Abstract
Repeated cocaine administration increases the dendritic arborization of nucleus accumbens neurons, but the underlying signaling events remain unknown. Here, we show that repeated cocaine negatively regulates the active form of Rac1, a small GTPase that controls actin remodeling in other systems. We show further, using viral-mediated gene transfer, that overexpression of a dominant negative mutant of Rac1, or local knockout of Rac1 from floxed Rac1 mice, is sufficient to increase the density of immature dendritic spines on nucleus accumbens neurons, whereas overexpression of a constitutively active Rac1 mutant, or light activation of a photoactivatible form of Rac1, blocks the ability of repeated cocaine to produce this effect. Downregulation of Rac1 activity in nucleus accumbens likewise promotes behavioral responses to cocaine, with Rac1 activation producing the opposite effect. These findings establish an important role for Rac1 signaling in mediating structural and behavioral plasticity to cocaine.
Experience-dependent structural plasticity within the adult brain has been extensively implicated in long-term adaptations that underlie several psychiatric syndromes including drug addiction1–3. Addiction is marked by long-lasting changes in behavior that persist despite prolonged abstinence4. Increasing evidence suggests that morphological changes in neurons that comprise the brain’s reward circuitry contribute to these lasting behavioral abnormalities. For example, repeated administration of cocaine or other psychostimulants induces a persistent increase in dendritic spine density and complexity of dendritic branching in medium spiny neurons of the nucleus accumbens (NAc), a key brain reward region2, 5, 6. Dendritic spines are protrusions of the dendritic membrane upon which more than 90% of excitatory synapses are formed7. However, the molecular mechanisms mediating these changes are poorly understood. While there have been several reports directly linking transcriptional mechanisms to cocaine-induced NAc dendritic plasticity8–13, the events more proximal to spine growth and actin remodeling remain largely unknown.
Dendritic spines are highly plastic and dynamic 7, 14 with spine growth and retraction implicated in experience-dependent plasticity in many neural systems15. The formation of new spines and reshaping of pre-existing spines is dependent upon remodeling of the actin cytoskeleton, and chronic cocaine administration has been shown to regulate actin turnover in the NAc, as inferred from complex, time-dependent changes in levels of F-actin and in the phosphorylated state of several actin-binding proteins and of the actin-severing protein cofilin16–18, (see Discussion). In other systems, regulation of actin turnover is governed in large part by small GTPases19, particularly the Rho family, which includes Rac1, RhoA, and Cdc4220. Rac1 is involved in dendritic remodeling in cortical and hippocampal neurons both in vitro21–23, as well as in vivo24, 25, and Rac1 activation regulates cytoskeleton reorganization, at least in part, through the modulation of cofilin26, 27. However, despite these insights, causal data directly linking Rac1 signaling to cocaine-induced spine plasticity in NAc is completely lacking. We show here that repeated cocaine exposure primes NAc neurons for transient drug-induced downregulation of Rac1 activity, and that such repression of Rac1, via signaling to cofilin, is responsible for the expansion of dendritic spines on NAc neurons and for enhanced cocaine reward.
Results
Regulation of Rac1 signaling by cocaine
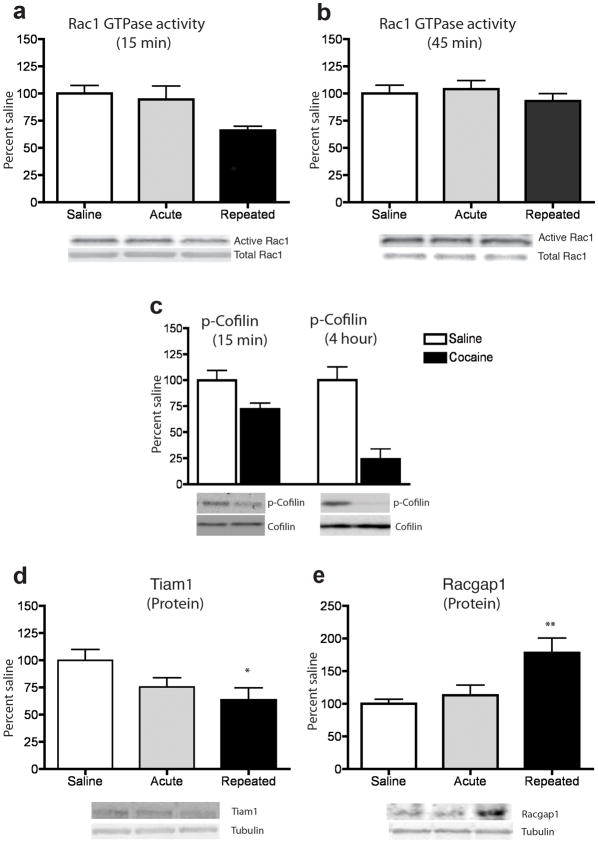
Since spine plasticity is regulated by the dynamic switching of Rac1 from its inactive GDP-bound form to the active GTP-bound form, we first investigated whether cocaine regulates the activity of Rac1 in the mouse NAc in vivo. Immunoprecipitation using an antibody specific for the active form of Rac1 revealed that repeated, but not acute, cocaine administration decreased the levels of active Rac1 15 minutes after a cocaine challenge, without a change in total Rac1 levels (Fig. 1a). This decrease was highly transient, as levels of active Rac1 returned to normal within 45 minutes (Fig. 1b) and remained unchanged 24 hours post-cocaine, (109.5 ± 10.1 for cocaine treated mice vs. 100.3 ± 9.2 for saline controls; n=8 mice per group; F1,15=0.5; p>0.05; analyzed by ANOVA), consistent with the known stimulus-dependent and transient nature of GTPase signaling in other systems28, 29. This decrease in GTPase activity was specific to Rac1 as there was no change in CDC42 activity (105.9 ± 11.65 for cocaine treated mice vs. 100.5 ± 8.43 for saline controls; n=6 mice per group; F1,11=0.02, p>0.05; analyzed by ANOVA). In line with the decreased Rac1 activity observed in response to repeated cocaine, levels of phosphorylated cofilin, but not total cofilin, were also decreased at the 15 minute and 4 hour time points examined (Fig. 1c). Since the phosphorylation of cofilin inhibits its actin-severing properties, these findings suggest that a cocaine challenge after repeated cocaine transiently increases the amount of active cofilin and thereby actin turnover in the NAc.
Figure 1. Cocaine regulation of Rac1 signaling in NAc.
Repeated, but not acute, cocaine administration: (a) transiently decreased Rac1 activity in NAc 15 min after the final drug injection (n=7–8 mice per group) (F2,21=4.50, *p<0.05), (b) but this decrease was no longer present at 45 min (n=7–8 mice) (p>0.05). At both the 15 and 45 min time points, there were no differences in total Rac1 levels (p >0.05). (c) The inactive (phosphorylated) form of cofilin (p-cofilin) was significantly decreased 15 min after repeated, cocaine (n=9 mice per group) (F1,17=6.29, *p<0.05), and remained decreased at 4 hours (n=5–6 mice per group) (F1,10=22.5, ***p<0.001). Total cofilin was unaltered by cocaine at both time points (p>0.05). (d) 24 hours after the final cocaine treatment, Tiam1 protein levels, normalized to tubulin (which was not altered), were significantly reduced by repeated cocaine (n=7–8 mice per group) (F2,22=3.54, *p<0.05). (e) RacGap1 protein levels, normalized to tubulin, were significantly increased at this same 24 hour time point (n=8–9 mice per group) (F2,14=6.73, **p<0.01). Full-length blots are presented in Supplementary Fig. 2). Data were analyzed by ANOVA and represented as mean ± s.e.m.
To identify a mechanism by which cocaine downregulates Rac1 activity, we examined the guanine nucleotide exchange factor (GEF) Tiam1, and the GTPase activating protein (GAP) RacGAP1 (also known as MgcRacGAP). Repeated, but not acute, cocaine administration significantly downregulated Tiam1 expression (Fig. 1d), but increased RacGap1 expression (Fig. 1f), in the NAc. These altered expression patterns are more lasting than the changes in Rac1 activity, being observed 24 hours after the last cocaine injection, which suggests that they prime the ability of a cocaine challenge to trigger transient suppression of Rac1 activity.
Rac1 activity in the NAc regulates reward behavior
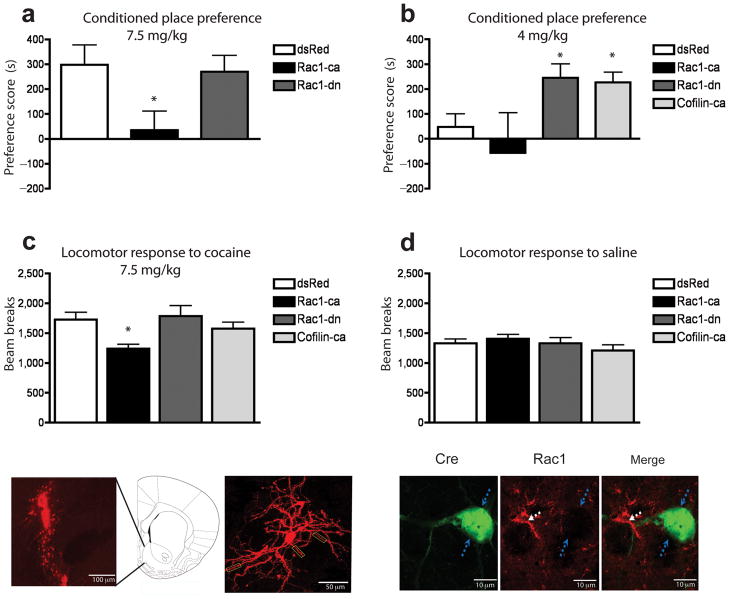
To assess the behaviorally relevant consequences of decreased Rac1 activity we used the conditioned place preference paradigm, which provides an indirect measure of cocaine reward. We bilaterally injected herpes simplex virus (HSV) vectors expressing either a dominant-negative (T17N) mutant of Rac1 (HSV-Rac1-dn) or a constitutively active mutant (L61) of Rac1 (HSV-Rac1-ca)30 into the shell of the mouse NAc. HSV-dsRed was used as a control. Intra-NAc injection of HSV-Rac1-ca blocked cocaine reward as measured by place conditioning, as well as the acute locomotor-activating effect of the drug, suggesting that cocaine’s suppression of Rac1 activity is required for normal rewarding and locomotor responses to the drug (Fig. 2a & 2c). Conversely, intra-NAc HSV-Rac1-dn promoted conditioned place preference to cocaine at a drug dose that did not induce a preference in HSV-dsRed injected control mice (Fig. 2b), without altering acute locomotor responses to the drug (Fig. 2c). As a decrease in Rac1 activity increases cofilin activity, we overexpressed a constitutively active mutant of cofilin (S3A), which cannot be phosphorylated and therefore increases actin remodeling31. Consistent with our HSV-Rac1-dn data, HSV-cofilin-ca injections into the NAc likewise increased the rewarding effects of cocaine without altering locomotor responses to the drug (Fig. 2b & 2c). Locomotor responses to saline were not changed by Rac1-ca, Rac1-dn, or cofilin-ca (Fig. 2d). Fig. 2e illustrates the ability to selectively target the NAc with our HSV vectors, and Fig. 2f illustrates the labeling of an NAc medium spiny neuron infected with HSV-GFP.
Figure 2. Rac1 signaling regulates behavioral responses to cocaine.
(a) HSV-mediated overexpression of Rac1-ca (n=10 mice) blocked cocaine conditioned place preference at 7.5 mg/kg compared to dsRed controls (n=7) (F2,23=4.00, *p<0.05), while HSV-Rac1-dn (n=9 mice) did not further enhance cocaine’s rewarding effects at this higher dose. (b) Using a lower dose of cocaine (4 mg/kg), which did not induce a conditioned place preference in control mice (n=13 mice), HSV-Rac1-dn (n=8) induced a robust preference to the drug (F3,37=3.33, *p<0.05 compared to control ). Overexpression of a constitutively active form of cofilin (HSV-cofilin-ca; n=10 mice) also increased the reward sensitivity to cocaine compared to controls (*p<0.05). (c) Rac1-ca (n=10 mice) attenuated locomotor activation following an acute injection of cocaine (7.5 mg/kg) (F3,41=3.06, p<0.05 compared to control), (d) but had no effect on locomotor responses to a saline challenge (p>0.05). (e) Anatomical placement of viral infection in NAc after HSV injection. Cartoon shows the location of the injection site at 1.77 mm from Bregma. (f) A representative HSV-infected NAc medium spiny neuron imaged at 40X. Rectangular boxes highlight areas used for dendritic spine analysis in Fig. 4. (g–i) High powered immunohistochemical images of Cre (green) and Rac1 (red) after HSV-Cre injection into the NAc of a floxed Rac1 mouse. Blue arrows highlight a Cre+ neuron in which Rac1 staining is completely absent, while white arrows indicate an adjacent non-Cre expressing neuron where Rac1 protein is strongly expressed. Consistent with previous reports of Rac1 cellular localization34, in NAc neurons, the Rac1 immunostaining concentrates at soma subplasma membrane. This result is representative of the analysis of numerous sections from 4 animals. Data were analyzed by ANOVA and represented as mean ± s.e.m.
To further study the role of Rac1 in cocaine-induced behavioral plasticity, and to ensure specificity of our Rac1-dn results (since Rac1-dn could conceivably interfere with the functioning of related small GTPases), adult homozygous floxed Rac1 mice32, 33 received bilateral intra-NAc injections of HSV vectors expressing Cre recombinase or GFP as a control. Overexpression of Cre significantly decreased Rac1 mRNA levels in the NAc as determined by quantitative PCR (qPCR) (21 ± 2.7% decrease compared to HSV-GFP controls; n=3–4 mice per group; t=2.60, df=6, p<0.05; analyzed by two-tailed t-test). The relatively small degree of knockdown likely reflects uninfected cells within the grossly dissected tissue, as our HSV vectors target neurons only and Rac1 is highly expressed in non-neural cells as well. Indeed, immunohistochemistry confirmed the complete loss of Rac1 from Cre+ medium spiny neurons of the NAc (Fig. 2g–i), while expression of GFP alone does not affect Rac1 staining (not shown). Using a low 4 mg/kg dose of cocaine, we found that floxed Rac1 mice injected with HSV-Cre showed a significant increase in cocaine preference—+238 ± 80.5 seconds compared to HSV-GFP injected floxed Rac1 control mice, which showed no preference to this cocaine dose (n=6 mice per group; t=2.35, df=10, p<0.05; analyzed by two tailed t-test). These data further support the ability of decreased Rac1 activity in NAc to increase sensitivity to cocaine reward.
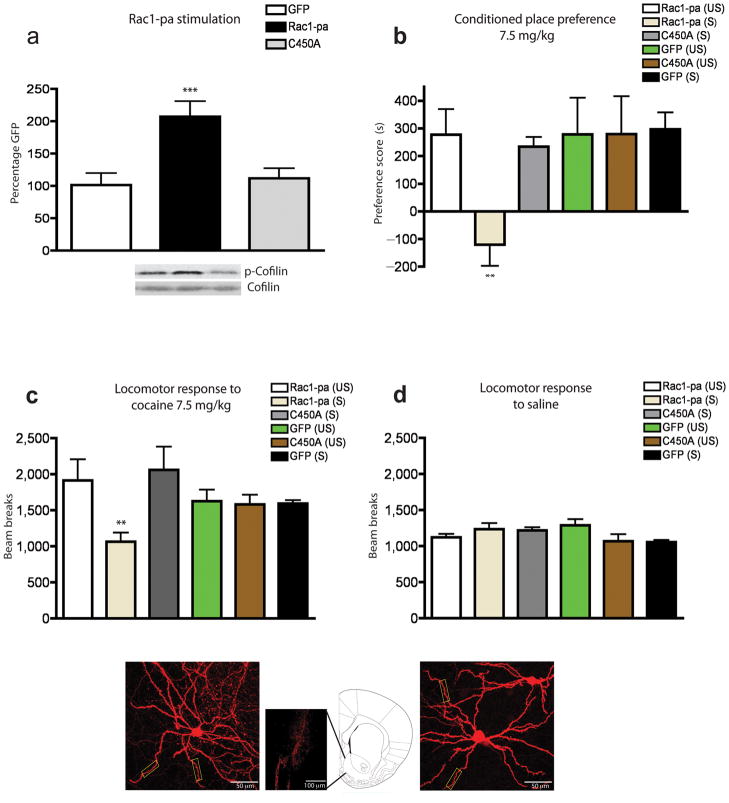
Since repeated cocaine induces a highly transient decrease in Rac1 activity in NAc that returns to normal levels within 45 minutes of a cocaine challenge, we sought to determine if a more temporally precise blockade of this decrease also blocks cocaine reward. To this end, we used a photoactivatible form of Rac1, where Rac1-ca is fused to the photoreactive LOV (light oxygen voltage) domain from phototropin; this prevents the interaction of Rac1 with effector proteins until stimulated with a 458–473 nm light34. This tool enabled us to test the behavioral consequences of discrete activation of Rac1 in NAc in vivo on a time scale that would functionally reverse the transient decrease in Rac1 caused by cocaine. Mice received intra-NAc injections of HSV-Rac1-photoactivatible (Rac1-pa), HSV-Rac1-pa(C450A)––a mutant that contains the identical LOV domain but is light-insensitive, or control HSV-GFP, and were fitted with cannulae to allow the passage of a fiber optic cable35. Light activation of Rac1-pa increased the phosphorylated form of cofilin when compared to both HSV-GFP or the light-insensitive mutant Rac1-pa(C450A), without a change in levels of total cofilin (Fig. 3a), demonstrating the efficacy of this construct in the NAc in vivo. Moreover, such discrete light activation of Rac1-pa prevented the formation of a conditioned place preference to cocaine (Fig. 3b) and suppressed locomotor activity to an acute injection of cocaine (Fig. 3c), without altering the locomotor response to saline (Fig. 3d), thus paralleling the effects of Rac1-ca. Overexpression of Rac1-pa without light stimulation had no effect on behavioral responses to cocaine. Similarly, overexpression and light stimulation of the control vectors (C450A or GFP) had no effect on place preference or locomotion. Overexpression of these Rac1-pa constructs had no effect on the structure of NAc medium spiny neurons (Fig. 3e–g). These data show that a highly temporally-discrete and transient activation of Rac1 activity and subsequent downstream signaling cascades in NAc is sufficient to attenuate the rewarding effects of cocaine, providing further evidence that the cocaine-induced transient downregulation of Rac1 activity in this brain region is required for normal rewarding effects of cocaine.
Figure 3. Temporal regulation of Rac1 signaling regulates behavioral responses to cocaine.
(a) Optical stimulation of Rac1 activity (by use of Rac1-pa) increased the phosphorylation state of cofilin (p-cofilin) (F2,13=8.72, ***p<0.005; n=4 mice) while light stimulation of the light insensitive mutant did not alter p-cofilin levels (p>0.05) compared to HSV-GFP controls (n=5 mice per group). Full-length blots are presented in Supplementary Fig. 3. (b) Such optical stimulation of Rac1 during cocaine pairing [Rac1-pa (S)] also prevented the formation of a conditioned place preference (n=8 mice) (F5,43=2.66, **p<0.01). This blockade required light activation of Rac1, since expression and stimulation of the light insensitive C450A mutant (n=7 mice) or HSV-GFP (n=7 mice) led to the formation of equivalent cocaine preferences as unstimulated (US) control mice (n=8 mice). (c) Light activation of the Rac1-pa construct blocked cocaine-induced increases in locomotion (n=7–8 mice per group) (F4,45=2.43, **p<0.01), (d) but had no effect on locomotor responses to saline (n=7–8 mice per group). (e) A representative infected NAc medium spiny neuron, imaged at 40X, infected with HSV-Rac1-pa. (f) Anatomical placement of viral infection in NAc after HSV injection. (g) A representative infected NAc medium spiny neuron, imaged at 40X, infected with HSV-Rac1-pa-C450A mutant. Rectangular boxes in f and g highlight areas used for dendritic spine analysis. Cartoon shows the location of the injection site at 1.77 mm from Bregma. Data were analyzed by ANOVA and represented as mean ± s.e.m.
Rac1 signaling regulates spine density and type on NAc neurons
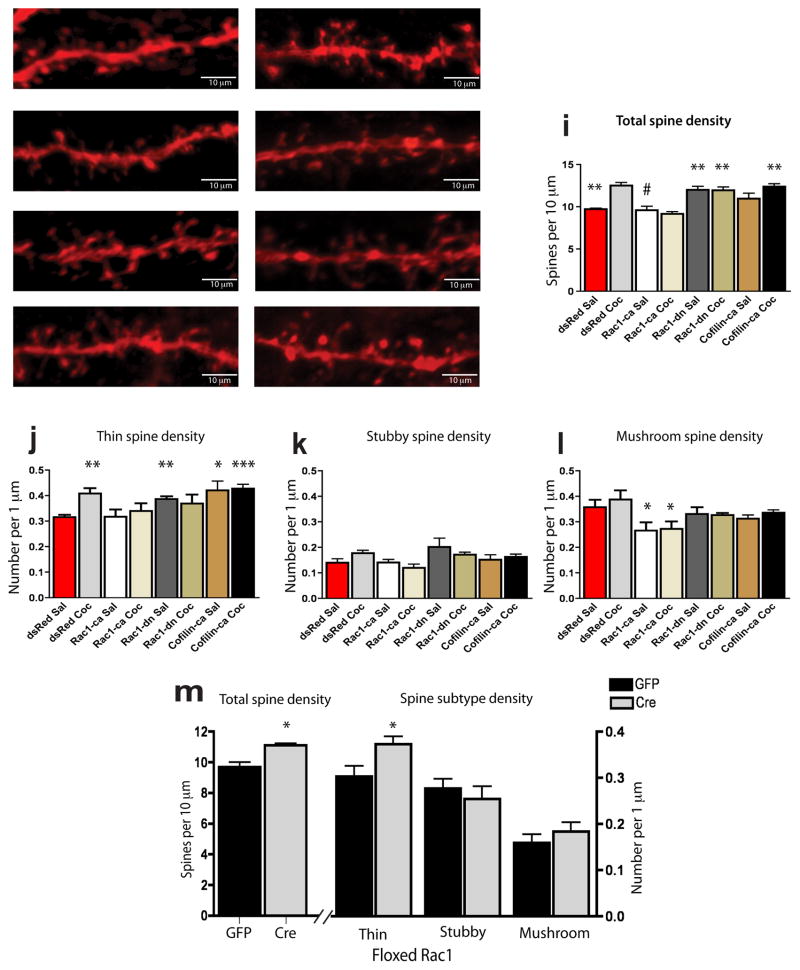
To directly examine the functional consequences of decreased Rac1 activity on spine morphology of NAc medium spiny neurons, we bilaterally injected HSV-Rac1-ca, HSV-Rac1-dn, or HSV-cofilin-ca, and compared results to injection of the HSV-dsRed control vector. Animals receiving these virus injections were then treated with repeated cocaine or saline. We found that, using this paradigm, repeated cocaine increased the number of dendritic spines on NAc medium spiny neurons compared to saline-treated mice, and that this increase was blocked completely by Rac1-ca, which had no effect on spine density under basal conditions. In contrast, overexpression of Rac1-dn induced an increase in spine density in saline-treated mice comparable to that observed in the HSV-dsRed cocaine group (Fig. 4a–i). The cocaine-induced increase in spine density in the HSV-dsRed cocaine group, compared to the HSV-dsRed saline group, was largely driven by an increase in thin spines (Fig. 4j), and this increase was mimicked by Rac1-dn overexpression, and blocked by Rac1-ca overexpression. In agreement with our Rac1-dn data, overexpression of HSV-cofilin-ca tended to increase total spine density under saline and cocaine conditions (Fig. 4i), which was significant when we looked specifically at the number of thin spines (Fig. 4j). In contrast, there was no significant effect of repeated cocaine on the density of more consolidated spines (i.e., stubby and mushroom) at the time point examined; however, we observed that Rac1-ca, but not Rac1-dn or cofilin-ca, by itself reduced the number of such spines (Fig. 4k–l).
Figure 4. Decreased Rac1 signaling mediates induction of NAc spines by cocaine.
Representative confocal images of viral-mediated dsRed expression after treatment with (a) saline or (b) cocaine; viral overexpression of Rac1-ca with (c) saline or (d) cocaine; viral overexpression of Rac1-dn with (e) saline or (f) cocaine; and viral overexpression of cofilin-ca with (g) saline or (h) cocaine. To correspond with the transient timescale of HSV expression, spine analyses were performed 4 hours after the last of 5 cocaine injections. (i) Cocaine significantly increased spine density of NAc medium spiny neurons (F1,16=6.50, p<0.001; main effect of cocaine effect by two-way ANOVA). Subsequent Tukey’s multiple comparison post-hoc test revealed that mice injected with dsRed and treated with cocaine (n=4 mice) had a significant increase in dendritic spine density compared with saline dsRed controls (n=4; **p<0.01). HSV-Rac1-ca (n=4 mice) blocked the cocaine-induced increase in spine density (#p<0.01 compared to dsRed cocaine), while HSV-Rac1-dn (n=3 mice) alone mimicked cocaine’s effects (**p<0.01) and this action was not further potentiated by cocaine (n=4 mice). There was a trend of HSV-cofilin-ca to increase spine number in the saline condition (n=5 mice), with the effect significant in the HSV-cofilin-ca cocaine group (n=5 mice; **p<0.01). (j) NeuronStudio spine type analysis of the same images (same n per group) revealed a main effect demonstrating that repeated cocaine alters the number of thin spines (F1,16=3.63, p<0.05). Further multiple comparisons post-hoc analysis revealed that, when compared to dsRed saline controls, dsRed cocaine treated mice had a significant increase in thin spines (**p<0.01) and this effect was mimicked by HSV-Rac1-dn (**p<0.01) as well as HSV-cofiliin-ca (*p<0.05), with no further effect of cocaine (***p<0.001). (k) There were no significant changes in the density of stubby spines in any of the treatment conditions. However, there was a trend for an increase in stubby spine density in the Rac1-dn saline group compared to dsRed saline. (l) Neither cocaine Rac1-dn nor cofilin-ca had any effect on mushroom spines, although in both the saline and cocaine conditions Rac1-ca overexpression reduced the density of mushroom spines (F2,20=2.83, *p<0.05). Data were analyzed by two-way ANOVA followed by Tukey’s multiple comparisons post hoc test and represented as mean ± s.e.m. (m) Total spine density in floxed Rac1 mice after HSV-Cre (n=3 mice) or HSV-GFP injection into NAc (n=4 mice). Data analysis by two-tailed t-test demonstrated that such local knockdown of Rac1 leads to an increase in spine density (t=3.71, df=5, *p<0.05), and (n) this increase is selective for thin spines (t=4.69, df=5, **p<0.01) with no change in the density of stubby spines or mushroom spines. Data are represented as mean ± s.e.m.
To further confirm the role of Rac1 in cocaine-induced spine plasticity in NAc, we repeated our morphological experiments using the floxed Rac1 mice. Consistent with our Rac1-dn results, HSV-Cre knockdown of Rac1 selectively in the NAc increased total spine density, and this increase was largely due to an increase in thin spines, with no changes observed in the density of stubby or mushroom spines (Fig. 4m).
We next used our photoactivatible Rac1 mutants to further explore the role of Rac1 in regulation of NAc neuron spine density in a more temporally precise manner. Brief light stimulation of animals injected intra-NAc with HSV-Rac1-pa following each injection of cocaine blocked the cocaine induction of spines (9.36 ± 0.306 spines per 10 μm (n=5 mice) vs. 9.78 ± 0.1059 for GFP saline controls (n=4 mice)) compared to the light insensitive mutant HSV-Rac1-pa(C450A) (12.0 ± 0.970 spines per 10 μm vs. 12.5 ± 0.343 for GFP cocaine; n=4 mice per group; F3,16=9.26, p<0.001; analyzed by ANOVA). These findings demonstrate that preventing the transient decrease in Rac1 activity in the NAc is sufficient to block cocaine-induced structural plasticity of medium spiny neurons.
Discussion
The findings of this study provide fundamentally novel insight into the molecular basis by which cocaine induces dendritic spine plasticity in the NAc. The data establish that downregulation of Rac1 activity induced by repeated cocaine is both necessary and sufficient for the cocaine-mediated increase in thin dendritic spines on NAc medium spiny neurons. The transient reduction in Rac1 signaling enhances actin turnover, as evidenced by the decrease in the inactive (phosphorylated) form of cofilin, which leads to more coflin activity and more thin spines. Our results also directly relate such downregulation of Rac1 signaling, and enhanced cofilin activity, in the NAc to greater sensitivity to the rewarding effects of cocaine. Indeed, the use of Rac1-pa, which allowed the novel manipulation of an intracellular signaling protein in real time in vivo, made it possible to examine the structural and behavioral consequences of the highly transient cocaine-induced decrease in Rac1 activity selectively within the adult NAc.
While our findings are in agreement with previous results demonstrating the importance of the cofilin pathway and actin dynamics in cocaine-induced structural plasticity, the precise details of cocaine regulation of cofilin phosphorylation are more complicated16–18. For example, Shen et al.17 reported no change in p-cofilin, but an increase in total cofilin, in response to a cocaine challenge in rats withdrawing from prior chronic cocaine exposure. This contrasts with our data showing a transient decrease in p-coflin, and no change in total cofilin, in response to a cocaine challenge. These apparent discrepancies may be due to several factors that are known to be critical for drug-induced plasticity, such as the use of rats vs. mice, the withdrawal times after the course of chronic cocaine (24 hours in our experiments and 3 weeks in Shen et al.), or methodological differences in measuring cofilin (total NAc extracts in our study and crude postsynaptic density fractions in Shen et al.). Importantly, however, the net effect—an increase in cofilin and actin dynamics—is the same in both studies. Moreover, the finding that inhibiting actin turnover by local injection of latrunculin into NAc blocks cocaine-induced increases in spine density and locomotor sensitization18, along with the report of RhoA GTPase downregulation by chronic cocaine36, further support the role of enhanced actin dynamics in mediating structural and behavioral plasticity to chronic cocaine.
Previous reports have suggested that Rac1 activity promotes spine development, while inhibition of Rac1 reduces spine number, in other neural systems37. However, the role of Rac1 in the regulation of actin dynamics and spine morphology is far more complicated and depends on many factors such as age and neuronal type, and may even vary between in vivo vs. in vitro systems22, 23, 25. Here, we demonstrate that decreased Rac1 signaling in the NAc in vivo increases spine formation, particularly of more immature, thin spines through a cofilin-mediated mechanism. Cofilin activity has been shown previously to increase actin depolymerization, nucleation, and branching, ultimately leading to thinner spines and new cellular protrusions38. However, it should be noted that the changes in Rac1 and cofilin activity observed here may not occur exclusively at the spine, but could instead occur throughout the entire neuron, including the soma where Rac1 has been shown to regulate gene transcription39.
Cocaine-induced behavioral and synaptic plasticity has been strongly associated with adaptations in excitatory glutamatergic transmission in the NAc6, 40–43. For example, at early withdrawal time points after the last cocaine exposure, including those examined here, there is an increase in thin (more highly plastic) spines and synaptic depression17, 44, perhaps representing an increased pool of silent synapses45. The role of Rac1 signaling in mediating silent synapse formation, which has not yet been investigated directly, now warrants examination. It will also be important in future studies to determine whether the influence of Rac1 on cocaine regulation of spine plasticity of NAc medium spiny neurons is selective for various subtypes of these neurons, which play distinct roles in the addiction process35.
Recently, cocaine has been reported to induce kalirin-7, another Rho GEF, and loss of kalirin-7 in knockout mice blocks cocaine’s induction of NAc spines and cocaine reward46. However, how these findings on kalirin-7 relate to Rac1 is unknown, since kalirin-7 induction would be expected to increase Rac1 activity and we show here that downregulation of Rac1, not activation, induces spines and cocaine reward. It is possible that the paradoxical effects seen in kalirin-7 knockout mice are mediated via loss of kalirin-7 in other brain regions or earlier in development, or perhaps mediated via actions of kalirin-7 on Rho GTPases other than Rac1.
While repeated administration of opiate drugs of abuse, like psychostimulants, causes sensitized behavioral responses to the drugs, opiates decrease dendritic spine density on NAc medium spiny neurons in striking contrast to the induction seen with psychostimulants5. Virtually nothing is known about the effect of opiate drugs on activity of the Rac1-cofilin pathway in the NAc, and the role of actin dynamics in mediating opiate-induced addictive behaviors remains unexplored. Moreover, while several prior studies that blocked cocaine-induced increases in spine density, through a variety of pharmacological and molecular manipulations, concomitantly observed blunted behavioral effects of cocaine10, 13, 18, several studies have seen the opposite9, 11, 12, 16. These findings highlight the need for further research to carefully delineate the likely complex and time-dependent role of spine plasticity of NAc medium spiny neurons in mediating distinct aspects of behavioral adaptations to cocaine. One key consideration is that most studies that have examined drug-induced morphological plasticity in the NAc have relied, as we do in the present investigation, on the use of investigator-administered drug. An important subject for future studies is to determine whether the molecular changes seen using such non-contingent drug administration are the same as those that occur with drug self-administration paradigms, highlighting the critical importance of investigating Rac1 signaling in these additional models of drug addiction47–49.
Together, our data support a scheme whereby repeated administration of cocaine leads to downregulation of the Rac1 GEF Tiam1 and upregulation of the Rac GAP RacGAP1, thereby priming NAc medium spiny neurons for transient reductions in Rac1 activity in response to a subsequent cocaine challenge. This cascade (Supplemental Fig. 1) may be a mechanism by which chronic cocaine exposure induces long-term changes in plasticity in NAc, and provides new directions for the development of novel therapies for cocaine addiction.
Methods
Animals and Treatments
All mice were housed four to five per cage in a colony with a 12 hour light/dark cycle (lights on from 7:00 A.M. to 7:00 P.M.) at constant temperature (23°C) with ad libitum access to water and food. 8- to 10-week-old male C57BL/6J mice received 7 daily i.p. injections of saline (7 treatments saline), acute cocaine (6 treatments saline + one treatment cocaine-HCl 20 mg/kg) or repeated cocaine (7 treatments cocaine). Mice were used 15 min, 45 min, 4 hours, or 24 hours after the last injection. All animal protocols were approved by the Mount Sinai School of Medicine IACUC.
We used the bi-cistronic p1005+ HSV vector, expressing dsRed alone or with Rac1-ca or Rac1-dn. Cofilin-ca(S3A), Rac1-pa, and Rac1-pa(C450A) were expressed with the same HSV vector where GFP replaced dsRed. Extensive prior work has shown that HSV vectors infect neurons only and, within the NAc, the vast majority of neurons infected are medium spiny neurons which comprise ~95% of all neurons in this region50. Mice were anesthetized with ketamine (100 mg/kg)/xylazine (10 mg/kg), placed in a small-animal stereotaxic instrument, and their skull surface was exposed. For Rac1 and cofilin vectors, thirty-three gauge syringe needles were used to unilaterally infuse 0.5 μl of virus into the NAc of both hemispheres at a 10° angle (AP + 1.6; ML + 1.5; DV − 4.4) at a rate of 0.1 μl/min. For Rac1-pa experiments, mice were cannulated as previously published35, and 0.5 μl of either HSV-Rac1-pa, HSV-Rac1-pa(C450A) or HSV-GFP was injected into the NAc of the right hemisphere at a 0° angle (AP + 1.4; ML + 1.3; DV − 4.3) at a rate of 0.1 μl/min. Next, a 20-gauge cannula, 4 mm in length from the cannula base, was implanted into the right hemisphere (AP + 1.4; ML + 1.3; DV − 3.9). Instant adhesive was placed between the base of the cannula and the skull, and skull fixture adhesive was used to cement the cannula in place.
To induce local deletion of the Rac1 transcript restricted to NAc neurons, we used mutant mice homozygous for a floxed Rac1 allele. The floxed allele contains loxP sites flanking exon 1 including the translation start site33. Mice were sterotaxically-injected into the NAc with HSV-GFP or HSV-Cre-GFP between the age of 10 and 13 weeks.
All injection/cannula placements were verified, and <5% of all animals with misplaced sites were excluded from subsequent analyses.
GTPase Activity Assay
Active Rac1 or CDC42 was immunoprecipitated using a monoclonal antibody that specifically recognizes the GTP-bound but not GDP-bound form of these proteins (Neweast Biosciences). The immunoprecipitate was then western blotted for total Rac1 or CDC42, respectively
Dendritic Spine Analysis
To study the role of Rac1 in the regulation of NAc medium spiny neuron morphology in vivo, we used methods previously described with the following modifications9, 10, 13. Briefly, two days after viral vector injections, when HSV expression is maximal, animals were injected 5 times over three days with either cocaine (20 mg/kg i.p.) or saline. For the Rac1-pa spine studies, 10 minutes following each cocaine injections, mice were stimulated with 473 nm blue laser diode for 20 minutes. Mice were sacrificed by transcardial perfusion four hours following the last treatment, and brains were later sectioned at 100 μm on a vibratome. Sections were then immunostained using an antibody against GFP (Invitrogen) or DsRed (Clontech) respectively. All HSV images were captured using a laser confocal microscope with a 100X oil-immersion objective. Images were acquired with the pinhole set at 1 arbitrary unit and a 1024 x 1024 frame size. Dendritic length was measured using NIH Image J software, and spine numbers were counted, blind by the primary experimenter, as slides were coded prior to experimental scanning. The average number of spines per 10 μm of dendrite was calculated. We measured the number of spines on 1–2 neurites per neuron equaling at least 300 μm of secondary dendrites from dsRed-expressing NAc medium spiny neurons. We examined 6–8 neurons per animal with 3–4 animals per group (7 groups), after which an average value was obtained for each animal for statistical analysis. Spine type analysis was performed using the semi-automated software NeuronStudio (http://research.mssm.edu/cnic/tools-ns.html)9.
Western Blotting
NAc punch dissections were homogenized in 30 μL of homogenization buffer containing 320 mM sucrose, 5 nM Hepes buffer, 1% SDS, phosphatase inhibitor cocktails I and II (Sigma), and protease inhibitors using an ultrasonic processor. Protein concentrations were determined using a DC protein assay and 30 μg of protein were loaded onto Tris-HCL polyacrylamide gels for electrophoresis. The samples were then transferred to a nitrocelluose membrane and blocked for one hour in Odyssey blocking buffer. Blocked membranes were incubated overnight at 4°C in Odyssey blocking buffer with the following primary antibodies: 1:500 phospho-cofilin and 1:1000 cofilin (Cell Signaling); 1:500 RacGap1 and Tocris, and 1:300 Tiam1 (Sigma); 1:500 total Rac1 (NewEastBio). After thorough washing, the membranes were incubated with respective IRDye secondary antibodies (1:5000 to 1:10000) dissolved in Odyssey blocking buffer for 1 hour at room temperature. All total proteins were normalized to tubulin (Cell Signaling 1:50000). The blots were imaged with the Odyssey Infrared Imaging system and quantified by ImageJ.
Quantitative PCR
NAc punch dissections were homogenized in Trizol (Invitrogen) and processed according to the manufacturer’s instructions. RNA was purified with RNAeasy Micro columns (Invitrogen). The RNA was reverse transcribed using a Bio-Rad iScript Kit. cDNA was quantified by qPCR using SYBR Green. Each reaction was run in triplicate and analyzed following the ΔΔCt method as previously described10. The Following primer pairs were used to confirm Rac1 knock down:
Rac1 F-CAGTGAATCTGGGCCTATGG
Rac1 R-ACAGTGGTGTCGCACTTCAG
Gapdh F-AGGTCGGTGTGAACGGATTTG
Gapdh R-TGTAGACCATGTAGTTGAGGTCA
Behavioral Assays
To examine the effect of Rac1 and cofilin on cocaine reward, we used an unbiased conditioned place preference paradigm similar to one used in a previous study13. Mice were conditioned for two days to saline injections (i.p.) in one chamber for 30 min during a morning session and to 7.5 or 4 mg/kg cocaine injections (i.p.) to the opposite chamber for 30 min during an afternoon session. The day after the second conditioning, mice were tested for place preferences during a 20-min session where they were allowed to freely explore all 3 chambers.
Locomotor activity was recorded automatically by infrared photobeams 30 minutes following an acute injection of saline or cocaine (7.5mg/kg i.p.).
Light Activation of Rac1-pa
For in vivo validation of the Rac1-pa constructs, mice were stereotaxically injected with HSV-Rac1-pa or an HSV expressing the light-insensitive mutant, HSV-Rac1-pa(C450A), and cannulated as previously published35. Three days later, an optic fiber was placed in the cannula. We used a 200 μm core optic fiber that was modified such that when the optic fiber was secured in vivo to the cannula, the fiber was flush with the length of the cannula with ~50 μm of the stripped 200 μm core exposed beyond the cannula35. Optic fibers were attached through an FC/PC adaptor to a 473 nm blue laser diode and continuous light was generated through a stimulator. Optic fiber light intensity was measured using a light sensor; light intensity was ~15mW/mm2 at the tip of the fiber.
For conditioned place preference experiments, mice were injected with either HSV-Rac1-pa, HSV-Rac1-pa(C450A), or HSV-GFP. The 473 light was turned on for the duration of the cocaine pairing. Conditions were identical during the saline pairing without light stimulation. To ensure specificity of the Rac1-pa construct, a group of mice was injected with the same constructs and underwent identical treatment with the exception that they remained “unstimulated” (i.e., cannulae and laser implantation but no light stimulation) during cocaine training.
Statistics
Two way ANOVAs (viral vector x drug injection) were used for dendritic spine analysis followed by Tukey’s multiple comparisons post-hoc analysis when appropriate. One-way ANOVAs were used for behavioral experiments and Rac1 activity assays followed by planned comparisons as post-hoc analysis. Student’s t-tests (2 sample, 2 tailed) were used for verification of gene knockdown, as well as spine and behavioral analysis of floxed Rac1 mice. These tests were chosen based on the design of each experiment according to established standards in the field.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse and National Institute of Mental Health. We thank Mr. Samuel Golden, Dr. Alfred Robison, and Dr. Vincent Vialou for helpful discussions and comments on the manuscript.
Footnotes
Competing financial interests: The authors declare no competing financial interests.
Author Information: Reprints and permissions information is available at www.nature.com/reprints.
Author Contributions D.M.D., S.J.R., and E.J.N. were responsible for overall study design. D.M.D., H.S., K.C.D., C.D., and I.M. designed and conducted GTPase activity assays and analyzed the data. D.M.D., M.E.C., J.K., and D.F. performed the stereotactic surgeries and performed behavioural experiments. D.M.D., M.S.R., D.D.W., V.G., and H.S. performed and analyzed the Western blots. D.M.D., D.C., and V.G. scanned, counted, and analyzed the spine data. D.M.D., M.K.L., H.S., K.N.S., G.E.H., S.J.R., Y.O., and K.H. designed and performed the necessary cloning, and conducted the optical Rac1-pa experiments. Y.Z. provided the floxed Rac1 mice and expertise in Rac1 signalling; R.L.N. constructed and provided the viral vectors for gene transfer. D.M.D. and E.J.N. wrote the paper with the help of the other authors.
References
- 1.Garey LJ, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo SJ, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soetanto A, et al. Association of anxiety and depression with microtubule-associated protein 2- and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch Gen Psychiatry. 2010;67:448–457. doi: 10.1001/archgenpsychiatry.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 5.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 7.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 8.Deng JV, et al. MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nat Neurosci. 2010;13:1128–1136. doi: 10.1038/nn.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaPlant Q, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maze I, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norrholm SD, et al. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulipparacharuvil S, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo SJ, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 15.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:759–759. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 16.Toda S, Shen HW, Peters J, Cagle S, Kalivas PW. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J Neurosci. 2006;26:1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen HW, et al. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toda S, Shen H, Kalivas PW. Inhibition of actin polymerization prevents cocaine-induced changes in spine morphology in the nucleus accumbens. Neurotox Res. 2010;18:410–415. doi: 10.1007/s12640-010-9193-z. [DOI] [PubMed] [Google Scholar]
- 19.Halpain S. Actin and the agile spine: how and why do dendritic spines dance? Trends Neurosci. 2000;23:141–146. doi: 10.1016/s0166-2236(00)01576-9. [DOI] [PubMed] [Google Scholar]
- 20.Penzes P, Jones KA. Dendritic spine dynamics - a key role for kalirin-7. Trends Neurosci. 2008;31:419–427. doi: 10.1016/j.tins.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi-Takagi A, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- 23.Tashiro A, Yuste R. Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol Cell Neurosci. 2004;26:429–440. doi: 10.1016/j.mcn.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Oh D, et al. Regulation of synaptic Rac1 activity, long-term potentiation maintenance, and learning and memory by BCR and ABR Rac GTPase-activating proteins. J Neurosci. 2010;30:14134–14144. doi: 10.1523/JNEUROSCI.1711-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo L, et al. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- 26.Yang N, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 27.Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 28.Shirazi Fard S, Kele J, Vilar M, Paratcha G, Ledda F. Tiam1 as a signaling mediator of nerve growth factor-dependent neurite outgrowth. PLoS ONE. 2010;5:e9647. doi: 10.1371/journal.pone.0009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyamoto Y, Yamauchi J, Tanoue A, Wu C, Mobley WC. TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc Natl Acad Sci U S A. 2006;103:10444–10449. doi: 10.1073/pnas.0603914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marinissen MJ, et al. The small GTP-binding protein RhoA regulates c-Jun by a ROCK-JNK signaling axis. Molecular Cell. 2004;14:29–41. doi: 10.1016/s1097-2765(04)00153-4. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Melendez J, Campbell K, Kuan CY, Zheng Y. Rac1 deficiency in the forebrain results in neural progenitor reduction and microcephaly. Dev Biol. 2009;325:162–170. doi: 10.1016/j.ydbio.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu Y, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 34.Wu YI, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lobo MK, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim WY, Shin SR, Kim S, Jeon S, Kim JH. Cocaine regulates ezrin-radixin-moesin proteins and RhoA signaling in the nucleus accumbens. Neuroscience. 2009;163:501–505. doi: 10.1016/j.neuroscience.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 37.Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh M, et al. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304:743–746. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]
- 39.Bosco EE, Mulloy JC, Zheng Y. Rac1 GTPase: a "Rac" of all trades. Cell Mol Life Sci. 2009;66:370–374. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 45.Huang YH, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiraly DD, et al. Behavioral and morphological responses to cocaine require Kalirin7. Biol Psychiatry. 2010;68:249–255. doi: 10.1016/j.biopsych.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen BT, et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrot M, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.