Abstract
Objective
To examine the hemodynamic determinants of dysregulated arginine metabolism in patients with acute decompensated heart failure and explore possible mechanism of arginine dysregulation in human heart failure.
Background
Accumulating methylated arginine metabolites and impaired arginine bioavailability have been associated with heart failure, but the underlying pathophysiology remains unclear.
Methods
We prospectively determined plasma levels of asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor, and global arginine bioavailability ratio [GABR=arginine/(ornithine+citrulline)] by tandem mass spectrometry in subjects with advanced decompensated heart failure in the intensive care unit (“ADHF”, n=68) and with stable chronic heart failure (“CHF”, n=57).
Results
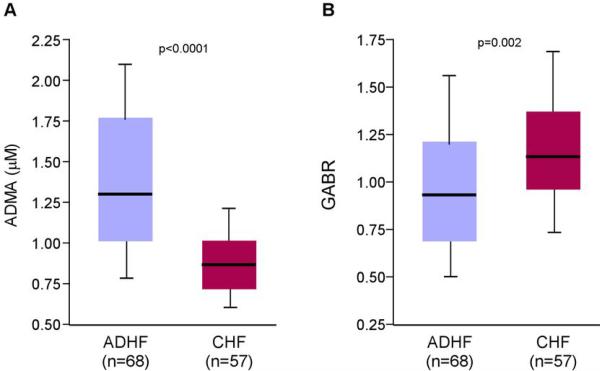
Compared to CHF subjects, plasma ADMA was significantly higher (median[interquartile range]: 1.29 [1.04–1.77] versus 0.87 [0.72–1.05] μM, p<0.0001), and GABR significantly lower (0.90 [0.69–1.22] versus 1.13 [0.92–1.37], p=0.002) in ADHF subjects. Elevated ADMA and diminished GABR were associated with higher systolic pulmonary artery pressure (sPAP) and higher central venous pressure, but not with other clinical or hemodynamic indices. We further observed myocardial levels of dimethylarginine dimethylaminohydrolase-1 (DDAH-1) were increased in CHF without elevated sPAP (<50mmHg), but diminished with elevated sPAP (≥50mmHg, difference with sPAP<50 mmHg, p=0.02).
Conclusions
Dysregulated arginine metabolism was observed in advanced decompensated heart failure, particularly with pulmonary hypertension and elevated intracardiac filling pressures. Compared to control hearts, we observed higher amounts of ADMA-degradation enzyme DDAH-1 (but similar amounts of ADMA-producing enzyme, PRMT-1) in the human failing myocardium.
Keywords: Nitric oxide synthase, asymmetric dimethylarginine, heart failure, pulmonary hypertension
Introduction
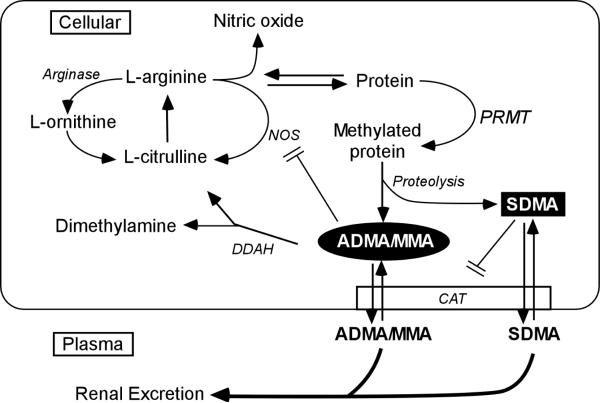
Endothelium-derived nitric oxide (NO) is an endogenous vasodilator which is essential to vascular homeostasis (1). NO is primarily synthesized from L-arginine and oxygen by various NO synthases to produce L-citrulline (Figure 1). As the sole nitrogen source of NO, L-arginine is tightly regulated. L-arginine can also be catabolized into L-ornithine by arginases, while L-arginine bound to proteins can be methylated to various forms of methylarginines (N-mono-methylarginine [MMA], asymmetric dimethylarginine [ADMA], or symmetric dimethylarginine [SDMA]). These methylarginines can become free amino acids after proteolysis, and migrate into extracellular space and circulation. ADMA and MMA, but not SDMA, are endogenous inhibitors of NO synthase (2–3) while all of them share with L-arginine the cationic amino acid transporter, and can thus inhibit intracellular L-arginine uptake (4). Hence, reduced L-arginine bioavailability or increased methylarginine production may reduce the production of NO and directly lead to endothelial dysfunction (5).
Figure 1. Schematic illustration of arginine metabolic pathways and nitric oxide production.
Abbreviations: NOS = nitric oxide synthase; PRMT = protein arginine methyltransferases; ADMA = asymmetric dimethylaminohydrolase; MMA = N-monomethylarginine; SDMA = symmetric dimethylarginine; DDAH = dimethylarginine dimethylaminohydrolase; CAT = cationic amino acid transport.
Dysregulated arginine metabolism can be readily detected in systemic circulation. In particular, diminished global arginine bioavailability ratio (GABR, defined by the ratio of L-arginine to the combination of its two major catabolites, L-ornithine plus L-citrulline) as well as increased ADMA levels have been associated with poorer long-term clinical outcomes stable patients with coronary artery diseases (6–8). As disease progresses, systemic levels of ADMA predict adverse outcomes in patients with acute coronary syndromes (9–10) and with post-infarction cardiogenic shock (11). Elevated levels of ADMA have been identified in both chronic systolic heart failure (12–13) and in acute decompensated heart failure (14–15). Previous studies have identified increases in the endothelial or erythrocyte expression of NO synthases and arginases may be directly responsible for diminished arginine bioavailability (16). However, the underlying mechanisms mediating arginine metabolic dysregulation in human heart failure, particularly at the level of the myocardium, is largely unknown. For example, levels of protein expression of enzymes that either produce ADMA (protein methyltransferases-1 [PRMT-1]) or eliminate ADMA (dimethylarginine dimethylaminohydrolase-1 and -2 [DDAH-1 and -2]) have not yet been established within the failing human heart. We therefore sought to test the hypothesis that pathways that regulate methylarginine metabolism and thus directly impact NO production are independently associated with disease progression, serving as a potential mechanism contributing to vascular dysfunction and hemodynamic compromise in the setting of advanced decompensated heart failure.
Methods
Study population
Three independent groups of adult subjects (≥18 years of age) were included in this study; all provided written informed consent approved by the Cleveland Clinic Institutional Review Board in their respective studies. The first group consisted of 68 consecutive patients with advanced systolic heart failure admitted for progressive decompensation (advanced decompensated heart failure, or “ADHF” group) at the Cleveland Clinic Heart Failure Intensive Care Unit receiving intensive medical therapy that were prospectively enrolled to participate in a serial blood draw, echocardiographic and hemodynamic evaluation study. Inclusion criteria included markedly impaired left ventricular systolic function defined by left ventricular ejection fraction (LVEF) ≤30% and New York Heart Association (NYHA) class III to IV. Patients on artificial ventilation, those who had undergone aortic and/or mitral valve repair or prosthesis, and cardiac transplantation patients were excluded. The second group consisted of 57 ambulatory patients with stable systolic heart failure (chronic heart failure, or “CHF” group) that were prospectively enrolled to participate in a blood draw, echocardiographic and metabolic evaluation study. To participate, patents had to have a clinical diagnosis of chronic (>6 months' duration) heart failure with LVEF ≤40% by echocardiography at the time of enrollment. We excluded subjects with a major cardiovascular event (myocardial infarction, unstable angina, stroke, transient ischemic attack, pulmonary embolism) within 30 days of enrollment. Other exclusion criteria including a history of significant chronic obstructive pulmonary disease (COPD), major surgery, hospitalization or emergency room visits for heart failure exacerbation, or use of inotropic agents within the month preceding enrollment. The third group consisted of patients with end-stage heart failure undergoing orthotropic heart transplantation (“HF-H” or “HF-L,” see definitions below) or unused heart transplant donors (“Controls”), whose myocardial tissue samples from their explanted hearts were available for experimental studies.
Hemodynamic and Echocardiographic Evaluation
For our ADHF group, hemodynamic and echocardiographic data were simultaneously collected at baseline (within 12 hours of admission) and at follow-up (after 48 hours of intensive medical therapy). Hemodynamic data, including systemic blood pressure, central venous pressure (CVP), systolic pulmonary artery pressure (sPAP), and pulmonary capillary wedge pressure (PCWP), represent the average of 5 cycles with balanced transducers. Cardiac index was calculated with the Fick equation through sampling of a mixed central venous blood gas taken at the level of the pulmonary artery while assuming standard metabolic rates. For both ADHF and CHF groups, a comprehensive two-dimensional echocardiography was performed with a commercially available system (Vingmed, System Seven, General Electric, Piscataway, NJ) by a single American Society of Echocardiography registered research sonographer (A.B.). Images were acquired in the left lateral decubitus position with a phased-array transducer in the standard parasternal and apical views. Standard two-dimensional and Doppler data, triggered to the QRS complex, were digitally stored in a cine-loop format.
Mass spectrometry (MS) analysis of arginine metabolites
Fasting blood samples were collected in ethylenediaminetetraacetic acid (lavender top) tubes and isolated plasma stored at −80°C. Samples were thawed on ice the day of analysis. Plasma levels of L-arginine, L-citrulline, L-ornithine, ADMA, SDMA and MMA were quantified using stable isotope dilution LC/ESI/MS/MS assays using an upgraded ABI 365 triple quadrupole mass spectrometer (Applied Biosystems Inc., Foster City, CA, USA) with Ionics EP 10+ redesigned source (Concord, Ontario, Canada) and electrospray ionization (ESI) needle connected to an Aria LX4 series multiplexed HPLC system with Flux pumps (Cohesive Technologies, Franklin, MA, USA), as previously described (6–7). MS analyses were performed online using LC/ESI/MS/MS in the positive ion mode with multiple reaction monitoring using unique parent→daughter ion transitions and retention times unique for each analyte and its stable isotope labeled internal standard. Intra-assay and inter-assay coefficients of variance were <10% for all analytes. Plasma B-type natriuretic peptide (BNP) was measured by the Architect ci8200 assay (Abbott Laboratories, Abbott Park IL).
Human heart tissue homogenate preparation
Upon arrival to the lab, human heart tissue was frozen in liquid nitrogen and stored at −80°C for later use. Samples were divided into three groups: 1) 10 from failing hearts with elevated systolic pulmonary artery pressure (sPAP ≥50mmHg), “HF-H”; 2) 10 from failing hearts without elevated systolic pulmonary artery pressure (sPAP <50 mmHg), “HF-L”; 3) 10 from non-failing hearts, “Controls”. For each sample, 0.25g of tissue was placed in cold buffer (50mM Tris, PH 7.4, 150mM NaCl, 1mM EDTA, 1%NP-40, 10% glycerol, 25mM β-Glycerolphosphate with protease and phosphatase inhibitors) and homogenized immediately using a PowerGen Model 125 homogenizer (Fisher Scientific). Samples were then centrifuged at 2000 rpm for 10 minutes at 4°C and supernatant was stored at −80°C for western blot analysis.
Western blot analysis
Protein concentrations of human heart homogenates were tested with Lowry protein assay (Bio-Rad). Proteins were separated by SDS-PAGE and then transferred to nitrocellulose membranes. Blots were blocked with 5% nonfat dry milk for 1 hour. Primary antibody concentrations were: anti-DDAH1 (Abcam, ab2231), 1:1000; anti-DDAH2 (Abcam, ab1383), 1:1000; anti-PRMT1 (Sigma, P1620), 1:1000 and anti-GAPDH (Affinity Bioreagent, LF-PA0018, GADPH = glyceraldehyde 3-phosphate dehydrogenase), 1:60,000. All incubations were done at 4°C overnight except anti-GAPDH for 1 hour at room temperature. LI-COR IRDye® 800CW donkey anti-goat IgG (926-32214), goat anti-mouse IgG (926-32210) and goat anti- rabbit IgG (926-32211) were used as secondary antibodies at 1:5000 dilution for 1 hour at room temperature. The membranes were scanned using an Odyssey infrared imager (LI-COR). The densities of target bands were quantified using the Odyssey application software (Odyssey V3.0). Band densities were normalized to those of the GAPDH bands (loading controls). For samples in different gels, relative band densities (to GAPDH) were then normalized to that of a common sample loaded in every gel.
Immunohistochemical staining of DDAH-1
Frozen human left ventricular myocardial sections (6 μm) were fixed in pre-cooled acetone for 10 minutes at room temperature and let dry in air completely. Then DDAH-1 staining was done using a rabbit anti-DDAH-1 antibody (Sigma, HPA006308), normal rabbit IgG (used as negative control, from Santa Cruz, sc-2027) and Dako EnVision+System-HRP (DAB) kit (K4010). After endogenous peroxidase was blocked for 5 min, the sections were washed with distilled water and incubated with the anti-DDAH-1 antibody (1:200) or normal rabbit IgG for 30 min at room temperature. The sections were then washed with TBST (0.05% Tween 20) and incubated with peroxidase-labeled polymer conjugated to goat anti-rabbit Ig for 30 min at room temperature. After washed with TBST, the sections were incubated with liquid DAB-chromogen solution for 10 min, and counterstained in hematoxylin for 2 min.
Statistical analyses
Continuous variables were summarized as mean ± standard deviation if normally distributed, and as median and interquartile range [IQR] if non-normally distributed. Normality was assessed by the Shapiro-Wilk W test. Differences in normally distributed continuous variables across clinical categories were assessed using the Student's t test or one-way analysis of variance (ANOVA), while differences in non-normally distributed variables were assessed using the Wilcoxon rank-sum test. Differences in clinical proportions across clinical categories were assessed by contingency table analysis. Relative protein expression levels among different groups of human heart tissues were compared with the Wilcoxon test. All p-values reported are from two-sided tests and a p-value <0.05 was considered statistically significant. Statistical analyses were performed using JMP 8.0.2 and SAS 9.0 (SAS Institute, Cary, NC).
Results
Study population
Table 1 illustrates the baseline clinical characteristics of our study population stratified into ADHF and CHF groups. There were no differences in age, gender, or history of hypertension, or history of diabetes mellitus between ADHF and CHF groups. ADHF subjects demonstrated a higher prevalence of ischemic etiology and lower rates of neurohormonal antagonist use (Table 1). Furthermore, ADHF subjects had more advanced echocardiographic abnormalities and higher plasma BNP levels compared to those of CHF subjects (Table 1).
Table 1.
Baseline characteristics of study population.
| Variables | ADHF (n=68) | CHF (n=57) | P value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 56 [47–65] | 55 [45–65] | 0.341 |
| Female, n (%) | 14 (21%) | 14 (25%) | 0.582 |
| BMI (kg/m2) | 27 [24–31] | 30 [26–33] | 0.021 |
|
| |||
| Heart failure history | |||
| NYHA class, n (%) | I 0 (0%) | I 5 (10%) | |
| II 3 (5%) | II 38 (73%) | ||
| III 24 (42%) | III 9 (17%) | ||
| IV 30 (53%) | IV 0 (0%) | ||
| Ischemic etiology, n (%) | 36 (53%) | 15 (29%) | 0.008 |
|
| |||
| Co-morbidities | |||
| Hypertension, n (%) | 29 (43%) | 26 (51%) | 0.367 |
| Diabetes Mellitus, n (%) | 25 (37%) | 15 (29%) | 0.8362 |
|
| |||
| Medications | |||
| ACEI or ARB, n (%) | 42 (61%) | 49 (92%) | <0.0001 |
| Beta-blocker, n (%) | 51 (74%) | 52 (88%) | 0.043 |
| Spironolactone, n (%) | 32 (46%) | 21 (40%) | 0.456 |
| Loop diuretics, n (%) | 58 (83%) | 32 (60%) | 0.005 |
| Digoxin, n (%) | 29 (42%) | 23 (43%) | 0.880 |
|
| |||
| Echocardiographic indices | |||
| LVEF (%) | 27 [22–32] | 30 [25–40] | 0.048 |
| LVEDVi (mL/m2) | 106 [78–136] | 85 [70–116] | 0.084 |
| LAVi (mL/m2) | 42 [33–53] | 31 [23–48] | 0.001 |
| E/Septal Ea | 23 [18–30] | 14 [9–21] | <0.0001 |
|
| |||
| Hemodynamic indices (ADHF only) | |||
| sPAP (mmHg) | 48 ± 14 | -- | -- |
| PCWP (mmHg) | 21 ± 7 | -- | -- |
| CVP (mmHg) | 13 ± 6 | -- | -- |
| CI (L/min/1.73m2) | 2.1 ± 0.7 | -- | -- |
|
| |||
| Laboratory data | |||
| eGFR (ml/min/1.73 m2) | 72 ± 42 | 72 ±25 | 0.865 |
| BNP (pg/mL) | 1412 [677–2136] | 79 [25–231] | <0.0001 |
|
| |||
| Arginine metabolites | |||
| ADMA (μM) | 1.29 [1.04–1.77] | 0.87 [0.72–1.05] | <0.0001 |
| SDMA (μM) | 2.72 [1.65–4.30] | 1.09 [0.81–1.48] | <0.0001 |
| MMA (nM) | 88 [53–213] | 34 [26–45] | <0.0001 |
| L-Arginine (μM) | 73 [50–91] | 67 [56–82] | 0.759 |
| L-Ornithine (μM) | 61 [42–81] | 46 [38–58] | 0.003 |
| L-Citrulline (μM) | 20 [11–28] | 14 [11–18] | 0.003 |
| GABR | 0.90 [0.69–1.22] | 1.13 [0.92–1.37] | 0.002 |
Abbreviations: ADHF, advanced decompensated heart failure; CHF, chronic systolic heart failure; BMI, Body mass index; NYHA, New York Heart Association; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction; LVEDVi, left ventricular end-diastolic volume index; LAVi, left atrial volume index; sPAP, systolic pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; CVP, central venous pressure; CI, cardiac index; eGFR, estimated glomerular filtration rate; BNP, B-type natriuretic peptide; ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; MMA, N-mono-methylarginine; GABR, global arginine bioavailability ratio.
Methylated arginine metabolites and arginine bioavailability in ADHF versus CHF
Compared to CHF subjects, ADHF subjects had higher plasma levels of methylated arginine metabolites, including ADMA (1.29 [1.04–1.77] versus 0.87 [0.72–1.05] μM, p<0.0001; Figure 2A), SDMA (2.72 [1.65–4.30] versus 1.09 [0.81–1.48] μM, p<0.0001) and MMA (88 [53–213] versus 34 [26–45] nM, p<0.0001). ADHF subjects also demonstrated lower overall GABR (0.90 [0.69–1.22] versus 1.13 [0.92–1.37], p=0.002; Figure 2B), with higher plasma levels of L-ornithine and L-citrulline compared to those in CHF subjects (Table 1).
Figure 2. Comparison of plasma ADMA and GABR levels between advanced decompensated and chronic heart failure patients.
Abbreviations: ADMA = asymmetric dimethylarginine; GABR = global arginine bioavailability ratio; ADHF = advanced decompensated heart failure; CHF = chronic systolic heart failure.
Clinical and hemodynamic determinants of dysregulated arginine metabolism in ADHF
Table 2 presents clinical and hemodynamic indices stratified by median plasma ADMA and GABR levels in our ADHF cohort. Elevated (above-median) ADMA and diminished (below-median) GABR were associated with higher sPAP and higher CVP, but not with other clinical or hemodynamic indices including age, plasma BNP levels, or PCWP or CI. Subjects with elevated sPAP (≥50 mmHg) demonstrated higher levels of plasma ADMA (1.50 [1.17, 1.82] versus 1.19 [0.88, 1.53], p=0.014) and lower GABR (0.72 [0.58, 1.08] versus 1.04 [0.84, 1.34], p=0.003) compared to those with lower sPAP (<50mmHg).
Table 2.
Clinical and hemodynamic indices stratified by median asymmetric dimethylarginine (ADMA, 1.29 μM) and median global arginine bioavailability ratio (GABR, 0.9) in patients with advanced decompensated heart failure.
| ADMA | GABR | |||||
|---|---|---|---|---|---|---|
| Parameters | < 1.29 μM (n=34) | ≥ 1.29 μM (n=34) | P value | < 0.90 (n=34) | ≥ 0.90 (n=34) | P value |
| Age (years) | 53 ± 13 | 58 ± 12 | 0.130 | 58 ± 10 | 53 ± 15 | 0.054 |
| BNP (pg/mL) | 1,125 [668–2,172] | 1,492 [661–2,143] | 0.556 | 1,279 [641–2,247] | 1,431 [679–2,079] | 0.654 |
|
| ||||||
| Hemodynamic indices | ||||||
| sPAP (mmHg) | 42 ± 12 | 54 ± 13 | <0.001 | 53 ± 15 | 43 ± 11 | 0.002 |
| PCWP (mmHg) | 20 ± 7 | 22 ± 7 | 0.490 | 22 ± 7 | 20 ± 7 | 0.223 |
| CVP (mmHg) | 11 ± 6 | 15 ± 5 | 0.009 | 15 ± 6 | 12 ± 5 | 0.050 |
| CI (L/min/1.73m2) | 2.0 ± 0.8 | 2.1 ± 0.5 | 0.219 | 2.1 ± 0.8 | 2.1 ± 0.5 | 0.537 |
Abbreviations: BNP, B-type natriuretic peptide; sPAP, systolic pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; CVP, central venous pressure; CI, cardiac index.
Myocardial expression levels of PRMT-1, DDAH-1 and DDAH-2 among three groups of human heart tissues
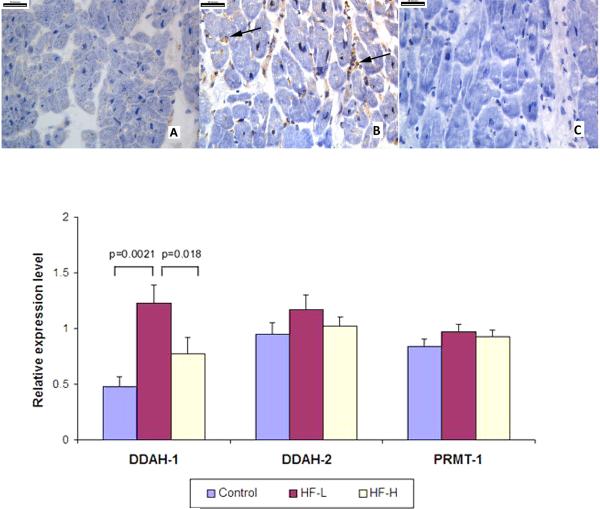
Immunohistochemical staining of DDAH-1 in donor hearts and failing hearts demonstrated remarkably enhanced DDAH-1 protein expression in failing heart tissures, which is mainly distributed in the interstitial and perivascular areas (Figure 3, upper panel). We observed significantly higher myocardial protein levels of DDAH-1 in our HF-L group compared with DDAH-1 levels in control hearts (1.23±0.49 versus 0.48±0.24, p=0.002). However, DDAH-1 levels became lower with elevated sPAP (HF-H versus HF-L: 0.77±0.47 versus 1.23±0.49, p=0.018). DDAH-2 trended toward an increase in the HF-L group relative to the Controls and HF-H groups but did not reach statistical significance. In contrast, myocardial expression levels of PRMT-1 were similar across all three groups (Figure 3, lower panel).
Figure 3. Increased Myocardial DDAH-1 Protein Levels in Human Failing Myocardium Caption: Upper Panel.
Upper Panel. Immunohistochemistry staining of DDAH-1 in donor hearts (A) and failing human hearts (B), as well as IgG control staining of failing human hearts (C). Arrows showing increased staining in the interstitial and perivascular areas. Lower Panel. Myocardial levels of DDAH-1/2 and PRMT-1 proteins among “Controls” (n=10), “HF-L” (“low” sPAP <50 mmHg, n=10) and “HF-H” groups (“high” sPAP ≥50 mmHg, n=10). Band densities were normalized to those of GAPDH.
Discussion
Endothelial dysfunction adversely influence myocardial function by impairing vasodilatation of myocardial capillaries (17–18) as well as pulmonary vasculature(19). We observed a direct association between dysregulated arginine methylation and elevated pulmonary artery pressures in the setting of ADHF. We further identified the higher myocardial levels of DDAH-1 in the failing myocardium in the absence of significant pulmonary hypertension compared to that of controls or those with heart failure and significant pulmonary hypertension. Linking these two observations, our findings support the notion that an up-regulation of DDAH-1 at the level of the myocardium may provide the reserve in response to increased systemic ADMA, presumably to maintain myocardial perfusion at the local level in the setting of rising intracardiac pressures. These findings may imply the potential benefit of promoting myocardial DDAH-1 counter-regulatory expression to deter progression or decompensation of heart failure.
Elevated circulating methylarginines and diminished arginine bioavailability have been observed in a wide variety of end-organ dysfunction closely linked to vascular dysfunction, such as idiopathic pulmonary hypertension (19–20), sickle-cell disease (16,21), renal failure (2,22), and diabetes mellitus (23). Generation and adequate balance of NO have long been recognized as important factor in maintaining myocardial function. Pertaining to heart failure, several studies have identified dysregulated arginine metabolism is present in patients with heart failure compared to healthy controls (15). In our own experience in an independent cohort of patients undergoing elective coronary angiography, we have observed higher median levels of ADMA in patients with history of heart failure compared to those without heart failure (1.17 vs. 0.96 μM)(7). We and others have also described higher ADMA levels with greater disease severity within CHF (13,24). It is therefore conceivable that disease progression is associated with imbalance of factors affecting local NO production. Our first observation that patients with ADHF had significantly higher plasma ADMA levels than CHF group is consistent with prior reports linking elevated ADMA levels (which was notably higher in the acute setting) to reduced NOx levels (14). We now demonstrate for the first time that systemic arginine bioavailability (estimated by GABR) in patients with ADHF was significantly lower than that in CHF patients. Thus, a relative deficiency of NO as a result of insufficient substrate (L-arginine) bioavailability or diminishing capacity of production by endogenous inhibition by local ADMA accumulation can in part contribute to disease progression and decompensation. These findings may in part explain why treatment studies with drugs that supplemental NO (such as isosorbide dinitrate or sodium nitroprusside) have been associated with favorable long-term outcomes in patients with advanced systolic heart failure (25–27).
Another novel finding of our study is that elevated ADMA and diminished GABR were associated with higher sPAP in the setting of ADHF. The presence of pulmonary hypertension secondary to left heart failure portends poor long-term morbidity and mortality and therapeutic options remain limited (28–29). Our finding therefore suggests the potential for endothelial dysfunction from impaired NO production to affect pulmonary vascular reactivity.
Accumulation of ADMA can either due to increased production (i.e. increase in PRMT) or reduced degradation (i.e. decrease in DDAH). The potential increase in DDAH-1 resulted in improved myocardial perfusion or hemodynamic function of the failing ventricle is speculative. Nevertheless, increase in DDAH-1 in the human failing myocardium has not been described before. Our findings therefore extended the role of DDAH-1 from animal studies to humans, and provide much needed human data to support future clinical investigations to explore how strategies to increase DDAH-1 may improve myocardial perfusion or hemodynamics in the failing heart. It is conceivable that compensatory expression of DDAH (in particular, DDAH-1) can serve as an important counter-regulatory mechanism in the failing myocardium in humans. We also do not have the data to explain why DDAH-1 did not increase further along with elevated systemic ADMA in the setting of HF-H. However, previously reported data showing improvement in DDAH following LVAD support provided indirect evidence, even though the intervention is not directly related to improved DDAH expression. We speculate that the lack of compensatory DDAH-1 in the setting of high sPAP, and further studies are needed to explore such mechanisms.
The critical role of DDAH activity in preserving endothelial function has been demonstrated in the transgenic mouse model (30–33), even though it is important to recognize that there might be differences in expression of DDAH isoforms among different species. Over-expression of DDAH-1 has been associated with increased eNOS in the myocardium (34). In the setting of heart failure, enhanced DDAH-1 expression has been previously demonstrated in a pacing-induced dog model, particularly at the level of smooth muscle cells and endothelium of coronary arteries and veins as well as coronary microvessels, and relatively weak staining of the sarcolemma and cytosol of the cardiac myocytes (35). Myocardial gene expression of DDAH-1 was also increased following unloading by mechanical circulatory support in patients with end-stage heart failure (36). Nevertheless, it is important to point out that our findings are in direct contrast with that observed in pulmonary arterial hypertension and in the pacing-induced heart failure dog model, whereby more significant suppression of DDAH-2 instead of DDAH-1 was observed (19,35). Regardless of which DDAH isoforms are altered, the ability to increase degradation of ADMA by enhancing myocardial DDAH expression may preserve endothelial function and maintain hemodynamic compensation in the human failing heart. Hence, treatment strategies to increase DDAH-1 expression in the failing myocardium should be considered for future investigations in advanced heart failure.
Study Limitation
Myocardial and blood samples were not simultaneously collected, and thus the association between altered DDAH levels and elevated ADMA/reduced GABR was made via their commonly associated pulmonary hypertension in the setting of systolic heart failure. Also, no direct invasive hemodynamic assessment in patients with CHF was performed, even though none of the subjects demonstrated significant pulmonary hypertension based on echocardiographic assessment of right ventricular systolic pressure estimated by tricuspid regurgitant jet velocity. We also cannot adjust for the impact of treatment on plasma levels of arginine metabolites, even though these methylated end-products are relatively stable. Our findings are associative and cannot establish a cause-and-effect relationship between myocardial DDAH-1 and systemic ADMA levels. We also do not have access to localized samples at the level of pulmonary artery as well as coronary sinus blood sampling available to identify trans-cardiac gradient of ADMA. Since a subset of patients were treated with oral nitrates or sodium nitroprusside at the time of blood draws, confirmation of NO deficiency with direct measurements of nitrate levels (NOx) cannot be a reliable estimate of systemic NO production. Interpretation of the findings on myocardial samples is complicated by the small sample size and potential heterogeneity of patient populations between the HF-L and the HF-H groups. Differences in variables such as disease severity, left ventricular ejection fraction, etiologies, duration of disease, co-morbidities, and concomitant medications could have affected pulmonary artery pressure, myocardial DDAH-1, or both. The fact that we can only sample human myocardial tissues at a single time-point has limited our analysis to cross-sectional comparison between failing and non-failing hearts regarding the relative amounts of production (PRMT-1) and degradation (DDAH-1 and DDAH-2) enzymes rather than true kinetics of ADMA at the level of the myocardium. Nevertheless, these hypothesis-generating observations warrant further investigations to determine if enhancing DDAH-1 is a viable strategy in this patient population that has limited treatment options.
Conclusion
Dysregulated arginine metabolism, either in the form of diminished substrate availability or increased endogenous inhibition of nitric oxide synthases, is observed in patients with systolic heart failure and increased pulmonary artery pressures. Compared to control hearts, we observed higher amounts of ADMA-degradation enzyme DDAH-1 (but similar amounts of ADMA-producing enzyme, PRMT-1) in the human failing myocardium.
Acknowledgments
Dr. Tang has previously received research grant support from Abbott Laboratories, Inc. Dr. Hazen is named as co-inventor on issued and pending patents filed by the Cleveland Clinic that relate to the use of biomarkers in inflammatory and cardiovascular disease. Dr. Hazen is the scientific founder of Cleveland Heart Lab.; he has received speaking honoraria or consulting fees from Pfizer, AstraZeneca, Merck, Merck Schering Plough, Lilly, Esperion.,Liposciences, Prognosti X Inc., Wyeth, BioPhysical and Abbott Laboratories; and has received research grant support from Abbott Laboratories, Liposciences, and Cleveland Heart Lab.
Sources of Funding This research was supported by National Institutes of Health grants 1RO1 HL103931-02 (W.H. T.), P01 HL076491-055328 (S.L.H.), P01 HL087018-020001 (S.L.H.), 1P01 HL098055-01 (S.L.H.), P50 HL077107-050004 (S.L.H.), the Cleveland Clinic Clinical Research Unit of the Cleveland Clinic/Case Western Reserve University CTSA 1UL1RR024989 (W.H.T., S.L.H., A.B.), American College of Cardiology Foundation (W.H.T.), and the American Society of Echocardiography (A.B.).
ABBREVIATIONS
- ADMA
asymmetric dimethylarginine
- ADHF
advanced decompensated heart failure
- CHF
chronic heart failure
- DDAH-1
dimethylarginine dimethylaminohydrolase-1
- GABR
global arginine bioavailability ratio
- LVEF
left ventricular ejection fraction
- MMA
N-mono-methylarginine
- NO
nitric oxide
- NYHA
New York Heart Association functional class
- PRMT-1
protein methyltransferase-1
- SDMA
symmetric dimethylarginine
- sPAP
systolic pulmonary artery pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures No other disclosures are reported.
References
- 1.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 2.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–5. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 3.Vallance P, Leone A, Calver A, Collier J, Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol. 1992;20(Suppl 12):S60–2. doi: 10.1097/00005344-199204002-00018. [DOI] [PubMed] [Google Scholar]
- 4.Closs EI, Basha FZ, Habermeier A, Forstermann U. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide. 1997;1:65–73. doi: 10.1006/niox.1996.0106. [DOI] [PubMed] [Google Scholar]
- 5.Boger RH, Bode-Boger SM, Szuba A, et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842–7. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 6.Tang WH, Wang Z, Cho L, Brennan DM, Hazen SL. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 2009;53:2061–7. doi: 10.1016/j.jacc.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Tang WH, Cho L, Brennan DM, Hazen SL. Targeted metabolomic evaluation of arginine methylation and cardiovascular risks: potential mechanisms beyond nitric oxide synthase inhibition. Arterioscler Thromb Vasc Biol. 2009;29:1383–91. doi: 10.1161/ATVBAHA.109.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavusoglu E, Ruwende C, Chopra V, et al. Relation of baseline plasma ADMA levels to cardiovascular morbidity and mortality at two years in men with diabetes mellitus referred for coronary angiography. Atherosclerosis. 2010;210:226–31. doi: 10.1016/j.atherosclerosis.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Zeller M, Korandji C, Guilland JC, et al. Impact of asymmetric dimethylarginine on mortality after acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:954–60. doi: 10.1161/ATVBAHA.108.162768. [DOI] [PubMed] [Google Scholar]
- 10.Cavusoglu E, Ruwende C, Chopra V, et al. Relationship of baseline plasma ADMA levels to cardiovascular outcomes at 2 years in men with acute coronary syndrome referred for coronary angiography. Coron Artery Dis. 2009;20:112–7. doi: 10.1097/MCA.0b013e328323982f. [DOI] [PubMed] [Google Scholar]
- 11.Nicholls SJ, Wang Z, Koeth R, et al. Metabolic profiling of arginine and nitric oxide pathways predicts hemodynamic abnormalities and mortality in patients with cardiogenic shock after acute myocardial infarction. Circulation. 2007;116:2315–24. doi: 10.1161/CIRCULATIONAHA.107.693986. [DOI] [PubMed] [Google Scholar]
- 12.Duckelmann C, Mittermayer F, Haider DG, Altenberger J, Eichinger J, Wolzt M. Asymmetric dimethylarginine enhances cardiovascular risk prediction in patients with chronic heart failure. Arterioscler Thromb Vasc Biol. 2007;27:2037–42. doi: 10.1161/ATVBAHA.107.147595. [DOI] [PubMed] [Google Scholar]
- 13.Tang WH, Tong W, Shrestha K, et al. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. Eur Heart J. 2008;29:2506–13. doi: 10.1093/eurheartj/ehn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saitoh M, Osanai T, Kamada T, et al. High plasma level of asymmetric dimethylarginine in patients with acutely exacerbated congestive heart failure: role in reduction of plasma nitric oxide level. Heart Vessels. 2003;18:177–82. doi: 10.1007/s00380-003-0715-y. [DOI] [PubMed] [Google Scholar]
- 15.Usui M, Matsuoka H, Miyazaki H, Ueda S, Okuda S, Imaizumi T. Increased endogenous nitric oxide synthase inhibitor in patients with congestive heart failure. Life Sci. 1998;62:2425–30. doi: 10.1016/s0024-3205(98)00225-2. [DOI] [PubMed] [Google Scholar]
- 16.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause CD, Yang ZH, Kim YS, Lee JH, Cook JR, Pestka S. Protein arginine methyltransferases: evolution and assessment of their pharmacological and therapeutic potential. Pharmacol Ther. 2007;113:50–87. doi: 10.1016/j.pharmthera.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Paulus WJ. Endothelial control of vascular and myocardial function in heart failure. Cardiovasc Drugs Ther. 1994;8:437–46. doi: 10.1007/BF00877920. [DOI] [PubMed] [Google Scholar]
- 19.Pullamsetti S, Kiss L, Ghofrani HA, et al. Increased levels and reduced catabolism of asymmetric and symmetric dimethylarginines in pulmonary hypertension. FASEB J. 2005;19:1175–7. doi: 10.1096/fj.04-3223fje. [DOI] [PubMed] [Google Scholar]
- 20.Kielstein JT, Bode-Boger SM, Hesse G, et al. Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2005;25:1414–8. doi: 10.1161/01.ATV.0000168414.06853.f0. [DOI] [PubMed] [Google Scholar]
- 21.Kato GJ, Wang Z, Machado RF, Blackwelder WC, Taylor JGt, Hazen SL. Endogenous nitric oxide synthase inhibitors in sickle cell disease: abnormal levels and correlations with pulmonary hypertension, desaturation, haemolysis, organ dysfunction and death. Br J Haematol. 2009;145:506–13. doi: 10.1111/j.1365-2141.2009.07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoccali C, Bode-Boger S, Mallamaci F, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–7. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 23.Hanai K, Babazono T, Nyumura I, et al. Asymmetric dimethylarginine is closely associated with the development and progression of nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant. 2009;24:1884–8. doi: 10.1093/ndt/gfn716. [DOI] [PubMed] [Google Scholar]
- 24.Seljeflot I, Nilsson BB, Westheim AS, Bratseth V, Arnesen H. The L-arginine-asymmetric dimethylarginine ratio is strongly related to the severity of chronic heart failure. No effects of exercise training. J Card Fail. 2011;17:135–42. doi: 10.1016/j.cardfail.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Mullens W, Abrahams Z, Francis GS, et al. Sodium nitroprusside for advanced low-output heart failure. J Am Coll Cardiol. 2008;52:200–7. doi: 10.1016/j.jacc.2008.02.083. [DOI] [PubMed] [Google Scholar]
- 26.Mullens W, Abrahams Z, Francis GS, et al. Usefulness of Isosorbide Dinitrate and Hydralazine as add-on therapy in patients discharged for advanced decompensated heart failure. Am J Cardiol. 2009;103:1113–9. doi: 10.1016/j.amjcard.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–57. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 28.Khush KK, Tasissa G, Butler J, McGlothlin D, De Marco T. Effect of pulmonary hypertension on clinical outcomes in advanced heart failure: analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) database. Am Heart J. 2009;157:1026–34. doi: 10.1016/j.ahj.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Shin JT, Semigran MJ. Heart failure and pulmonary hypertension. Heart Fail Clin. 2010;6:215–22. doi: 10.1016/j.hfc.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dayoub H, Achan V, Adimoolam S, et al. Dimethylarginine dimethylaminohydrolase regulates nitric oxide synthesis: genetic and physiological evidence. Circulation. 2003;108:3042–7. doi: 10.1161/01.CIR.0000101924.04515.2E. [DOI] [PubMed] [Google Scholar]
- 31.Dayoub H, Rodionov RN, Lynch C, et al. Overexpression of dimethylarginine dimethylaminohydrolase inhibits asymmetric dimethylarginine-induced endothelial dysfunction in the cerebral circulation. Stroke. 2008;39:180–4. doi: 10.1161/STROKEAHA.107.490631. [DOI] [PubMed] [Google Scholar]
- 32.Jacobi J, Sydow K, von Degenfeld G, et al. Overexpression of dimethylarginine dimethylaminohydrolase reduces tissue asymmetric dimethylarginine levels and enhances angiogenesis. Circulation. 2005;111:1431–8. doi: 10.1161/01.CIR.0000158487.80483.09. [DOI] [PubMed] [Google Scholar]
- 33.Hu X, Atzler D, Xu X, et al. Dimethylarginine dimethylaminohydrolase-1 is the critical enzyme for degrading the cardiovascular risk factor asymmetrical dimethylarginine. Arterioscler Thromb Vasc Biol. 2011;31:1540–6. doi: 10.1161/ATVBAHA.110.222638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuhlinger MC, Conci E, Haubner BJ, et al. Asymmetric dimethyl L-arginine (ADMA) is a critical regulator of myocardial reperfusion injury. Cardiovasc Res. 2007;75:417–25. doi: 10.1016/j.cardiores.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Li Y, Zhang P, et al. Dimethylarginine dimethylaminohydrolase and endothelial dysfunction in failing hearts. Am J Physiol Heart Circ Physiol. 2005;289:H2212–9. doi: 10.1152/ajpheart.00224.2005. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Park S, Li Y, et al. Alterations of gene expression in failing myocardium following left ventricular assist device support. Physiol Genomics. 2003;14:251–60. doi: 10.1152/physiolgenomics.00022.2003. [DOI] [PubMed] [Google Scholar]