Abstract
Axon regeneration in the central nervous system is severely hampered, limiting functional recovery. This is in part because of endogenous axon regeneration inhibitors that accumulate at the injury site. Therapeutic targeting of these inhibitors and their receptors may facilitate axon outgrowth and enhance recovery. A rat model of spinal cord contusion injury was used to test the effects of two bacterial enzyme therapies that target independent axon regeneration inhibitors, sialidase (Vibrio cholerae) and chondroitinase ABC (ChABC, Proteus vulgaris). The two enzymes, individually and in combination, were infused for 2 weeks via implanted osmotic pumps to the site of a moderate thoracic spinal cord contusion injury. Sialidase was completely stable, whereas ChABC retained>30% of its activity in vivo over the 2 week infusion period. Immunohistochemistry revealed that infused sialidase acted robustly throughout the spinal cord gray and white matter, whereas ChABC activity was more intense superficially. Sialidase treatment alone resulted in improved behavioral and anatomical outcomes. Rats treated exclusively with sialidase showed significantly increased hindlimb motor function, evidenced by higher Basso Beattie and Bresnahan (BBB) and BBB subscores, and fewer stepping errors on a horizontal ladder. Sialidase-treated rats also had increased serotonergic axons caudal to the injury. ChABC treatment, in contrast, did not enhance functional recovery or alter axon numbers after moderate spinal cord contusion injury, and dampened the response of sialidase in the dual enzyme treatment group. We conclude that sialidase infusion enhanced recovery from spinal cord contusion injury, and that combining sialidase with ChABC failed to improve outcomes.
Key words: axon regeneration, chondroitin sulfate, ganglioside, motor behavior, serotonergic axons
Introduction
After spinal cord injury (SCI) axon regeneration is severely limited, hampering functional recovery. This failure of axon regeneration is caused, at least in part, by endogenous axon regeneration inhibitors that accumulate at the injury site.1,2 These include molecules found on residual myelin debris (Nogo, myelin-associated glycoprotein [MAG], and oligodendrocyte-myelin glycoprotein [OMgp]) and chondroitin sulfate proteoglycans (CSPGs) expressed primarily by reactive astrocytes in the glial scar. Axon regeneration inhibitors bind to complementary receptors on axons, initiating signaling cascades that halt axon growth. Targeting these inhibitors, their receptors, or their associated signaling pathways may reduce inhibition and release axons to sprout and/or regenerate, enhancing functional recovery. This concept has been validated in mechanism-based pre-clinical SCI injury models.3–8
Among axon regeneration inhibitor-targeted experimental therapies, two bacterial enzymes have emerged as potential drugs to treat SCI. Chondroitinase ABC (ChABC) cleaves inhibitory CSPGs,9 and sialidase cleaves sialoglycan receptors for MAG.6 ChABC infusion enhanced recovery after experimental nigrostriatal lesions10 and in several (but not all) animal models of SCI.8,11,12 Sialidase enhanced recovery in experimental brachial plexus avulsion,13 and after spinal cord contusion6 in the rat. As ChABC and sialidase act on different substrates, we hypothesized that their therapeutic effects may be additive or synergistic. To test this hypothesis we delivered ChABC, sialidase, and combination (ChABC/sialidase) therapy to the site of a spinal cord contusion in the adult rat. Behavioral, physiological, and anatomical outcomes were measured, allowing us to compare these two treatments when delivered independently and in combination.
Methods
Enzymes
Recombinant Vibrio cholera sialidase was overproduced in Escherichia coli from a plasmid kindly provided by Dr. Garry Taylor (University of St. Andrews, St. Andrews, U.K.) and purified as previously described.6 Chondroitinase ABC (Proteus vulgaris, protease free) was purchased from Seikagaku (Tokyo, Japan). Sialidase activity was determined using 2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (Sigma-Aldrich, St. Louis, MO),14 with 1 U defined as release of 1 μmol 4-methylumbelliferone per min at 37°C and pH 7.3. ChABC activity was determined using a colorimetric assay15 or by ΔA23216 as previously described, with 1 U defined as production of 1 μmole of unsaturated disaccharide from shark cartilage chondroitin sulfate C per min at 37°C and pH 8.0. Sialidase, ChABC, or both enzymes were diluted in carrier (137 mM NaCl, 2.7 mM KCl, 6.4 mM Na2HPO4, 1.5 mM KH2PO4, 0.5 mM CaCl2, and 1 mg/mL fatty acid free rat serum albumin [Sigma-Aldrich]) at a final concentration of 2 U/mL of each enzyme. Infusion solutions were filter-sterilized before use. Enzymes and carrier components were screened for the presence of endotoxin (LAL Endotoxin Assay, GenScript, Piscataway, NJ) according to the manufacturer's instructions.
SCI and treatment
All procedures were approved by the Johns Hopkins Animal Care and Use Committee and were consistent with federal law and NIH regulations. Johns Hopkins Medical Institutions are accredited by the American Association for Accreditation of Laboratory Animal Care.
Rats were randomly assigned to one of four treatment groups: carrier control (saline containing rat serum albumin), 2 U/mL ChABC in carrier, 2 U/mL sialidase in carrier, or 2 U/mL each (sialidase and ChABC) in carrier. Treatments were coded, and evaluators were blinded to the treatment groups.
Female Sprague–Dawley rats (250–275 g) were anesthetized with an intraperitoneal mixture of ketamine (60 mg/kg, Phoenix Pharmaceutical, St. Joseph, MD) and medetomidine (Domitor, 40 mg/kg, Pfizer Animal Health, New York, NY). Under aseptic conditions, the dorsal skin was shaved and sterilized; and a laminectomy was performed at the T9 and T13 vertebrae. A rat intrathecal catheter (Durect Corp., Cupertino, CA) was inserted subdurally and guided rostrally to T10. Silk sutures and cyanoacrylate were used to anchor the catheter in place. A moderate contusion injury (200 kdyne) was performed at T9 using the Infinite Horizons Impactor (Precision Systems and Instrumentation, Lexington, KY). Immediately after injury, a bolus injection of 50 μL of treatment solution was administered via the intrathecal catheter, which was then connected to a Lynch coil reservoir containing the same solution.17 The reservoir was attached to a subcutaneous osmotic pump pre-filled with Evan's blue dye solution (Alzet Model 2002, 200 μl, 0.5 μL/h, 14 days; Durect Corp.). After skin closure, animals were revived from anesthesia using Antisedan (atipamezole hydrochloride; 1.3 mg/kg, i.m., Pfizer Animal Health, New York, NY). Fluid supplementation (Lactated Ringer's) and gentamicin (5 mg/kg, Quality Biological, Inc., Gaithersburg, MD) were administered daily. Bladder expression was performed twice daily until bladder function returned.
Two weeks following injury, rats were anesthetized with a mixture of ketamine (15 mg/kg) and Domitor (10 mg/kg) and the catheter, coil, and osmotic pump were removed. Animals were revived from anesthesia using Antisedan (1.3 mg/kg). The distance of Evan's blue infusion into the Lynch coil was measured to determine pumping efficacy, and residual treatment solution in the distal coils was recovered and tested for remaining enzyme activities.
Motor behavior and autonomic responses
Behavioral tests and analyses were performed by observers blinded to the treatments. On days 1, 4, and 7 post-injury, and weekly thereafter, hindlimb motor function was assessed using the open-field Basso Beattie and Bresnahan (BBB) locomotor test.18 BBB subscores were calculated using a method previously described.19 Animals that obtained a BBB score of ≥7 at day 4 post-injury were considered to have insufficient initial injury and were removed from the study. Forty-four of 61 animals that entered the protocol were retained for subsequent analyses.
To evaluate fine motor control, rats were assessed for their ability to cross a 70 cm horizontal ladder with a fixed set of randomly spaced support bars. Animals were tested at 5 weeks post-injury. Two trials were video recorded and the total number of steps and hindlimb foot slip errors was recorded in each trial. When an attempted placement failed to make contact with the ladder rung, it was noted as missed, and further characterized as complete (to the hip joint), major (to the knee joint), or minor.
To assess the properties of baroreceptor regulation, we measured changes in renal sympathetic nerve activity (RSNA) resulting from induced changes in arterial blood pressure (AP) as previously described6,20 on a randomly selected subset of animals. Rats were initially anesthetized with isoflurane, which was replaced by intravenous doses of α-chloralose (100 mg/kg) sufficient to maintain a surgical plane of anesthesia for the duration of experiments. Arterial catheters were placed for measurement of AP, and venous catheters were placed for venous infusions of drugs used to manipulate AP. The left renal nerve was approached through a left dorsal laparotomy and dissected free of fat and connective tissue. After either ligating or cutting the renal nerve proximal to the kidney (to prevent recording of afferent nerve activity from the kidney), the intact portion of the nerve was placed on bipolar electrodes in a mineral oil pool. Differential recordings were amplified and stored for off-line processing. Increases in RSNA were measured in response to decreases in AP to 60 mmHg below baseline levels, produced by ramped intravenous infusions of sodium nitroprusside. Decreases in RSNA in response to increases in AP to 60 mmHg above baseline levels were then produced by ramped intravenous infusions of phenylephrine. From these measurements, we calculated the maximum excursions of RSNA in response to maximal changes in AP and the maximum slopes of the AP–RSNA relationships.
Histology and immunohistochemistry
At 5–6 weeks post injury, rats were anesthetized and transcardially perfused with Dulbecco's phosphate buffered saline (PBS) followed by 4% paraformaldehyde in phosphate-buffered saline (PBS). Spinal cords were dissected and post-fixed 12–16 h in the same fixative, then cryoprotected in 30% sucrose. The spinal cord was embedded in Shandon M-1 Embedding Matrix (Thermo Scientific, Waltham, MA) and serial 15 μm cryosections were cut in sets on a longitudinal plane and thaw-mounted onto Super-Frost Plus slides (Thermo Scientific, Pittsburgh, PA). Transverse cryosections of spinal cord were collected 7–8 mm rostral and caudal to the injury site.
For histochemical analyses of lesion areas, 12 longitudinal sections (1.5 cm length and 15 μm/section thickness) spaced 150 μm apart were rehydrated and stained with myelin-specific Eriochrome cyanine and cresyl violet (cell bodies), dried, then mounted under Krystalon mounting medium (EMD Chemicals, Gibbstown, NJ). Digitized photographs of the stained sections were taken using a Nikon 90i microscope with automated stage. A 4.5×4.5 mm boxed region of interest (ROI) was placed around the injury epicenter and individual areas pertaining to white matter, gray matter, lesioned tissue, and cavity were digitially traced within the ROI to generate cross-sectional areas. Lesioned tissue was visually identified according to the presence of necrotic tissue and infiltration of inflammatory cells. Injured penumbra denoted the boundary of lesioned tissue, whereas the injury cavity was demarked by the absence of tissue. The total cross-sectional areas of the lesioned tissue and cavity were calculated using NIS-Elements software (Nikon, Melville, NY). The individual subvolumes were obtained by multiplying the cross-sectional areas of lesioned tissue or cavity by the distance between sections, and the subvolumes were added to obtain the total volume of lesioned tissue or cavity. All analyses were performed by investigators blind to the treatment groups.
For immunohistochemistry, transverse spinal cord sections (15 μm thickness) were pretreated for 2 h at ambient temperature with a solution of 5% goat serum, 10 mg/mL bovine serum albumin, and 0.5% Triton X-100 in PBS, then were incubated in primary antibodies against serotonin (5-hydroxytryptamine [5-HT]) (1:5000, ImmunoStar, Inc., Hudson, WI), tyrosine hydroxylase (TH) (1:500, Chemicon, Temecula, CA), or calcitonin gene-related peptide (CGRP) (1:1000, Peninsula Laboratories Inc., Belmont, CA) overnight at 4°C. After three washes in PBS, sections were incubated with appropriate Cy3-conjugated secondary antibody (1:200, 2 h, ambient temperature; Jackson ImmunoResearch, West Grove, PA). Sections were washed and mounted as described. Fluorescently labeled 5-HT fibers were detected in bilateral ventral horns of individual sections under 10×magnification and imaged at equal intensities. A 0.5 mm×0.5 mm boxed ROI was placed over each individual ventral horn in three transverse sections (spaced 150 μm apart) 7.5–8.0 mm caudal to the lesion epicenter. 5-HT positive fibers were distinguished from background staining and quantified within the ROI using NIS-Elements software. Relative pixel areas represented by 5-HT were converted into total fiber immunoreactive areas based on internal calibration. Areas collected from six individual ventral horns across three sections were averaged. All analyses were performed by investigators blind to the treatment group.
Enzyme efficacy in vivo
To test the efficacy of intrathecally delivered enzymes during treatment, spinal cords from one set of rats were collected 12 days into the treatment protocol. Twelve days after injury and treatment (as described), the rats were perfused, spinal cords dissected and cryoprotected, and 20 μm longitudinal sections were collected onto slides. Sialidase efficacy in vivo was determined using antibodies to the sialidase substrate, ganglioside GT1b, and the sialidase product, ganglioside GM1.21 Sections were blocked in PBS containing 10 mg/mL bovine serum albumin and 5% goat serum in PBS for 5 h at 4°C, and then were incubated in the same buffer containing 1 μg/mL of monoclonal antibodies against GT1b (GT1b-1) or GM1 (GM1-1) for 16 h at 4°C. Slides were washed with PBS, and then incubated in the same buffer containing Cy3-labeled goat anti-mouse immunoglobulin G (IgG) (7 μg/mL, Jackson Immunoresearch) for 16 h at 4°C. Sections were washed with PBS and water, dried, and mounted as described previously. ChABC efficacy in vivo was determined using a monoclonal antibody that binds to unique unsaturated uronic acid epitopes created by the enzyme. Sections were blocked in a solution of 10 mg/mL bovine serum albumin, 10% goat serum, and 0.3% Triton X-100 in PBS for 2 h at ambient temperature, and then with primary monoclonal antibody 2-B-6 (1:200, Seikagaku) overnight at 4°C. Sections were washed in PBS and incubated in secondary antibody for 2 h, ambient temperature, then washed and mounted as described previously. Composite fluorescent images were acquired at equal intensities and compiled using a Nikon Eclipse 90i microscope.
Statistical analysis
Statistical analyses were performed using Systat 13 software (Systat Software, San Jose, CA). Statistical significance was evaluated using one way and two way repeated measures analysis of variance (ANOVA) followed by post-hoc pairwise multiple comparisons using Fisher least significant difference (LSD) method when appropriate. Data showing an unequal distribution were analyzed using nonparametric Kruskal–Wallis test followed by Dunn's post-hoc analysis. Significance was set at p<0.05. Data are presented as mean±SEM unless otherwise indicated.
Extreme deviant individual single data points (single values in any one group, e.g. Fig. 4C) were rejected using Dixon's Q parameter22 with stringent criteria (>98% confidence) based on Rorabacher's critical values.23
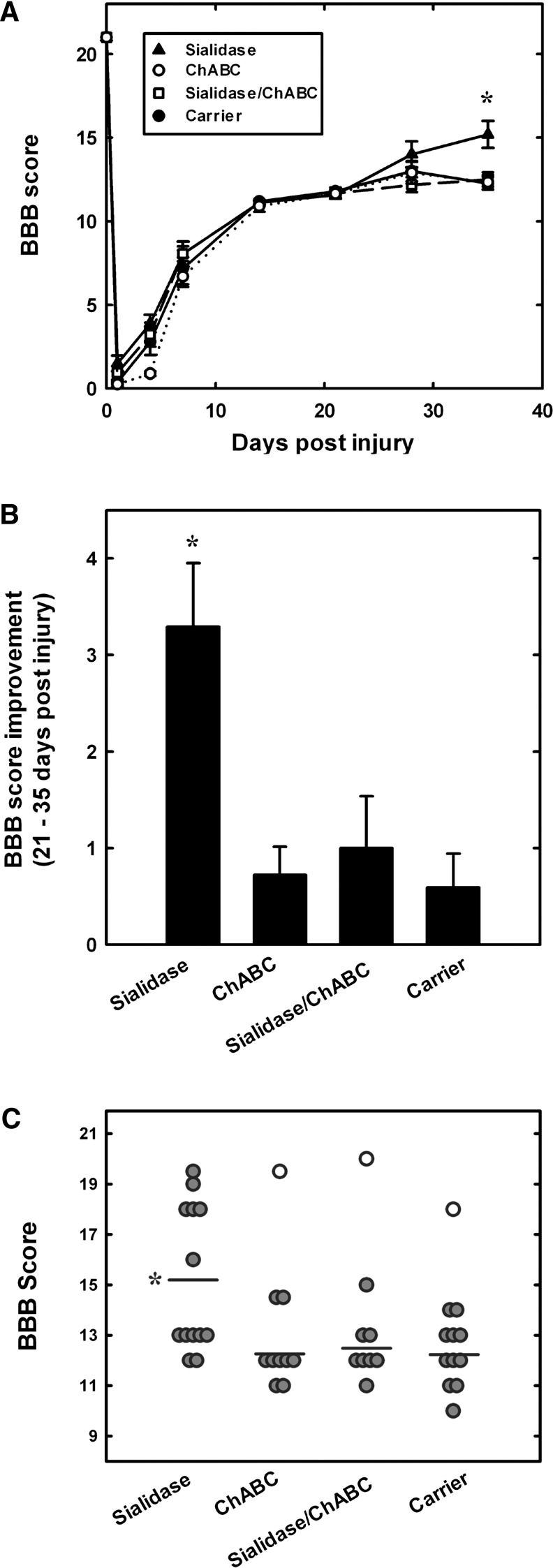
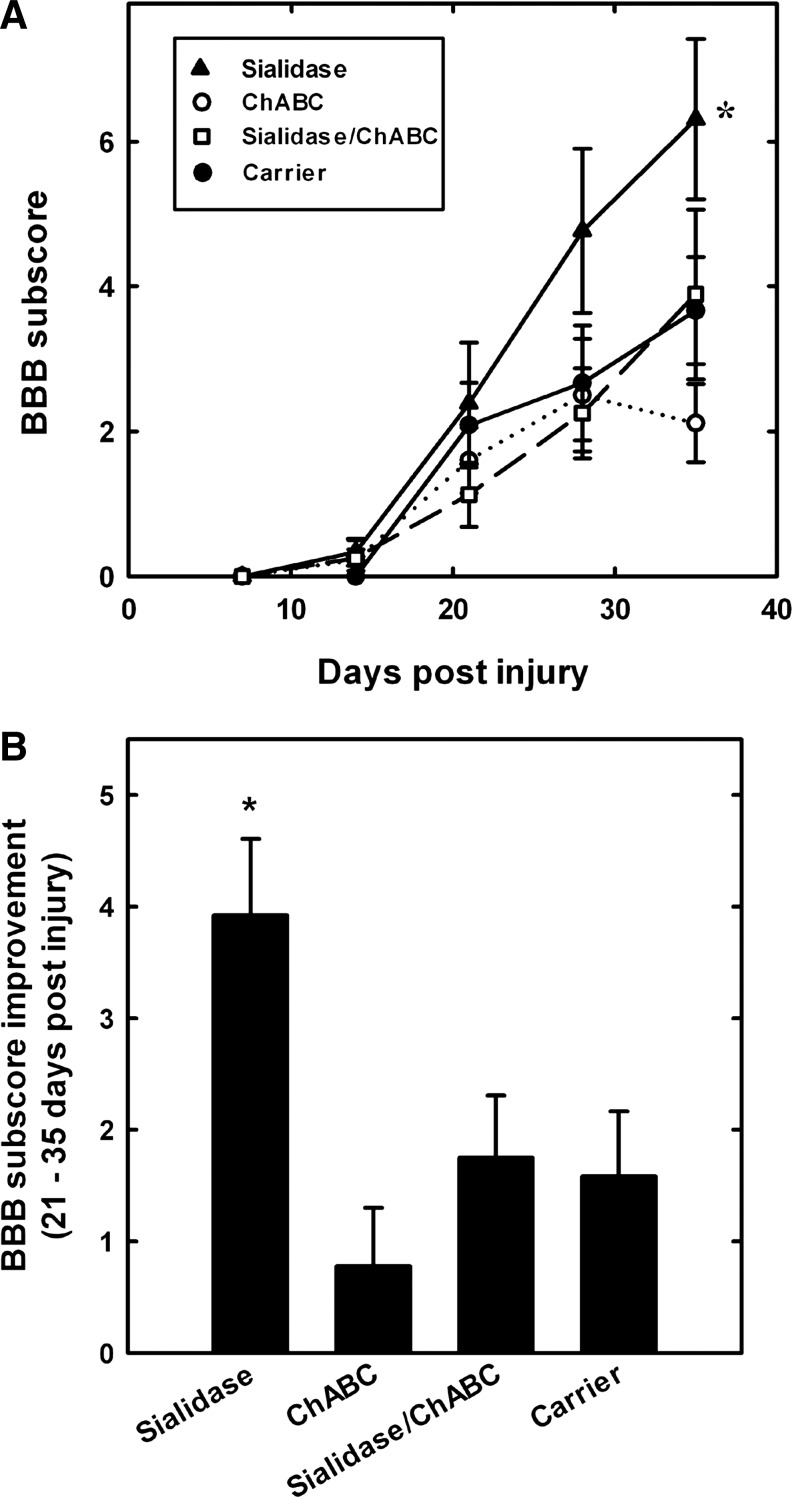
FIG. 4.
Sialidase treatment enhances recovery of hindlimb locomotor function after spinal cord injury. Rats received intrathecal treatment with sialidase (n=13), chondroitinase ABC (ChABC) (n=10), combination sialidase/ChABC (n=9), or carrier (n=12) for 2 weeks. Hindlimb motor function was quantified using the Basso Beattie and Bresnahan (BBB) scale periodically for 35 days after injury. (A) Average BBB scores (mean±SEM) as a function of time after injury. All groups displayed the same partial recovery (BBB=11.7) during the first 3 weeks, then diverged over the last 2 weeks (treatment effect: F3, 273=3.044, p<0.05; time effect: F7, 273=871.8, p<0.001; F 21, 273=1.88, p<0.05). Pairwise post-hoc comparisons revealed a significant difference between sialidase-treated and each other group at 35 days (*p<0.005). (B) BBB score improvement (mean±SEM) over the period 21–35 days after injury. Treatment group differences in improvement were detected by one-way ANOVA (p<0.001); pairwise post-hoc comparisons revealed a significant difference between sialidase-treated and each other group (*p<0.005). (C) Individual BBB scores at 35 days after injury for each rat in the trial. Outlier values (open symbols) were rejected based on>98% confidence using Rorabacher's critical values of Dixon's Q parameter.22,23 Post-hoc analyses revealed significant differences between sialidase-treated (*) and each other group (p<0.005).
Results
SCI metrics
The measured contusion impact force was highly reproducible (207±4 kdyne, mean±SD) and did not vary significantly among the four treatment groups. Likewise, cord displacement during injury was consistent animal to animal (1.2±0.1 mm, mean±SD) and did not vary significantly among the groups. The average BBB score indicated that the contusion injury resulted in marked impairment in over-ground walking in the days immediately following the contusion (BBB score 1.1±0.2 at day 1 and 4.2±0.3 at day 4). Individual rats that reached a BBB score of ≥7 on days 1 or 4 were excluded from the study because of insufficient injury.
Enzyme delivery, stability and efficacy
Sialidase and ChABC, formulated for maximum stability, were infused separately and in combination to the site of a spinal cord contusion injury in the rat. Preliminary experiments (data not shown) indicated that, as formulated, sialidase activity was completely stable when incubated alone or in combination with ChABC for at least 2 weeks at 37°C in vitro. ChABC had a half-life of>8 days at 37°C in vitro when the enzyme was incubated alone or mixed with sialidase. ChABC stability was enhanced markedly by the inclusion of fatty acid-free rat serum albumin to the formulation (Fig. S1)(see online supplementary material at http://www.liebertonline.com)
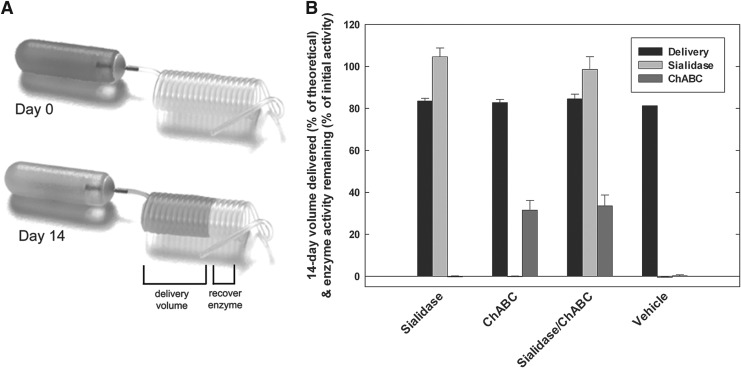
The volume of enzyme solution delivered to the spinal cord contusion injury site over the 14 day infusion period, and the stability of the enzymes at the end of the infusion period were directly determined. Delivery volume was highly consistent within and between experimental groups, with average delivery over 14 days equaling 0.42 μL/h (83% of theoretical, Fig. 1). Sialidase retained 100% of its enzyme activity in the implanted Lynch coil over the 14 day infusion (Fig. 1). ChABC retained 33% of its initial enzyme (Fig. 1), corresponding to a half-life of 8.6 days, consistent with our in vitro observations (Fig. S1). Average recovered activities of sialidase and ChABC were the same in infusion coils containing the mixture of two enzymes compared with those with each enzyme alone, demonstrating that mixing the enzymes neither inhibited nor enhanced enzyme activity or enzyme stability.
FIG. 1.
Sialidase and chondroitinase ABC (ChABC) retain enzymatic activity after 14 days in vivo. (A) Implanted Lynch coils and osmotic pumps were removed from animals after 14 days. (B) Delivery volume was determined based on the migration of Evans Blue solution into the Lynch coil, and residual infusion solution was recovered and tested for sialidase and ChABC activities. Bars represent percent of predicted volume delivered and residual enzyme activity compared with measured initial activity. Mean±SEM; n=13 (sialidase), 10 (ChABC), 9 (combination sialidase/ChABC) and 12 (carrier).
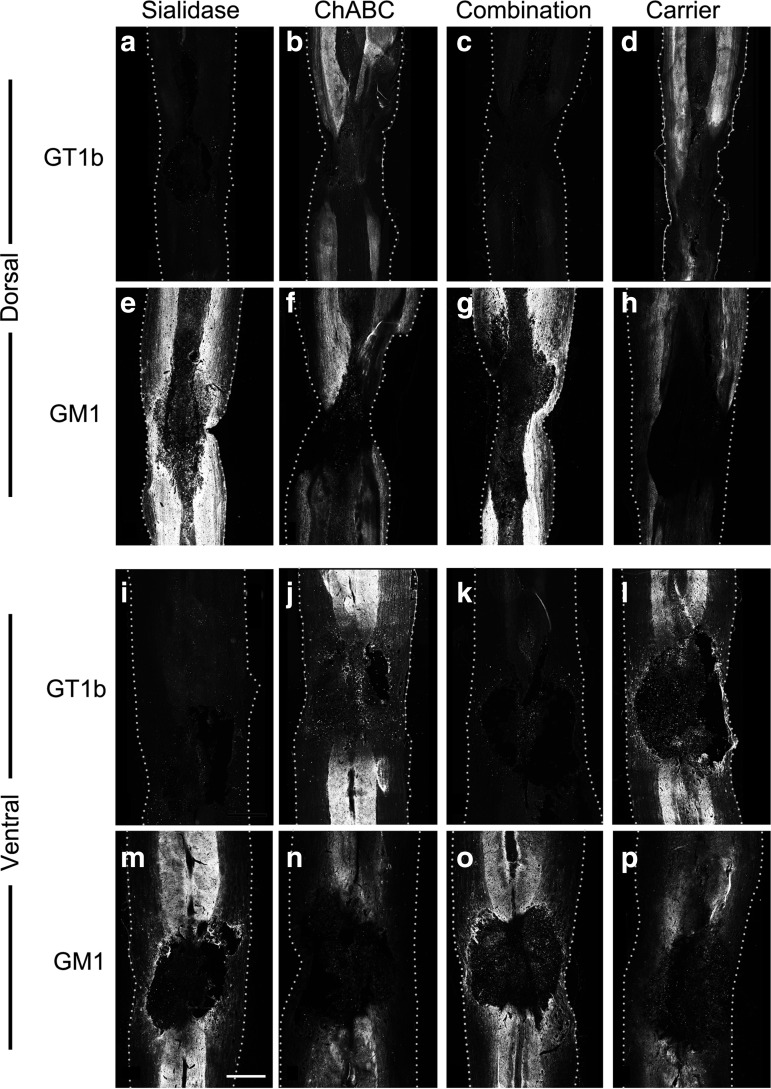
Enzyme efficacy at the injury site was determined via immunohistochemistry. For determination of sialidase efficacy, recovered spinal cord tissue was assessed for expression of ganglioside GT1b, a MAG receptor and sialidase substrate, and for ganglioside GM1, the product of sialidase treatment of GT1b and other major nerve gangliosides (Fig. 2). Sialidase penetrated deep within the spinal cord tissue resulting in extensive loss of GT1b from both gray and white matter tracts of both the dorsal and ventral cord. Sialidase cleavage of GT1b extended rostral and caudal from the lesion center, covering a distance ∼1.5 cm around the cavity (extent not shown). Combination (sialidase/ChABC)-treated animals showed the same loss of GT1b at the site of enzyme delivery; whereas rats treated with ChABC alone showed the same pattern of GT1b immunostaining as controls. Consistent with these findings, sialidase- and sialidase/ChABC-treated rats had increased GM1 immunostaining in both gray and white matter, whereas ChABC-treated rats did not have increased GM1 staining compared with control-treated animals (Fig. 2).
FIG. 2.
Sialidase efficacy on spinal cord tissue sialoglycans in vivo. Spinal cord contused rats were treated with sialidase, chondroitinase ABC (ChABC), combination sialidase/ChABC or carrier (control) as described in the text. At 12 days of treatment, rats were killed and perfusion fixed, and their spinal cords were recovered for immunohistochemical analyses. Dorsal (upper panels) and ventral (lower panels) sections were stained for the presence of GT1b, a sialidase substrate, and GM1, a sialidase product. Sialidase penetrated the spinal cord, desialylating GT1b from gray and white matter and increasing GM1. Bar=1 mm. Dotted lines denote the edges of each longitudinal section. (a–d and i–l) anti-GT1b antibody; (e–h and m–p) anti-GM1 antibody; (a–h) dorsal spinal sections; (i–p) ventral spinal sections; (a,e,i,m) sialidase-treated; (b,f,j,n) ChABC-treated; (c,g,k,o) combination sialidase/ChABC-treated; and (d,h,l,p) carrier-treated.
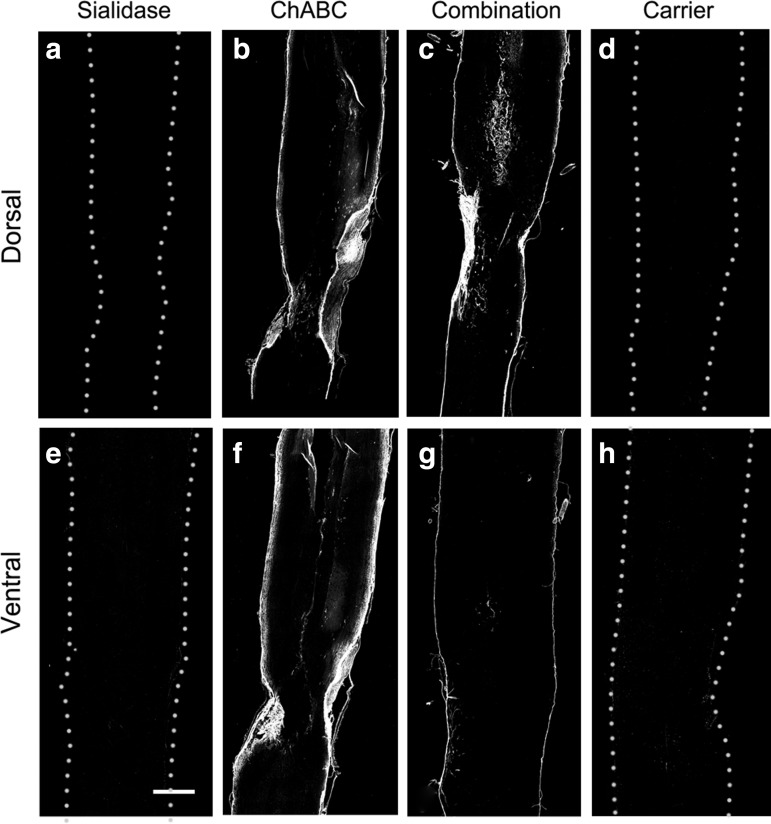
ChABC efficacy was determined by immunostaining the unique unsaturated uronic acid on the chondroitin sulfate “stub” remaining after enzyme treatment using the monoclonal antibody 2-B-6 (Fig. 3). Treatment with ChABC and combination (sialidase/ChABC) resulted in strong and equivalent 2-B-6 staining at the site of infusion that was most intense at the surface of the spinal cord. Based on the different staining patterns, we conclude that sialidase but not ChABC penetrates deep into spinal cord tissues. Increasing the ChABC concentration failed to enhance tissue penetration (data not shown) and high concentrations (>5 U/mL) resulted in cyst formation at the delivery site, consistent with prior reports.24 As expected, spinal cord tissue from carrier and sialidase-treated rats were devoid of positive 2-B-6 staining (Fig. 3).
FIG. 3.
Chondroitinase ABC (ChABC) efficacy on spinal cord tissue chondroitin sulfate proteoglycans (CSPGs) in vivo. Spinal cord contused rats were treated with sialidase, ChABC, combination sialidase/ChABC or carrier (control) as described in the text. At 12 days of treatment, rats were killed and perfusion fixed, and their spinal cords were recovered for immunohistochemical analysis. Dorsal and ventral sections were stained for the presence of unique unsaturated uronic acid “stubs” that are a product of ChABC cleavage (mAb 2-B-6). Bar=1 mm. Dotted lines denote the edges of carrier-treated and sialidase-treated sections. On ChABC-treated and combination-treated sections, immunostaining is at the edge of each section. (a–d) dorsal spinal sections; (e–h) ventral spinal sections; (a,e) sialidase-treated; (b,f) ChABC-treated; (c,g) combination sialidase/ChABC-treated; and (d,h) carrier-treated.
Sialidase treatment improves hindlimb motor and sensorimotor function after spinal cord contusion
As expected from the moderate severity of the contusion injury selected for these studies, all rats spontaneously recovered partial hindlimb overground walking ability within 3 weeks of injury, reaching an average BBB score of 11.7±0.11 at 21 days that varied little among treatment groups (Fig. 4A). After this time, the treatment groups diverged (ANOVA, p<0.05), with sialidase-treated rats improving significantly more than any other group (Fig. 4B). On average, sialidase-treated rats increased their hindlimb walking ability by a more than three point increase in their BBB score compared with a one point or less increase for control-treated rats. ChABC alone and sialidase/ChABC combination-treated animals did not show enhanced improvement over the final 2 weeks of testing compared with control-treated rats (Fig. 4B). Analysis of the BBB profiles revealed both treatment group and time differences (two way repeated measures ANOVA, p<0.05). At 35 days (Fig. 4C), control rats remained near the 3 week level (BBB score, 12.3±0.4), whereas rats treated with sialidase monotherapy exhibited improved over-ground walking ability (BBB score, 15.2±0.8). Post-hoc analyses revealed significant differences at 35 days between sialidase-treated and carrier-treated (p<0.001), ChABC-treated (p<0.002), or combination-treated (p<0.004) groups. In contrast, the combination-treated group was not significantly improved.
BBB subscores were determined to evaluate fine motor functions (Fig. 5A). Consistent with BBB scores, differences were detected with treatment group over time (treatment group×time effect, p<0.01), and post-hoc analysis indicated that sialidase treatment resulted in significant enhancement at 35 days over each other group. Evaluation of improvement in BBB subscores over the final 2 weeks (Fig. 5B) revealed significant treatment group differences (p<0.004), with post-hoc analyses revealing greater than twofold enhanced improvement of sialidase-treated rats compared with control-treated (p<0.01), ChABC-treated (p<0.001), or combination-treated (p<0.025) rats. Again, combination-treated rats did not show significantly enhanced recovery compared with controls (Fig. 5B).
FIG. 5.
Sialidase improves Basso Beattie and Bresnahan (BBB) subscores after spinal cord injury. After contusion spinal cord injury and treatment, BBB subscores were tabulated weekly to separate hindlimb mechanics from hindlimb coordination. Treatment groups and numbers of animals are as in the legend to Figure 4. (A) Average BBB subscore (mean±SEM) as a function of time after injury. All groups showed similar subscore improvements up to 3 weeks, then diverged (treatment effect: F3, 152=1.98, p>0.13; time effect: F4, 152=42.53, p<0.001; interaction treatment×time: F12, 152=2.44, p<0.01).Post-hoc analysis reveals significant differences at 35 days between sialidase-treated (*) and each other group (p<0.02). (B) BBB subscore improvement (mean±SEM) over the period 21–35 days after injury. Group differences were detected by one way ANOVA (p<0.005) and post-hoc comparisons revealed significant differences between sialidase-treated (*) and each other group (p<0.025).
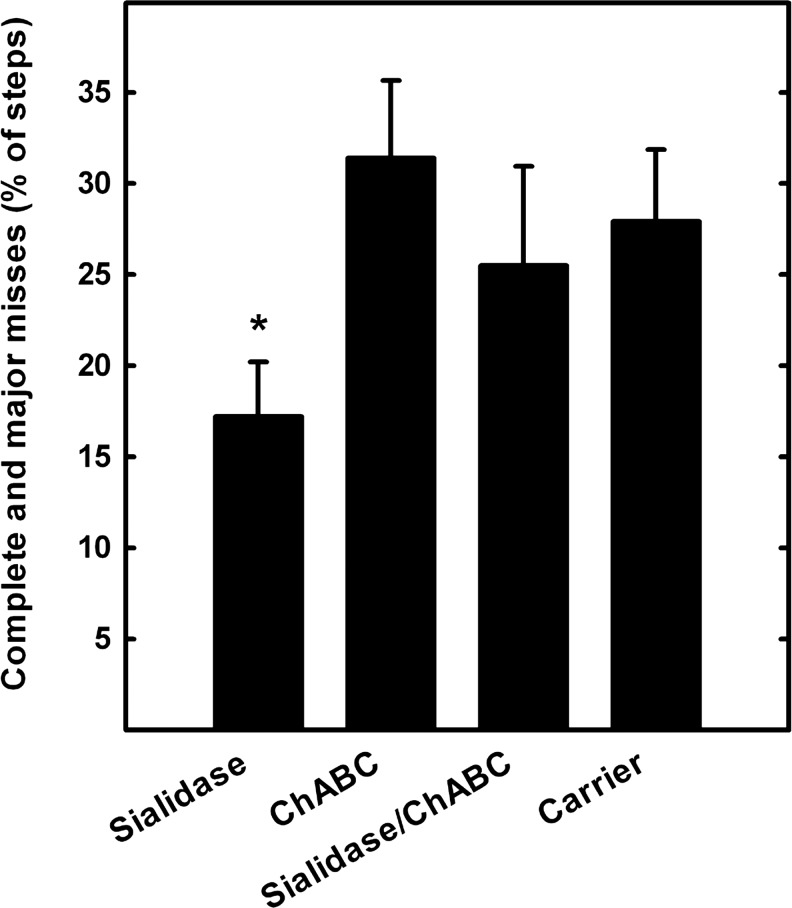
In addition to BBB evaluation, rats were tested for hindlimb sensorimotor function using a horizontal ladder with randomly spaced support bars. Uninjured animals traversed the ladder readily, making coordinated forelimb and hindlimb steps with few errors (data not shown). SCI animals crossed the ladder with difficulty, evidenced by quantifiable hindlimb foot fault errors. Summing major (fall through to the knee joint) and complete (fall through to the hip joint) misses at 5 weeks after injury, group differences were detected (ANOVA, p<0.05). Whereas the rate for sialidase-treated rats was ∼17%, the error rates for each other group was>25% (Fig. 6).
FIG. 6.
Sialidase treatment improves sensorimotor function. Five weeks after spinal cord injury and treatments (see legend, Fig. 4), rats were assessed for their ability to traverse a horizontal ladder with irregularly spaced rungs. Foot slip errors were counted and calculated as a percent of total steps. Bars represent the sum of complete (to the hip) and major (to the knee) foot slips. Group differences were detected by one way ANOVA on ranks (p<0.05). Pairwise post-hoc analyses revealed significant differences between sialidase-treated (*) and chondroitinase ABC (ChABC)-treated groups (p<0.02), with a strong trend between sialidase-treated and carrier-treated groups (p=0.052).
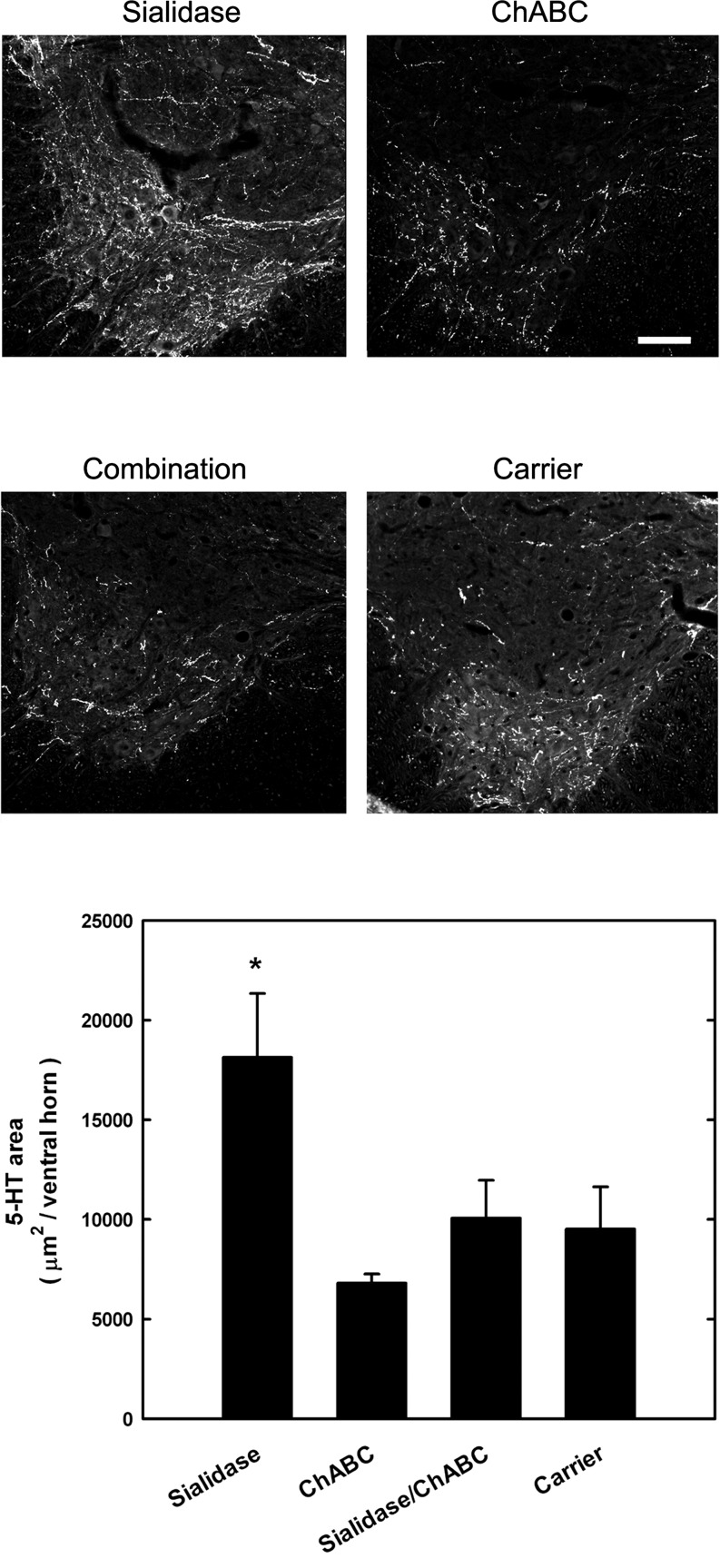
Sialidase treatment promotes 5-HT axon outgrowth
Axons from specific neuronal subsets were immunohistochemically labeled in the spinal cord, revealing positive effects of sialidase treatment on selected spinal pathways (Fig. 7). Antibody to 5-HT was used to label descending axons from the brainstem. Group differences were detected (ANOVA, p<0.05). Post-hoc analyses revealed that sialidase treatment resulted in a nearly twofold increase in 5-HT positive axon immunostaining in the ventral horns of the spinal cord ∼7 mm caudal to lesion when compared with control-treated rats (p<0.02), ChABC-treated rats (p<0.01), or combination-treated rats (p<0.05). In contrast, neither ChABC nor combination sialidase/ChABC treatment increased 5-HT axon immunostaining compared with controls. CGRP-positive axons in the dorsal horns of the spinal cord (a marker for a subtype of sensory axons) were also labeled and quantified (data not shown). Although sialidase and combination treatments both resulted in ∼70% increases in CGRP-positive axon immunostaining caudal to the injury compared with control-treated or ChABC-treated rats, statistical group differences were not reached (ANOVA). Sprouting of catecholaminergic axons (tyrosine hydroxylase-positive) in spinal cord ventral horns was not affected by any of the treatments (data not shown).
FIG. 7.
Sialidase treatment increases serotonergic axons caudal to a contusion spinal cord injury. Rats received contusion spinal cord injuries and treatments (see legend to Fig. 4). At 35 days post-injury, rats were perfusion fixed and their spinal cords dissected. Transverse sections 7.5 mm rostral and caudal to the lesion were immunostained for serotonergic fibers and immunoreactivity quantified in the ventral horns. Top: Representative images of animals from the different treatment groups. Bar=100 μm. Bottom: Serotonin (5-HT) immunopositive pixel areas caudal to the lesion (mean±SEM) for sialidase (n=6), chondroitinase ABC (ChABC) (n=6), combination sialidase/ChABC (n=4), and carrier (n=6) treated rats. Group differences were detected by one way ANOVA (p<0.05) and post-hoc comparisons revealed significant differences between sialidase-treated (*) and each other group (p<0.05).
The volume of spared tissue was similar among treatment groups
None of the treatments resulted in sparing of white or gray matter, nor did we find a reduction in lesion or cavity volume among groups (Fig. S2)(see online supplementary material at http://www.liebertonline.com). These results suggest that the anatomical and functional improvements observed in sialidase-treated rats were not caused by a reduction in inflammation or tissue damage. Similarly, the differences between the sialidase and the combination sialidase/ChABC groups were not caused by a difference in lesion severity.
Enzyme treatments did not significantly improve autonomic function
The regulation of AP by sympathetic activity in general, and by RSNA in particular, is mediated exclusively by pathways that descend from the brainstem to the spinal cord. Therefore, the properties of the inverse relationship between AP and RSNA provide a measure of the integrity of these bulbospinal tracts.20,25 We characterized the sigmoidal relationship between AP and RSNA by measuring the maximum increases and decreases in RSNA in response to changes in AP and the maximum slope of this relationship. Using identical methods, we previously reported that sialidase treatment (alone) improved the responsiveness of RSNA to induced changes in AP following SCI.6 In the current study, however, none of the parameters of baroreceptor regulation was significantly improved by any of the treatments (data not shown, see Discussion).
Discussion
The injured spinal cord is an inhibitory environment for axon regeneration. Agents that diminish endogenous inhibitory mechanisms may enhance recovery from traumatic central nervous system (CNS) injury. The current study compared and combined two agents that have shown promise in prior studies, sialidase and ChABC. Sialidase removes sialic acid residues that act as receptors for the axon regeneration inhibitor MAG, whereas ChABC depolymerizes chondroitin sulfate chains on inhibitory CSPGs. The hypothesis that these are independent treatments and that therefore their benefits would be additive failed. Instead, we found that ChABC was neither fully stable (enzymatically) nor did it enhance recovery from mild spinal cord contusion injury when delivered for 2 weeks via implanted osmotic pump, that sialidase was fully stable and enhanced recovery, and that adding ChABC to sialidase was counterproductive.
The finding that sialidase provided enhanced function and anatomical recovery after spinal cord contusion injury in the rat is consistent with our previous report,6 and extends those observations with the additional finding that sialidase is completely stable 14 days in an in vivo implanted Lynch coil; and that different motor behavioral outcomes (BBB, BBB subscore, horizontal ladder) support the conclusion that sialidase enhances function after spinal cord contusion injury.
Prior studies establish that ChABC promotes axon sprouting and enhances hindlimb and forelimb function after SCI.3,4,10 However, the current study found that intrathecal delivery of ChABC under identical conditions as sialidase did not enhance functional or anatomical recovery in the moderate contusion SCI model used here. The difference in the outcomes between the current and prior studies may be the result of several contributing factors. One factor may be the delivery method of ChABC, in that prior studies used repeated delivery of fresh enzyme via an indwelling catheter. However, our formulation had an average half-life of nearly 9 days in vivo and a recovery of>30% of the enzyme activity after 14 days in vivo, and showed enzyme efficacy by immunohistochemistry.
A more likely contributing factor is the injury itself. A study comparing the effects of ChABC on axon sprouting in hemisection versus contusion SCI found that ChABC induced corticospinal tract (CST) axon sprouting after hemisection but not after contusion despite reduced CSPG levels in both injury groups.12 In a separate study,11 ChABC did not improve hindlimb function in rats that received a moderate spinal cord contusion injury similar to the one used here (control group recovering to a BBB score of ∼10), but resulted in BBB score improvements in rats receiving more severe contusion injuries (control groups recovering to BBB scores of<7). In a model of brachial plexus injury, in which we measured spinal motor axon sprouting into a peripheral nerve graft, we found equal enhancement by infused sialidase and ChABC.13 Additional studies will be needed to compare sialidase and ChABC treatments in different types and severities of SCI.
An aspect of therapeutic enzyme delivery that may be affected by the type of injury is the penetration of enzymes into spinal tissues. In contused spinal cord, intrathecally infused sialidase was active throughout the spinal cord, whereas ChABC activity appeared to be greater in more superficial layers, consistent with previous reports.26 As the two enzymes have similar shapes and dimensions,27,28 the reasons for their differential enzyme activities in spinal cord tissues remains to be addressed. The lower thermal stability of ChABC may be a contributing factor to both its relatively low tissue penetration and low efficacy in this study. Although ChABC retained>30% of its original activity in the Lynch coil, once released into the cerebrospinal fluid its activity may have diminished. Formulations that further thermostabilize ChABC, for example, via microencapsulation and small molecule protein stabilizers, promise to enhance ChABC efficacy.29
An unexpected finding of the current study was that ChABC in combination with sialidase reversed or prevented the positive effects of sialidase alone. Sialidase cleaves terminal sialic acids from sialoglycoproteins and gangliosides (sialoglycolipids), of which the latter are the predominant form of sialic acid containing molecules in the nervous system. ChABC, in contrast, depolymerizes chondroitin sulfate chains on CSPGs, collapsing the extracellular perineuronal net. Although sialic acid-terminated glycan chains and chondroitin sulfate glycan chains are occasionally carried on the same core protein, they are typically considered structurally and functionally independent. The antagonism of ChABC to sialidase efficacy was not caused by direct enzyme–enzyme interference, as sialidase activity was equal after 14 days implanted in Lynch coils with and without ChABC (Fig. 1). Furthermore, staining of sialidase substrate (GT1b) and product (GM1) revealed the same in vivo efficacy of sialidase when delivered with and without ChABC (Fig. 2). Although the data may infer a direct interaction of CSPGs and sialoglycans in the spinal cord, it seems equally likely that the ChABC-induced collapse of the perineuronal net resulted in large-scale changes in the extracellular environment that indirectly reduced improvements gained with sialidase alone.
Sialidase treatment did not enhance baroreceptor regulation of renal sympathetic nerve activity, in contrast to our prior study.6 The spinal pathways that mediate baroreceptor regulation of sympathetic activity in intact and spinally injured rats are complex,20,25 and all of the parameters of baroreceptor regulation were more variable in our current study than in our prior one. This may have been because of the more severe impact used (200 vs. 175 kdyne) masking any therapeutic benefits.
The current study confirms and extends the finding that sialidase treatment induces axon sprouting and enhances motor function after a moderate spinal cord contusion injury. Together with evidence of the long-term stability of sialidase in an implanted delivery system, the findings further establish the feasibility of producing and delivering sialidase to the contused spinal cord for potential therapeutic benefit.
Supplementary Material
Acknowledgments
We thank Dr. Garry Taylor, University of St Andrews, Fife, Scotland, for providing the plasmid for sialidase expression. This work was supported by National Institutes of Health Grants NS057338 (to R.L.S.) and HL016315 (to L.P.S.).
Author Disclosure Statement
No competing financial interests exist
References
- 1.Silver J. Miller J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 2.Yiu G. He Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradbury E.J. Moon L.D. Popat R.J. King V.R. Bennett G.S. Patel P.N. Fawcett J.W. McMahon S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 4.Garcia–Alias G. Barkhuysen S. Buckle M. Fawcett J.W. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat. Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- 5.Hurtado A. Podinin H. Oudega M. Grimpe B. Deoxyribozyme-mediated knockdown of xylosyltransferase-1 mRNA promotes axon growth in the adult rat spinal cord. Brain. 2008;131:2596–2605. doi: 10.1093/brain/awn206. [DOI] [PubMed] [Google Scholar]
- 6.Mountney A. Zahner M.R. Lorenzini I. Oudega M. Schramm L.P. Schnaar R.L. Sialidase enhances recovery from spinal cord contusion injury. Proc. Natl. Acad. Sci U. S. A. 2010;107:11561–11566. doi: 10.1073/pnas.1006683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tohda C. Kuboyama T. Current and future therapeutic strategies for functional repair of spinal cord injury. Pharmacol. Ther. 2011;132:57–71. doi: 10.1016/j.pharmthera.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Wang X. Duffy P. McGee A.W. Hasan O. Gould G. Tu N. Harel N.Y. Huang Y. Carson R.E. Weinzimmer D. Ropchan J. Benowitz L.I. Cafferty W.B. Strittmatter S.M. Recovery from chronic spinal cord contusion after nogo receptor intervention. Ann. Neurol. 2011;70:805–821. doi: 10.1002/ana.22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradbury E.J. Carter L.M. Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res. Bull. 2011;84:306–316. doi: 10.1016/j.brainresbull.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Moon L.D. Asher R.A. Rhodes K.E. Fawcett J.W. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat. Neurosci. 2001;4:465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- 11.Caggiano A.O. Zimber M.P. Ganguly A. Blight A.R. Gruskin E.A. Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J. Neurotrauma. 2005;22:226–239. doi: 10.1089/neu.2005.22.226. [DOI] [PubMed] [Google Scholar]
- 12.Iseda T. Okuda T. Kane–Goldsmith N. Mathew M. Ahmed S. Chang Y.W. Young W. Grumet M. Single, high-dose intraspinal injection of chondroitinase reduces glycosaminoglycans in injured spinal cord and promotes corticospinal axonal regrowth after hemisection but not contusion. J. Neurotrauma. 2008;25:334–349. doi: 10.1089/neu.2007.0289. [DOI] [PubMed] [Google Scholar]
- 13.Yang L.J. Lorenzini I. Vajn K. Mountney A. Schramm L.P. Schnaar R.L. Sialidase enhances spinal axon outgrowth in vivo. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11057–11062. doi: 10.1073/pnas.0604613103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potier M. Mameli L. Belisle M. Dallaire L. Melancon S.B. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal. Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- 15.Yamagata T. Saito H. Habuchi O. Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J. Biol. Chem. 1968;243:1523–1535. [PubMed] [Google Scholar]
- 16.Prabhakar V. Capila I. Bosques C.J. Pojasek K. Sasisekharan R. Chondroitinase ABC I from Proteus vulgaris: cloning, recombinant expression and active site identification. Biochem. J. 2005;386:103–112. doi: 10.1042/BJ20041222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch H.J. Rivest R.W. Wurtman R.J. Artificial induction of melatonin rhythms by programmed microinfusion. Neuroendocrinology. 1980;31:106–111. doi: 10.1159/000123059. [DOI] [PubMed] [Google Scholar]
- 18.Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 19.Basso D.M. Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J. Neurotrauma. 2004;21:395–404. doi: 10.1089/089771504323004548. [DOI] [PubMed] [Google Scholar]
- 20.Zahner M.R. Schramm L.P. Spinal regions involved in baroreflex control of renal sympathetic nerve activity in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011b;300:R910–R916. doi: 10.1152/ajpregu.00646.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnaar R.L. Fromholt S.E. Gong Y. Vyas A.A. Laroy W. Wayman D.M. Heffer–Lauc M. Ito H. Ishida H. Kiso M. Griffin J.W. Sheikh K.A. Immunoglobulin G-class mouse monoclonal antibodies to major brain gangliosides. Anal. Biochem. 2002;302:276–284. doi: 10.1006/abio.2001.5540. [DOI] [PubMed] [Google Scholar]
- 22.Dixon W.J. Analysis of extreme values. Ann. Math. Statist. 1950;21:488–506. [Google Scholar]
- 23.Rorabacher D.B. Statistical treatment for rejection of deviant values: critical values of Dixon's "Q" parameter and related subrange ratios at the 95% confidence level. Anal. Chem. 1991;63:139–146. [Google Scholar]
- 24.Huang W.C. Kuo W.C. Cherng J.H. Hsu S.H. Chen P.R. Huang S.H. Huang M.C. Liu J.C. Cheng H. Chondroitinase ABC promotes axonal re-growth and behavior recovery in spinal cord injury. Biochem. Biophys. Res. Commun. 2006;349:963–968. doi: 10.1016/j.bbrc.2006.08.136. [DOI] [PubMed] [Google Scholar]
- 25.Zahner M.R. Kulikowicz E. Schramm L.P. Recovery of baroreflex control of renal sympathetic nerve activity after spinal lesions in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R1584–R1590. doi: 10.1152/ajpregu.00295.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barritt A.W. Davies M. Marchand F. Hartley R. Grist J. Yip P. McMahon S.B. Bradbury E.J. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J. Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crennell S. Garman E. Laver G. Vimr E. Taylor G. Crystal structure of Vibrio cholerae neuraminidase reveals dual lectin-like domains in addition to the catalytic domain. Structure. 1994;2:535–544. doi: 10.1016/s0969-2126(00)00053-8. [DOI] [PubMed] [Google Scholar]
- 28.Huang W. Lunin V.V. Li Y. Suzuki S. Sugiura N. Miyazono H. Cygler M. Crystal structure of Proteus vulgaris chondroitin sulfate ABC lyase I at 1.9A resolution. J. Mol. Biol. 2003;328:623–634. doi: 10.1016/s0022-2836(03)00345-0. [DOI] [PubMed] [Google Scholar]
- 29.Lee H. McKeon R.J. Bellamkonda R.V. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3340–3345. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.