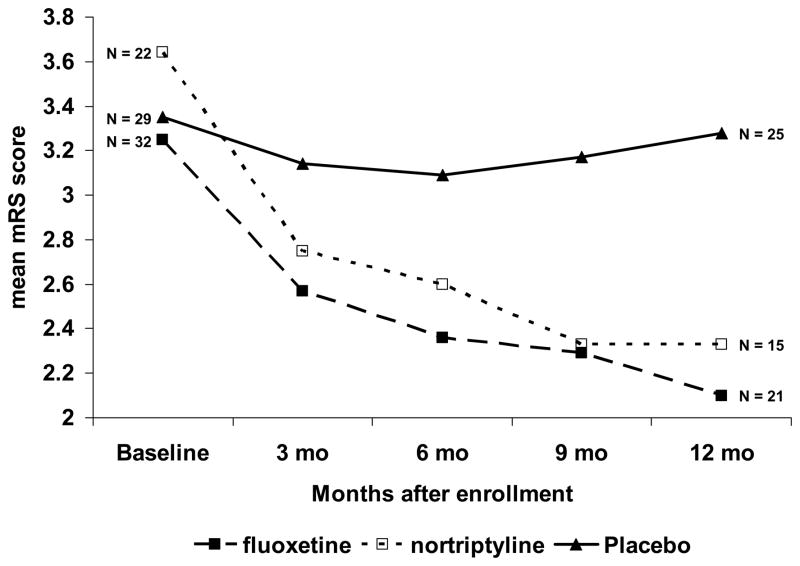
We compared the background characteristics of the patient treated with fluoxetine (N = 32) and those treated with nortriptyline (N = 22) and found no significant differences except there were significantly fewer women in the fluoxetine compared to the nortriptyline group (Fisher’s exact test, p = 0.04). Furthermore, mixed model analysis was performed on the mRS of the nortriptyline and fluoxetine groups controlling for age, total hours of physical rehabilitation, baseline NIHSS score, and baseline HDRS score and there were no significant intergroup differences (time by treatment t [90] = −1.06, p = 0.291, Figure 2). Therefore, to increase the power of our analysis, we combined the nortriptyline and fluoxetine subjects into a single active treatment group.
FIGURE 2.
Change in modified Rankin scores over 1 year following a recent stroke. Patients with or without initial depression were treated double blind from baseline to 3 months with fluoxetine (10–40 mg/d) or nortriptyline (25–100 mg/d), or placebo. Patients were followed at 6, 9 and, 12 months after treatment. Mixed model analysis showed a significantly better recovery in the patients given either fluoxetine (t [119] = 2.14, p = 0.035) or nortriptyline (t [105] = 2.91, p = 0.004), compared with placebo treatment. Note that the recovery in patients given fluoxetine or nortriptyline continued throughout the 12 months, although treatment was stopped at 3 months.
The demographic characteristics and stroke characteristics for both the fluoxetine/nortriptyline and placebo groups are shown on Table 1. Subjects who received either fluoxetine or nortriptyline were younger than those in the placebo group and physical rehabilitation time at baseline and more than 12-months were lower in the treatment group compared to the placebo group (Table 1). Otherwise, there were no significant differences between the active and placebo groups. MRS scores and FIM scores at baseline were not significantly different between the two groups (Table 1).
TABLE 1.
Demographic characteristics of the treatment and placebo groups
| Treatment (N = 54) | Placebo (N = 29) | Test | p | |
|---|---|---|---|---|
| Number (%) | ||||
| Age, years, mean (SD) | 65.7 (12.4) | 72.5 (9.4) | MWU (Z = −2.39) | 0.017 |
| Men | 34 (63.0) | 17 (58.6) | Fisher | 0.814 |
| White | 52 (96.3) | 26 (89.7) | Fisher | 1.0 |
| Married | 36 (66.7) | 15 (51.7) | Fisher | 0.238 |
| Education, years, mean (SD) | 13.8 (2.8) | 12.6 (3.7) | MWU (Z = −1.45) | 0.147 |
| Hollingshead class IV, V | 23 (42.6) | 11 (37.9) | Fisher | 0.816 |
| Personal history of depression | 7 (13.0) | 3 (10.3) | Fisher | 1.0 |
| Family history of depression | 6 (11.1) | 3 (10.3) | Fisher | 1.0 |
| DSM-IV major or minor depression | 29 (53.7) | 17 (58.6) | Fisher | 0.817 |
| Inpatients at enrollment | 44 (81.5) | 28 (96.6) | Fisher | 0.087 |
| Days since stroke, median (interquartile range) | 32.5 (22.0–59.5) | 34.0 (20.5–43.5) | MWU (Z = −0.81) | 0.416 |
| Baseline HDRS score, mean (SD) | 15.3 (8.2) | 12.3 (6.2) | MWU (Z = −1.42) | 0.156 |
| Baseline physical rehabilitation hours, mean (SD) | 6.46 (4.6) | 9.36 (2.8) | MWU (Z = −2.68) | 0.007 |
| Total physical rehabilitation hours, mean (SD) | 8.29 (9.0) | 11.03 (5.1) | MWU (Z = −2.31) | 0.021 |
| Baseline mRS score, mean (SD) | 3.4 (0.8) | 3.3 (0.7) | MWU (Z = −0.50) | 0.617 |
| Baseline FIM score, mean (SD) | 52.6 (13.1) | 50.1 (7.7) | MWU (Z = −1.55) | 0.122 |
| Stroke Characteristics | ||||
| Location—Left hemisphere | 14 (25.9) | 10 (34.5) | Fisher | 0.453 |
| Type —Hemorrhage | 8 (14.8) | 1 (3.4) | Fisher | 0.151 |
| Infarction | 46 (85.2) | 28 (96.6) | ||
| Mechanism of infarction—Large artery | 23 (42.6) | 11 (37.9) | Fisher | 0.317 |
| Small artery | 15 (27.8) | 14 (48.3) | ||
| Cardioembolism/other | 8 (14.8) | 3 (10.3) | ||
| Baseline NIHSS score, mean (SD) | 5.4 (4.4) | 7.1 (4.9) | MWU (Z = −1.68) | 0.094 |
Abbreviations: NIHSS: National Institute of Health Stroke Scale; HDRS: Hamilton Depression Rating Scale; mRS: modified Rankin Scale; FIM: Functional Independence Measure; MWU: Mann-Whitney U test; Fisher: Fisher’s exact test
After completing 3 months of treatment, 17 subjects were continued on antidepressants by treating physicians (usually 3 months more) in the treatment group. There was one subject in whom continued use of antidepressants was unknown in the treatment group. We therefore examined the effect of continued treatment (i.e., 17 patients) compared with those given only 3 months of treatment (i.e., 54 total minus 17 continued minus 1 unknown = 36) among the treatment group. Mixed model analysis was performed using the same covariates as Table 2. There was no significant interaction between time and group (i.e., continuation antidepressants versus no continuation therapy) in mRS scores over the 12 months of observation (t [87] = 0.96, p = 0.340). Also, there was no significant interaction effect between time and presence or absence of continuation therapy in FIM scores (t [88] = −0.78, p = 0.440). Because the recovery of patients given 3 months versus about 6 months of antidepressants was not significantly different, for our further analysis, we combined subjects who received antidepressants for 3 months with those who received about 6 months of treatment.
TABLE 2.
Mixed model analysis of longitudinal data (outcome variable: modified Rankin Scale)
| Variable | Slope | Standard Error | DF | t | p |
|---|---|---|---|---|---|
| Intercept | 1.354 | 0.563 | 66 | 2.41 | 0.019 |
| Treatment | 0.137 | 0.195 | 156 | 0.70 | 0.486 |
| Time | −0.020 | 0.018 | 55 | −1.09 | 0.279 |
| Treatment × Time | −0.076 | 0.024 | 156 | −3.17 | 0.002 |
| Age | 0.019 | 0.008 | 156 | 2.55 | 0.012 |
| Total hours of physical rehabilitation | −0.004 | 0.015 | 156 | −0.24 | 0.808 |
| Baseline NIHSS score | 0.085 | 0.019 | 156 | 4.51 | <.0001 |
| Baseline HDRS score | 0.002 | 0.011 | 156 | 0.15 | 0.881 |
Abbreviations: NIHSS: National Institute of Health Stroke Scale; HDRS: Hamilton Depression Rating Scale
Course of Disability Measures
The course of change in mRS more than 1 year was compared between active and placebo treatment using linear mixed model controlling for age, total hours of physical rehabilitation, baseline NIHSS score, and baseline HDRS score. A statistically significant interaction between time and placebo versus active treatment was found (Table 2). Subjects in the active treatment group gradually improved their mRS scores over time whereas mRS scores in the placebo group were little changed during the same period (Figure 2). We also examined the separate effects of fluoxetine and nortriptyline versus placebo. Controlling for age, total hours of physical rehabilitation, baseline NIHSS score, and baseline HDRS score, fluoxetine versus placebo showed a significant time by treatment interaction as did nortriptyline versus placebo (Figure 2). Thus, both fluoxetine and nortriptyline were associated with recovery as measured by mRS. In addition to antidepressant use, both age and baseline NIHSS score were also independent factors associated with change in mRS.
Because there were nine subjects with hemorrhagic stroke, we repeated the time by treatment analysis excluding these nine patients. There continued to be a significant time by treatment interaction on mRS score over 1 year among patients with ischemic stroke (t [145] = −2.92, p = 0.004).
To conduct a nonparametric sensitivity analysis, Kendall’s τb correlation between time and mRS scores were obtained for each subject. Among 83 patients, 18 patients had only baseline measures so their Kendall’s τb coefficients were not obtained. The mean τb correlation coefficient (SD) in the active (N = 40) and placebo (N = 25) groups were −0.600 (0.417) and −0.231 (0.611), respectively. A negative τb indicates that mRS scores decreased over time. We then used rank-transformed τb as the outcome variable in an ANCOVA. Covariates were group indicator, age, total hours of physical rehabilitation, baseline NIHSS score, and baseline HDRS score. Model results are shown in Table 3. The effect of treatment on outcome using this nonparametric analysis was almost identical to our finding using the mixed model for longitudinal mRS data, although, in this model, age, and baseline NIHSS score were not significant.
TABLE 3.
Sensitivity analysis using non-parametric approach
| Variable | Mean square | F (1,51) | p |
|---|---|---|---|
| Treatment | 2070.65 | 7.46 | 0.009 |
| Age | 703.73 | 2.53 | 0.118 |
| Total hours of physical rehabilitation | 385.69 | 1.39 | 0.244 |
| Baseline NIHSS score | 53.24 | 0.19 | 0.663 |
| Baseline HDRS score | 179.73 | 0.65 | 0.425 |
Abbreviations: NIHSS: National Institute of Health Stroke Scale; HDRS: Hamilton Depression Rating Scale
The Effect of Treatment on ADL as Measured by the FIM
Using mixed model analysis and the covariates as shown in Table 4, the longitudinal course of FIM scores were analyzed. FIM scores ranged from 13 to 71 and appeared to be normally distributed, so a nonparametric analysis was not necessary.
TABLE 4.
Mixed model analysis of longitudinal data (Outcome variable: Functional Independence Measure)
| Variable | Slope | Standard Error | DF | t | p |
|---|---|---|---|---|---|
| Intercept | 85.975 | 5.989 | 65 | 14.36 | <.0001 |
| Treatment | −3.578 | 2.104 | 153 | −1.70 | 0.091 |
| Time | 0.318 | 0.165 | 52 | 1.92 | 0.060 |
| Treatment × time | 0.366 | 0.214 | 153 | 1.71 | 0.089 |
| Age | −0.290 | 0.080 | 153 | −3.63 | 0.0004 |
| Total hours of physical rehabilitation | −0.340 | 0.154 | 153 | −2.21 | 0.029 |
| Baseline NIHSS score | −1.062 | 0.199 | 153 | −5.33 | <.0001 |
| Baseline HDRS score | −0.094 | 0.119 | 153 | −0.79 | 0.430 |
Abbreviations: NIHSS: National Institute of Health Stroke Scale; HDRS: Hamilton Depression Rating Scale
The subjects treated with fluoxetine or nortriptyline showed improvement, but the interaction between treatment and time failed to reach statistical significance after controlling for age, total hours of physical rehabilitation, baseline NIHSS score, and baseline HDRS score (Table 4). Age, total hours of physical rehabilitation and baseline NIHSS score, however, did show a significant relationship to FIM outcome.
DISCUSSION
This study found that the administration of either nortriptyline and fluoxetine for 3 months significantly reduced disability from stroke over 1 year compared with placebo, even after controlling for age, total hours of physical rehabilitation, baseline severity of stroke (using the NIHSS), and baseline HDRS score. To our knowledge, this is the first time that, independent of depression, antidepressant medication has been shown using double blind methodology to be associated with improved physical recovery from stroke over 1 year.
Before discussing these findings, the limitations imposed by the methods used in the study should be acknowledged. First, the majority of subjects were high school or college-educated, white, married, and were Hollingshead social classes I to III. The results of this study, therefore, may not be applicable to all patients with stroke. Second, 18 of 54 subjects receiving active treatment and 4 of 29 subjects receiving placebo dropped out during the 1-year study period. Although there were no statistically significant differences in background characteristics or NIHSS, or FIM scores between those that completed the study and those who prematurely stopped participation, attrition might have influenced our findings. Third, the group sizes were relatively small and this may have limited our statistical power to find some intergroup differences. To increase our statistical power for some analyses, we combined the data from the two active treatment groups. Fourth, the recruitment was done primarily at Yonkers Rehabilitation in Des Moines. Although all recruitment sites were in Iowa, this unequal recruitment may have influenced the generalizability of our findings. Finally, the level of rehabilitation care (hospital or outpatient), the type of social support and residence of each patient may have affected the mRS or FIM scores.
Despite these limitations, the study findings have some important implications. Using both parametric and nonparametric analytical techniques, both fluoxetine and nortriptyline significantly reduced disability over a 1-year period compared with the placebo group after adjusting for the major confounding variables.
Although the FIM scores went in the same direction as the mRS score, we failed to find a statistically significant effect of antidepressant treatment on recovery in ADLs. This may be explained by the fact that the mRS is a more global measure of disability, whereas the FIM assesses specific elements of daily activities or that the mRS is influenced by the demands of the environment in which the patient was residing whereas the FIM examined more specific functions.
This study raises the obvious question of why antidepressants reduced disability over the first year after stroke independent of depression. Although this question will require further research, we do know that antidepressants produce complex signaling cascades that result in increased expression of neurotrophic factors, the inhibition of inflammatory cytokines, the proliferation of neural and glial precursor cells, increased axonal sprouting and the development of new synapses.20–22 The serotonin reuptake inhibitors (SSRIs), such as fluoxetine, increase hippocampal neurogenesis probably by 5HT1A receptor mediated effects.23,24 Second, there is a large literature on the role of neuroplasticity on functional reorganization and recovery following stroke. Third, several studies have shown that both the tricyclic antidepressants and the selective serotonin reuptake inhibitor antidepressants inhibit the microglial production of proinflammatory cytokines.25–27 Thus improved recovery of some specific functions may result from inhibition of inflammatory cytokines leading to augmentation of neurogenesis and synaptic plasticity, which is ultimately manifested in physical and cognitive recovery from stroke. Although other explanations might be proposed, antidepressants appear to exert numerous effects on neuronal function not related to depression.
The major implication of this study is that elderly patients with stroke related disability may benefit from administration of antidepressants independent of depression. Larger studies aimed at examining the potential benefit of antidepressant mediations in physical recovery from stroke are clearly needed.
Acknowledgments
The authors would like to thank Stephanie Rosazza, B.A., study coordinator, and Teresa Kopel, M.A., The University of Iowa.
This work is supported in part by NIH grants R01-MH 40355 and R01-MH65134. Dr. Adams received research support from NIH-NINDS (Consultant R01NS19632, and coinvestigator, 5U01NS041895, both 2001–2009), Mayo Foundation for Medical Education/Research (Coinvestigator, 2000–2009), Boehringer–Ingelheim Pharm (Coinvestigator 2003–2009), Merck, Scherling-Plough, and MNT Medical (2003–2009). Dr. Robinson is a consultant for Avanir Pharmaceutical. Dr. Mikami was supported by a grant from Tokai University, Kanagawa, Japan. Drs. Jorge, Davis, Leira and Ms. Jang have no disclosures to report.
References
- 1.Paolucci S, Grasso MG, Antonucci G, et al. One-year follow-up in stroke patients discharged from rehabilitation hospital. Cerebrovasc Dis. 2000;10:25–32. doi: 10.1159/000016021. [DOI] [PubMed] [Google Scholar]
- 2.Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31:1829–1832. doi: 10.1161/01.str.31.8.1829. [DOI] [PubMed] [Google Scholar]
- 3.Dam M, Tonin P, De Boni A, et al. Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy [comment] Stroke. 1996;27:1211–1214. doi: 10.1161/01.str.27.7.1211. [DOI] [PubMed] [Google Scholar]
- 4.Palomaki H, Kaste M, Berg A, et al. Prevention of poststroke depression: 1 year randomised placebo controlled double blind trial of mainserin with 6 month follow up after therapy. J Neurol Neurosurg Psychiatry. 1999;66:490–494. doi: 10.1136/jnnp.66.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chemerinski E, Robinson RG, Arndt S, et al. The effect of remission of poststroke depression on activities of daily living in a double-blind randomized treatment study. J Nerv Ment Dis. 2001;189:421–425. doi: 10.1097/00005053-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Acler M, Robol E, Fiaschi A, et al. A double blind placebo RCT to investigate the effects of serotonergic modulation on brain excitability and motor recovery in stroke patients. J Neurol. 2009;256:1152–1158. doi: 10.1007/s00415-009-5093-7. [DOI] [PubMed] [Google Scholar]
- 7.Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short term recovery after stroke: a placebo controlled, double-blind study. Am J Psychiatry. 2000;157:351–359. doi: 10.1176/appi.ajp.157.3.351. [DOI] [PubMed] [Google Scholar]
- 8.De Renzi E, Faglioni P. Normative data and screening power of a shortened version of the Token Test. Cortex. 1978;14:41–49. doi: 10.1016/s0010-9452(78)80006-9. [DOI] [PubMed] [Google Scholar]
- 9.Wing JK, Cooper JE, Sartorius N. The Measurement and Classification of Psychiatric Symptoms: An Instructional Manual for the PSE and CATEGO Programs. New York: Cambridge University Press; 1974. [Google Scholar]
- 10.Robinson RG, Parikh RM, Lipsey JR, et al. Pathological laughing and crying following stroke: validation of measurement scale and double-blind treatment study. Am J Psychiatry. 1993;150:286–293. doi: 10.1176/ajp.150.2.286. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton MA. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chemerinski E, Robinson RG. The neuropsychiatry of stroke. Psychosomatics. 2000;41:5–14. doi: 10.1016/S0033-3182(00)71168-6. [DOI] [PubMed] [Google Scholar]
- 13.Robinson RG, Starr LB, Kubos KL, et al. A two-year longitudinal study of post-stroke mood disorders: findings during the initial evaluation. Stroke. 1983;14:736–744. doi: 10.1161/01.str.14.5.736. [DOI] [PubMed] [Google Scholar]
- 14.Robinson RG, Szetela B. Mood change following left hemispheric brain injury. Ann Neurol. 1981;9:447–453. doi: 10.1002/ana.410090506. [DOI] [PubMed] [Google Scholar]
- 15.vanSwieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 16.Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1988;19:1497–1500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- 17.Ottenbacher KJ, Mann WC, Granger CV, et al. Inter-rater agreement and stability of functional assessment in the community-based elderly. Arch Phys Med Rehabil. 1994;75:1297–1301. [PubMed] [Google Scholar]
- 18.Kunitz SC, Gross CR, Heyman A, et al. The pilot stroke data bank: definition, design, and data. Stroke. 1984;15:740–746. doi: 10.1161/01.str.15.4.740. [DOI] [PubMed] [Google Scholar]
- 19.Arndt S, Turvey C, Coryell WH, et al. Charting patients’ course: a comparison of statistics used to summarize patient course in longitudinal and repeated measures studies. J Psychiatr Res. 2000;34:105–113. doi: 10.1016/s0022-3956(99)00044-8. [DOI] [PubMed] [Google Scholar]
- 20.Malberg JE, Eisch AJ, Nestler EJ, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 22.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 23.Banasr M, Hery M, Printemps R, et al. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- 24.Radley JJ, Jacobs BL. 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res. 2002;955:264–267. doi: 10.1016/s0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- 25.Hashioka S, Klegeris A, Monji A, et al. Antidepressants inhibit interferon-gamma-induced microglial production of IL-6 and nitric oxide. Exp Neurol. 2007;206:33–42. doi: 10.1016/j.expneurol.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Obuchowicz E, Kowalski J, Labuzek K, et al. Amitriptyline and nortriptyline inhibit interleukin-1 release by rat mixed glial and microglial cell cultures. Int J Neuropsychopharmacol. 2006;9:27–35. doi: 10.1017/S146114570500547X. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko M, Stellwagen D, Malenka RC, et al. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]