Abstract
Few studies have explored how overall general health care and HIV/STI testing experiences may influence receipt of “Seek, Test, Treat, and Retain” (STTR) HIV prevention approaches among Black men in the southern United States. Using in-depth qualitative interviews with 78 HIV-negative/unknown Black men in Georgia, we explored factors influencing their general health care and HIV/STI testing experiences. The Andersen behavioral model of health care utilization (Andersen model) offers a useful framework through which to examine the general health care experiences and HIV testing practices of Black men. It has four primary domains: Environment, Population characteristics, Health behavior, and Outcomes. Within the Andersen model framework, participants described four main themes that influenced HIV testing: access to insurance, patient–provider communication, quality of services, and personal belief systems. If STTR is to be successful among Black men, improving access and quality of general health care, integrating HIV testing into general health care, promoting health empowerment, and consumer satisfaction should be addressed.
Introduction
HIV/AIDS disproportionately affects Black Americans, who account for 44% of new HIV cases in the United States despite constituting only 14% of the population.1 Of all new HIV diagnoses among Black men in 2010, heterosexual men accounted for 19%, and the number of new diagnoses among Black men who have sex with men (MSM) increased 48% between 2006 and 2009.2 In 2010, among Black women, 88% of HIV diagnoses were heterosexually acquired.2 Approximately 22% of all Black men with HIV are estimated to be unaware of their infection.3 Southern states demonstrate the highest prevalence rates of HIV as well as new diagnoses, and the distribution of HIV cases includes nonurban areas to a higher degree than in other areas of the country.4 Among newly diagnosed individuals, Blacks were less likely than Whites to have had a negative HIV test prior to their diagnosis.5
In response to this stark racial disparity in HIV, an increased emphasis on HIV testing and identification of HIV-positive individuals has taken center stage. Recent studies, including mathematical modeling,6 ecological studies,7,8 and trials using antiretroviral therapy (ART) to prevent transmission among heterosexual couples9 and mother-to-child transmission,10 have all promoted “Seek, Test, Treat, and Retain” (STTR) as a strategy for diagnosis and treatment of HIV to curb transmission.11–13 In fact, the Centers for Disease Control and Prevention (CDC) has recommended routine screening for HIV in health care settings.14,15
Despite this focus, there has been little consideration given to how these initiatives will be implemented within the larger contexts of the overall health care experiences of Black men. Given the recent push to routinize HIV screening in general health settings,15 it is critical that we understand the factors that drive general health care utilization and how HIV testing fits into general health care for Black men in the South. An improved understanding of health care utilization and HIV testing among Black men can be used to enhance implementation of STTR strategies for them.
Life expectancy among Black men is lower than that of White men, Black women, and White women,16 and racial disparities in chronic disease outcomes, such as hypertension control,17–21 prostate cancer screening,22 and stroke treatment23 continue to persist among Black men. There are many intersecting reasons postulated for these disparities, including poverty,24 decreased access to health insurance and high-quality care,24,25 lower quality of health care experiences,18,26 and decreased health-seeking behavior. Men receive preventive health services less often than women (particularly men aged 20–39 years).27,28 They are more likely to avoid seeking care even with symptoms,29 and Black men seek care less often than Black women.30 To explore this further, a survey conducted among community-dwelling Black men (Michigan, Georgia, and North Carolina) showed that factors associated with scheduling a routine health examination included having a usual source of care and exposure to health-promoting male subjective norms.31 Given this potent mix of structural, institutional, and individual level barriers to overall health care utilization among Black men, and the recent emphasis on STTR HIV prevention strategies, a more complete understanding of factors that affect general health care and HIV testing behavior among Black men is needed.
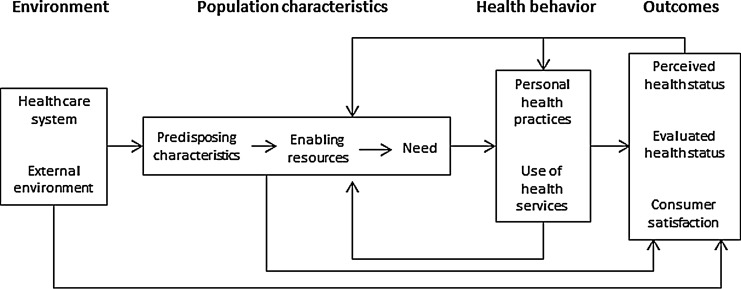
The Andersen behavioral model of health care utilization (Andersen model) offers a useful framework through which to examine the general health care experiences and HIV testing practices of Black men and has four primary domains: Environment, Population characteristics, Health Behavior, and Outcomes (Fig. 1).32 Environment includes both external environment (physical environment, political, and economic components) and health care systems. Population characteristics include enabling resources and demographics specific to the population. Health Behavior includes personal health beliefs and practices and use of health services. The outcomes category includes consumer satisfaction and clinical outcomes (perceived and evaluated health status). This model has been used to study health care utilization in a wide variety of populations, including routine health screening for Black men,31 breast cancer screening,33 spinal cord injury,34 and for HIV patients.35
FIG. 1.
Andersen model of health care utilization. From Ref. 32.
Understanding the factors that influence the health care utilization experiences and behaviors of Black men has been understudied.36,37 There are limited data that examines the influence of general health care experiences on HIV testing experiences. Qualitative methods have previously been used to understand health services utilization among HIV-positive and HIV-negative Black MSM in New York26 and HIV-positive Black women in rural South Carolina.38 Due to limited existing data on Black men's health care and HIV testing experiences, qualitative inquiry is ideally suited to provide context on the topic that may be missed by quantitative methods. The primary aims of this analysis were to: (1) describe Black men's general health care and HIV testing practices and experiences in Georgia; and (2) identify how emerging themes of these experiences reflect domains of the Andersen Model among Black men.
Methods
Participants and recruitment
We conducted semi-structured qualitative interviews in three cities in Georgia (Atlanta, Columbus, and Valdosta) between April 2010 and June 2010 as part of Project Adofo (a Ghanaian boy's name meaning “courageous one” or “one who loves”), a study exploring the relationships between demographic factors, mental health determinants, coping strategies in the context of HIV risk behavior among Black men. We chose these three locations because they represented diverse metropolitan statistical areas (MSAs) where Blacks constituted over 40% of the overall population39 and had high HIV prevalence rates (between 300 and 1,300 cases per 100,000 population).40 Population estimates from the 2010 United States Census were as follows: Atlanta 420,005, Columbus 189,885, and Valdosta 54,763.39 Study participants were recruited from barbershops, community-based organizations and college campuses using flyers, internet websites, word-of-mouth, and snowball sampling methods. Inclusion criteria were age ≥18, self-identification as “Black” or “African-American,” and reported HIV-negative or unknown status. The institutional review board and hospital research oversight committee of the primary author's institution approved the study.
Procedure
We trained 4 research staff interviewers on qualitative interview techniques using didactic and role-play approaches as described in previous studies.41 We obtained written informed consent and conducted interviews with an initial total of 90 participants (30 each in Atlanta, Columbus, and Valdosta) in private areas such as offices, conference rooms at schools, and hotel meeting rooms. Each interview was digitally audio-recorded and lasted from 70 min to 2 h in length. As part of the interview guide, participants answered the open-ended question “Tell me about your experiences with getting medical care in (name of city),” followed by the probe “How do you get along with medical staff?” Participants were also asked about frequency, motivators of and experiences with HIV testing. At the conclusion of the interview, participants completed a brief cross-sectional survey including demographics, condom use, and HIV testing practices. Participants received a $50 gift card for compensation at the end of the interviews.
Analysis
All interviews were transcribed by a professional transcription agency and imported into Nvivo version 8 (QSR International, Cambridge, MA), a qualitative management and analysis software package. Four members of the research team independently coded 3 initial interviews using a standard set of codes developed prior to coding. We determined inter-coder reliability by kappa statistic (>0.80 between all coders), and subsequently the remaining 87 interviews were coded separately by the same 4 research staff. This method of coding the interviews is consistent with methods described in the qualitative data analysis literature.42,43
A grounded theory approach to qualitative data undergirded the data collection and analytic process of this study.44,45 Following this method, topics for discussion were selected based on based on theoretical relevance to research questions, constant comparative analysis (e.g., comparing people with themselves or others, data referring to different points in time, or incidents with other incidents), and rigorous coding and restructuring of data into themes and patterns organized around a central storyline.42,44,45
We conducted three types of coding: (1) open coding; (2) axial coding; and (3) selective coding.42,46 During open coding we documented our first impressions and thoughts about the data, in which codes took the form of brief conceptual labels or categories that represent the text (e.g., “health care experiences” or “motivators for HIV testing”). During axial coding, we verified relationships between the categories and subcategories generated in open coding, in order to generate notes that explicitly describe connections between the categories and subcategories that were generated during open coding. For this sample, we utilized Andersen Model domains (Environmental factors, Population Characteristics, Health Behavior, and Outcomes) as categories through which we framed participant responses to interview questions related to health care experiences.32 Finally, we incorporated selective coding in which concepts were integrated around a core theme (HIV testing in past year), and analyzed the matrix of similarities and differences in codes and themes among participants' responses. Survey responses to the question “Have you been tested for HIV in the past year?” were coded in a dichotomous fashion, and those who responded “yes” were designated as “tested,” and those responding “no” were designated as “nontested.”
Results
Demographic characteristics
A total of 90 men were interviewed for the study. Our final sample consisted of 78 participants; the first 12 participants were excluded because they did not receive the survey section on HIV testing practices. Ages ranged from 19 to 60 years (mean 37.6, SD 14.7), and there was a high reported prevalence of unemployment (48/78, or 62%) and poverty (52/78, or 67%, reported annual incomes<$15,000). Most participants (69/78, or 87%) reported heterosexual orientation (sex with women only). HIV testing in the past year was reported by 50/78 (64%) of the participants. Additional demographics can be found in Table 1. We incorporated the Andersen model to conceptualize the themes emerging from the qualitative analysis. A comparison of themes between tested and nontested participants is included in Table 2.
Table 1.
Demographics for Project Adofo Participants
| Tested (n=50) (%) | Nontested (n=28) (%) | Total (n=78) (%) | |
|---|---|---|---|
| Age (years), mean (SD) | 39.8 (14.6) | 33.7 (14.4) | 37.6 (14.7) |
| Heterosexual | 43 (86) | 26 (96) | 69 (88) |
| Education | |||
| Less than high school | 8 (16) | 3 (10) | 11 (14) |
| Completed HS / GED | 12 (24) | 8 (29) | 20 (26) |
| Technical or some college | 22 (44) | 9 (32) | 31 (40) |
| College degree | 7 (14) | 7 (25) | 14 (18) |
| Graduate degree | 1 (2) | 1 (4) | 2 (3) |
| Currently employed | 16 (33) | 14 (50) | 30 (38) |
| Annual income <$15,000 | 32 (64) | 20 (71) | 52 (67) |
Table 2.
Summary of Thematic Findings Among Tested and Nontested Participants Along Andersen Model Domains
| Andersen model domains | Tested for HIV in past 12 months | Nontested for HIV in past 12 months |
|---|---|---|
| Environment | Hard to navigate | Hard to navigate |
| Used creativity to manage obstacles | Frustrated with care | |
| Confidentiality an issue | Confidentiality an issue | |
| Population characteristics | Peer norms | Trust in private doctor |
| Networks in testing locations | ||
| Fraternities | ||
| Health behavior | Want to live | Low perceived risk |
| High perceived risk | No regular testing | |
| Emphasis on health maintenance | Test result fear | |
| Fear of transmitting HIV to others | Symptoms-based testing | |
| Outcomes: consumer satisfaction | Mostly positive experiences | Positive and negative experiences |
| Proactive with health care |
Environmental factors
Health care system
Several health care system factors either promoted or detracted from HIV testing among the participants. Having health insurance was an important factor in promoting access to HIV testing. Barney (tested, Columbus, 30) said, “If I didn't have medical insurance, I probably wouldn't be getting tested.” Having insurance also increased the frequency of HIV testing. For example, Noah (tested, Atlanta, 30) said, “If I had insurance, yeah. It'd be like every 6 months now or something like that, instead of once a year or once every 2 years, or something like that.” Several participants endorsed that having HIV testing routinely part of general health care settings, such as within a patient-centered medical home, supported regular HIV testing habits.47 Adam (tested, Atlanta, 52) said, “They draw like ten vials of blood, and they test for everything. So I wasn't looking for an HIV test. It was just part of the physical that I took.”
The larger social contexts of poverty and incarceration certainly influenced how participants experienced HIV testing within the health care system. Some tested participants mentioned that both HIV and sexually transmitted infection (STI) testing were easy to obtain from venues that offered free testing, such as homeless shelters or a college campus health fair, or with incentives like a subway card. Many participants were tested for HIV repeatedly because they were mandatorily tested when transferring in or out of correctional settings, substance abuse programs, or transitional housing. Overall, HIV testing was either mandatory or incentivized in settings that targeted poor, homeless, or incarcerated individuals.
Difficulty in accessing safety net health care systems was mentioned more often among nontested participants. Gaining initial access to a safety net system required several documents to prove identity, local residence, and financial need, and resulted in an access card. While safety net systems can be quite beneficial for those in need, the short duration of the access card proved to be a challenge to many participants, including Juan (non-tested, Atlanta, 48). He said,
“Well, I don't have [the card] now, but uh when I, you know they give you, once you apply for food stamps and stuff, you can go get you a card. But the card only lasts maybe three months. Sometimes they give it for 30 days and so if I ever had to go back, I would have to take the paperwork down there and get another card.”
Other barriers noted by participants in obtaining health care from safety net hospitals included long waits and quality of care received. Several nontested participants expressed uncertainty about their willingness to get care from free or safety net clinics due to perceived inferior quality of care and poor treatment by staff. When probed about the availability of free health care, Eli (nontested, Columbus, 20) said, “Yeah, but you don't want to deal with everybody [medical staff], I mean from previous experience of how people, you know, treat you and act towards you.” This sentiment highlights how access to care for the uninsured with the presence of a safety net clinic can be limited by institutional rules, long waiting times, and perceived poor quality.
External environment
Among external environment characteristics, concerns about confidentiality emerged as a major theme for both tested and nontested participants, especially those who sought care in clinic settings (and not by participants receiving care in private doctors' offices). Participants worried that health care providers could potentially disclose an HIV/STI diagnosis to others in the clinic or members of the local community. Some were also embarrassed to seek HIV/STI testing locally because they feared recognition by peers while entering a care location specifically designated for HIV/STI testing, such as public health departments or school clinics. Interestingly, this theme of confidentiality emerged only among participants in Columbus and Valdosta, not Atlanta, which may reflect perceived lack of anonymity in smaller towns.
“You don't really want to go get tested. The main problem is that people can see you going in the Health Department. They got different sections. And you're dealing with sharing information. Somebody in the Health Department, the nurse, tell somebody else out in the street.” – Kyle (tested, Columbus, 54)
“I was just sitting in there, and then like one of my other home boys came in there…He was like, ‘Oh, man. What you doing going back there? I know what happen when you go back there!’” – Landon (non-tested, Valdosta, 24)
Perceptions of the inferior appearance or cleanliness of health care facilities along racial lines presented another external environment factor that may affect testing for HIV:
“I don't think that you know, health clinics are really serving the needs of the Black community. The ones that I've seen, they usually like run down. They're not as clean as like a hospital or something like that.” – Austin (non-tested, Valdosta, 27)
The perceived quality of external environments was equally important as the access itself. If health care facilities were perceived as shoddy, unclean, or environments where confidentiality could be compromised, participants described reluctance in accessing both general and sexual health services there.
Population characteristics
The Andersen model domain of Population Characteristics reflects predisposing characteristics (including personal health beliefs), enabling resources, and need. Responses from study participants primarily reflected personal health beliefs and enabling resources.
Personal health beliefs
Knowledge of one's HIV status was discussed differently by tested and nontested participants, and differences between knowledge of HIV testing as power versus fear was readily apparent. Jason (tested, Valdosta, 30) said, “You should always feel proud of yourself for being a mature adult to know that you know, I'm sexually active so I need to take these steps [take an HIV test] so I need to know my status.” In contrast, Connor (nontested, Columbus, 38) emphasized fear as a significant factor influencing his testing practices. He said, “Of course it's scary because you're wondering, you know, what the results is. AIDS is, you know, it ain't like they calling you to tell you, you got the common cold.”
When asked about their motivation to be tested for HIV, several tested participants expressed a strong desire to be “healthy” and emphasized the importance of regular health check-ups to maintain health. In this context, regular HIV testing was seen as part of this overall health care maintenance, and early diagnosis of HIV was valued:
“Well my, at my age, 60, I'm very concerned about my health. So any time something, I think something's going on with my body or even my mind, I want to address it…So it's [HIV testing is] a yearly thing that needed to be [done].” - Brody (tested, Atlanta, 60)
In the setting of having a chronic disease, maintaining good health was often described as requiring a combination of vigilance and regular use of medications. Diego (tested, Atlanta, 63) described the importance of not missing refills for his high cholesterol and hypertension: “I never run out of my medicine. It's very important to me.” His attitude towards controlling his chronic diseases by staying vigilant of medication refills was consistent with his approach to HIV testing.
Some personal health beliefs around health care access and HIV testing described by participants were driven by consideration of romantic partners and familial responsibilities. Preventing transmission of HIV was noted by several tested participants. Joe (tested, Atlanta, 62) said,
“I could meet somebody you know, that I you know am really into and spend some time with them. I don't want to lay down with them and pass on something.”
Moreover, fatherhood and men's family responsibilities were described by some tested participants as motivators for staying healthy and taking an HIV test.
“I go pretty often to the doctor. You want to know that you're healthy…if you want to live your life uh, and have a family and everything, you gonna have to be strong for your family as a male.” - Eric (tested, Columbus, 19)
Several tested participants cited their own sexual behavior, dislike of condoms, and multiple partners, as reasons for regular HIV testing. Many described managing the risk of choosing unprotected sex with partners by getting routine testing:
“I like to have sex and I'm the type of guy I'm not very interested in using no condom…and I know I might be having sex with you know two or three different women…I know I got to go get tested.” - Aaron (tested, Columbus, 31)
In contrast, nontested participants perceived themselves to have low risk for acquiring HIV or STI for a multitude of reasons. For example, Brian (nontested, Columbus, 21) said “But I ain't never had…any legit reason to go [get HIV tested]…I use protection every time,” suggesting that testing is not necessary when one uses condoms regularly. Perceptions that sexual partners were low risk were also endorsed by several nontested participants as reasoning for not testing, even when acknowledging some uncertainty. Cole (nontested, Atlanta, 51) said, “I haven't ever been tested for HIV. Because I feel like the women that I've been with they've been clean, but you never know.” Finally, participants like Brian and Lucas (nontested, Columbus, 21 and 39), both stated that they got regular medical check-ups but not an HIV test, suggesting perhaps either a disconnect between general medical care and knowing one's HIV status, or perceiving themselves at low risk for any of the reasons cited above. Consistent with their reasons for not getting HIV tested, some nontested participants also endorsed lack of medical management of their chronic diseases:
“Yeah, I was on two types [of] little pills [for high cholesterol] he had me on, but uh I took ‘em for about a couple of years, and then I just kept working out, physically…I felt fine, I didn't feel like I needed it.” – Logan (nontested, Columbus, 48)
For HIV testing and general health maintenance, nontested participants' approaches contrasted starkly with tested participants and were couched in perceptions of: (1) their own health or that of their sexual partners; and (2) how they were feeling. For tested participants, high personal risk perception, concern for transmission of HIV to sexual partners, and a general sense of empowerment in health maintenance were predominant health belief themes, while nontested participants described low personal risk perception, did not mention protecting sexual partners, and appeared to deny the importance of medications for other general health issues.
Enabling resources
Tested participants described peer norms and convenience of location as factors that encouraged HIV testing. In particular, younger participants who were students described group testing experiences that took place during health fairs on their college campuses. Having a knowledgeable peer educator was described by Mason (tested, Valdosta, 25) who said, “The brother that was hooking the test up, good people, good man to talk to.” Peer norms were also echoed as a factor influencing Julian (tested, Valdosta, 20):
“It's just, it literally was like a group of friends, like, you know I think we should all go out there and get tested today. So like you have it, it's free testing. So we all went. And you find out like within 20, 30 minutes.”
Both Mason and Julian speak to the importance of familiarity and comfort with styles of HIV testing from the people offering testing themselves, as well as with friends and social networks encouraging testing as a normative behavior.
Rapid testing with results given within the time frame of the health fairs, oral testing (as opposed to blood testing), and free testing, were also mentioned as enabling resources of testing in these venues. Conversely, several tested participants who sought medical care (general, HIV, and STI tests) from student health facilities also stated that because they paid a fee to participate in the plan, they were motivated to use the available services. Their descriptions of these motivators, particularly monetary (whether free or paid), speaks to the varied resources that influence individual level approaches to accessing health care and HIV testing.
In contrast to tested participants, group testing experiences and similar peer norms were not described by any of the nontested participants. Instead, several nontested participants stated that they would take an HIV test if their private doctors offered a test to them, making medical providers a primary enabling resource for them. In this context, if these same participants do not seek regular health care, opportunities for medical providers to offer HIV is moot. Moreover, given both medical providers and patients' reluctance to bring up honest sexual behavior discussions in health care settings,48 waiting to get HIV testing until a provider suggests it may not be a reliable enabling resource for HIV testing.
Health behavior
Personal health practices and use of health services
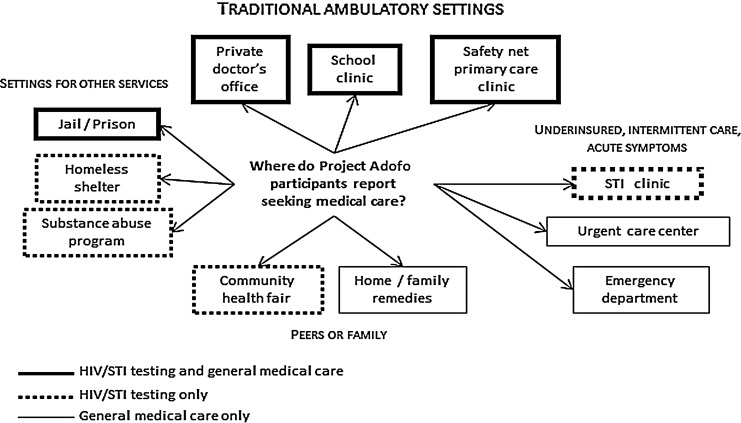
Fragmentation of care locations was a theme commonly described by both testers and nontesters, with general health care, HIV testing, and STI testing and treatment often occurring in different locations, but overlapping for some participants (Fig. 2). The participants sought general health care in a variety of venues, including primary care clinics/safety net systems, private offices, student health centers, correctional facilities, urgent care centers, and emergency departments (EDs). Men obtained HIV testing in some of these venues, including primary care clinics and safety net systems, private offices, and student health centers. However, urgent care centers and EDs were not mentioned as places to obtain HIV testing by any of the participants. Conversely, some men took HIV tests at homeless shelters, substance abuse programs, and local health fairs but did not describe receiving general health care at these venues. Both tested and nontested participants experienced general health care and HIV testing as separate entities.
FIG. 2.
Locations for use of health care services among HIV-negative/unknown Black men in Georgia.
In addition to the fragmentation of HIV testing being separate from general medical care, there was also a sentiment that HIV testing was not often done with STI testing; this was described by tested and nontested participants and included participants who had access to general health care. Adam (tested, Atlanta, 52), the same participant who received HIV testing along with general health care, discussed the importance of availability of neighborhood STI clinics for diagnosis and treatment of STI: “I've been burned [got a STI] a couple of times, you know, and right there, we have a little clinic right up the street.” Kevin (tested, Valdosta, 21) stated that he received care for his STI in an ED, even though he had access to a clinic. This could be interpreted as related to confidentiality issues as noted earlier or the belief that STI diagnosis and treatment is best done in a relatively anonymous, quick and easy setting, such as an ED or local clinic.
While fragmentation was a problem for both tested and nontested participants, there were some notable differences. Many tested participants sought innovate ways to obtain health care, even in the face of obstacles such as low finances. Some of these methods included negotiating costs with health care providers, seeking care from family members, using prior knowledge of the health care system to obtain good quality care, or setting aside money on an annual basis to use towards health care. For example, Casey (tested, Columbus, 28) described how as a barber he lacked health insurance, but said, “I get all of the guys together and be like, ‘Look man this doctor said we can do this for this price.” In comparison, none of the nontested participants described alternative methods to obtain health care when faced with obstacles.
Outcomes
Responses from study participants fit most neatly into the Andersen model Outcomes domain subsection of consumer satisfaction because a probe asking about “getting along with medical staff” was included in the interview guide.
Consumer satisfaction
Many of the tested participants said they had good experiences with health care providers. Aiden (tested, Atlanta, 56) did not encounter difficulties with medical staff and said, “No problem with me because if I was there I needed them; when you've been shot you're glad to see the doctor and the nurse.” Familiarity was also important to several tested participants. Cooper (tested, Atlanta, 50) highlighted the importance of having a relationship with a primary care doctor:
“My doctor is my friend, you can't ask for nothing better. My doctor knows my wife. He's talked to my mom when I got put into the hospital for the first time in my life…my doctor was in that room that next day. A friend will do that for you.”
Justin and Jeremiah (tested, Valdosta, both 20) had been going to the same physician since they were children and were happy with their care for similar reasons of familiarity and comfort.
Several tested participants took it upon themselves to maintaining positive relationships with medical staff, even in adverse conditions. Adam (tested, Atlanta, 52) said, “I don't bring a lot of attention like that. I'm not Hell-raising.” Diego (tested, Atlanta, 63) also noted, “if you respect them [medical providers], they respect you. If you go in with a cocky attitude with anybody, you're going to have a problem, you know? You need them; they don't need you.”
While personal responsibility in provider–patient relationships was described, several tested participants also emphasized good communication skills and showing genuine concern as measures of a good provider. Specifically, tested participants mentioned clear explanations for health conditions, receiving counseling on healthy living, and being treated with respect as factors that they valued:
“They're [Good providers are] going to look at you. They gonna be like okay; they'll sit down and explain to you okay well this might be the cause and you know this here might be the cause…them be the good ones there.” - Aaron (tested, Columbus, 31)
Sean (tested, Columbus, 20) directly connected the level of respect and his likelihood of returning for more care. He said, “They [medical providers] pretty nice. Yeah. They um treat you with respect, you know. Be courteous to you…It make you feel comfortable, going and getting checked up and stuff like that.”
Both the provider's role and the patients' roles in a good partnership were described by tested participants. Adrian (tested, Valdosta, 19) described the active role that patients should play in giving a medical history. Jordan (tested, Valdosta, 32) was similarly happy with his physician and their collaborative approach to medications:
“The kind of doctor I have um like if he diagnoses me with something then what I'll do is I'll research it and figure out what medicine I want to take or what medicine I want to try and I'll tell him and if he's heard of it he'll prescribe it to me.”
Several nontested participants also described positive experiences with provider–patient interactions, but they also more frequently cited dissatisfaction with services than the tested men in our sample. Connor (nontested, Columbus, 38) thought his doctor was good because, “he don't keep you in there all day, and uh, he talks to you, you know, he's a little personal with you.” However, many other nontested participants described negative interactions with medical personnel, like Jayden (nontested, Atlanta, 28) describing his struggle with pain management:
“Those folks [doctors] didn't even care. Like I'm…I'm still be in pain now so I mostly go to the doctor when I be in a lot of pain…’How does it make me feel?’ It make me feel uncomfortable.”
Several nontested participants also described a distrust of their medical providers' professional abilities and motivations that was not mentioned among tested participants:
“You know student health doctors—unfortunately are kind of the, the rejects unfortunately, like that's how it feels…I had a recent experience, where I went in and was mistakenly diagnosed with Herpes.” - Tristan (nontested, Atlanta, 26)
“I feel for too long doctors have had the rank of uh, sainthood, anything they tell you you're supposed to believe that. And a lot of time they be pushing different pills…after you because the pharmaceutical company court doctor.” - Blake (Columbus, non-tested, 60)
Customer satisfaction was diverse among both tested and nontested in our sample, as both groups described both good and bad experiences. However, nontested participants described more negative experiences and mentioned distrust of the abilities and motivations of providers.
Discussion
In this study, we identified several themes regarding the health care experiences and HIV testing practices of Black men in Georgia within the framework of the Andersen model of health care utilization. First, both tested and nontested participants described fragmented experiences obtaining general health care and HIV/STI testing, while also describing similar challenges navigating complex health care systems. Confidentiality emerged as a primary external environmental issue, particularly for participants from the smaller towns of Columbus and Valdosta. Population characteristics such as peer norms, convenience, and male gender role responsibilities were commonly described motivators for HIV testing. Tested participants tended to embody an empowered approach to HIV testing that mirrored their approach to their overall health care. Nontested participants described few enabling resources and were often reliant upon the medical provider to suggest HIV testing. Finally, both tested and nontested participants described satisfactory levels of health care experiences overall that were primarily driven by the doctor–patient relationship, but nontested participants noted more negative experiences.
The similarities emerged from common experiences with barriers in the Andersen model domain of Environmental Factors (e.g., health care systems, external environment) that the majority of participants endorsed, particularly issues surrounding poverty, lack of insurance, and the confidentiality of services. Both tested and nontested participants gave similar descriptions of difficulty with navigating safety net health care systems, lack of insurance, and medical “silos,” reflecting general deficits in the US health care system.26 Participants also mentioned experiences with inferior quality health services within Black communities, including confidentiality concerns (particularly in Valdosta and Columbus), consistent with previous research noting how intersecting contexts of race, racism, geography, and poverty influence racial disparities in health outcomes, particularly HIV.49–53 Confidentiality was a particularly important component of HIV testing among our participants, and the findings support its presence among Black men that may be part of overall distrust of medical systems that have persisted over time despite public health outreach efforts.54–62 Policies such as the Affordable Care Act should improve access to insurance and care. However, even with these improvements, quality within these systems and institutions and perceived lack of confidentiality will affect both overall health care utilization and HIV testing within Black communities.
Despite these similarities in the domain of Environmental Factors between tested and nontested participants, there were several notable contrasts in other domains of the Andersen model. In the domain of Population characteristics (i.e., predisposing characteristics, personal health beliefs, and enabling resources), tested participants described a strong sense of empowerment about approaches to their own health and HIV testing in spite of health system and external environmental obstacles. Specifically, they described assessing their own risk behavior, concern for risk to their sexual partners, and expectations of being a responsible man as reasons to seek HIV testing. Conversely, nontested participants placed less priority on HIV testing due to low self-perceived risk and did not mention consideration of the welfare of their sexual partners. Interestingly, if participants' descriptions of approaches to HIV testing and general health care were vigilant about seeking and prioritizing their health care in general, they also expressed similar proactive approaches to HIV testing. Conversely, if they did not prioritize seeking general preventive health care, they also did not prioritize regular HIV testing.
Several tested participants explicitly stated that they sought preventive health care and HIV testing because it was part of their image of being a strong man. These findings also suggest that health empowerment should be promoted as masculine attributes. While masculinity in the United States is traditionally touted as a barrier to general health care access, HIV testing and condom use among Black men,26,63–65 we note that a paradigm shift emphasizing health empowerment as a component of masculine expectations could be crucial in future interventions for Black men. This is consistent with limited prior literature that has associated higher masculinity scores with increased usage of preventive health services.37
Tested participants appeared to have enabling resources such as social and familial support for their health care decisions, while nontested participants did not. Peer norms, convenient access, and social support have been found to be positively associated with both condom use practices and HIV testing among samples of Black MSM,66–69 but comparable convenience or population-based studies among predominantly heterosexual samples of American Black men are limited. Moreover, many nontested participants cited physician recommendations as the main enabling resource for HIV testing, without the social support that tested participants described. It is equally important that nontested participants mentioned relying often on physician recommendations, which, given medical provider discomfort with sexuality discussions with patients in general70–73 and time constraint factors,74 has implications for future programs that focus on increasing comfort with sexuality discussions in medical institutions targeting both providers and patients alike.75,76 Peer norms, familial contexts, social support, and provider education should be integrated within STTR initiatives when considering programs that encourage HIV testing among Black men.77
Our analysis of themes within the Health Behavior domain (i.e., personal health practices, use of health services) revealed that both tested and nontested participants often described seeking general health care, HIV tests, and STI tests in different locations (Fig. 2), reflecting fragmentation of care. Among this sample, however, the venues where participants described receiving general health care and both HIV and STI testing included: (1) school clinics; (2) jails and prisons; (3) private doctor's offices; and (4) safety net/primary care clinics. While school and correctional clinics may be designed to cover several areas of health care (including general health care and HIV testing), participants' experiences with private doctors' offices and some safety net clinics suggested that they may not be offering all of these services. Moreover, given the decreased emphasis on annual physicals or recommended screenings,78 integrating HIV testing into other clinical service areas (e.g., Emergency Departments, Mobile Clinics) may be successful in capturing individuals who are not necessarily going to traditional primary care settings. Participants also noted that despite accessing primary care services, they did not get HIV tests, exposing the apparent incomplete adherence of CDC recommendations to test all patients presenting for routine care.3,15,74,79 This underscores the need to promote HIV testing among Black men within varied approaches and care settings and the need for new approaches that emphasize provider training to successfully adopt and integrate routine HIV testing into primary care settings.
The Andersen model Outcomes domain included themes related to consumer satisfaction and the importance of medical provider–patient relationships. Not surprisingly, tested participants described more positive personal qualities (e.g., good communication, concern, and respect) in their relationships with their providers and greater overall satisfaction, while nontested voiced more concerns about provider competence and distrust in motivations with medication prescribing. This is consistent with previous studies citing distrust as a barrier to HIV testing, acceptance of prevention messages and adherence to HIV medications.60–62,80,81 Better physician–patient relationships have shown to improve adherence to ART among HIV positive persons,82 while fear of stigma83 and perceptions of poor communication about sexual behavior with providers84 have also been barriers to HIV testing. These differences in the narratives between tested and non-tested participants among a predominately heterosexual sample of Black men highlight how general doctor–patient dynamics may influence HIV testing practices. They also provide context to the types of communication styles and relationships Black men desire with their providers that may improve health care utilization, and ultimately, HIV testing practices among this population.
There are several implications for our findings in the context of STTR HIV prevention and testing strategies. STTR shifts the emphasis away from HIV-negative persons and places it squarely on identifying those who are positive on getting them in treatment. However, questions about STTR implementation into effective HIV public policy still remain, including concerns about treating patients who may have poor adherence, thus increasing the risk of developing viral resistance, high costs of ART, toxicity and interactions of ART with concurrent medications, and shortages of HIV-experienced clinical providers.85 Our findings suggest that not only these HIV-specific considerations are important, but a richer appreciation of how general medical experiences affect receipt of HIV testing, particularly among Black men, is needed when developing STTR strategies. In addition, approaches that consider personal belief systems, peer norms, familial/social support, and how to make medical experiences welcoming to patients will be important in rapidly identifying HIV-positive individuals and successfully linking them to medical care. This may be especially challenging to implement among Black men, who currently experience disparities compared with men of other races/ethnicities in chronic disease outcomes, health care access, treatment, and research literature attention. For STTR HIV prevention approaches to be effective, we cannot ignore aspects of general health care systems in which these approaches will be integrated.
There are some limitations to this study. First, this study focused primarily on heterosexual Black men in Georgia, making it difficult to generalize the results. Second, social desirability might have influenced responses to our interview questions. This may be especially due to the fact that the survey questions were asked at the conclusion of the in-depth interviews. However, because survey questions were asked after the in-depth interviews, after (presumably) trust had been established between the interviewer and the research participant, more honest responses could have been obtained. Finally, an inter-geographical analysis between Atlanta, Columbus, and Valdosta was not conducted, since our primary focus was comparing tested versus nontested participants, but this could be the focus of a future analysis. Despite these limitations, this study contributes to an important gap in the literature, specifically concerning health care experiences and HIV testing among Black men in the South.
Successful implementation of STTR HIV prevention strategies among Black men in the South requires a nuanced understanding of facilitators and barriers to general health care utilization as well as HIV testing. As routine HIV testing becomes an integral part of HIV prevention initiatives, parallel efforts to improve the access, quality and retention of these men in general medical care should be prioritized as well. Improving both access and quality of health systems/institutions, integrating HIV/STI testing into general medical services, promoting health empowerment, and addressing consumer satisfaction may be keys to improving HIV outcomes for this population successfully.
Acknowledgments
Rupali Doshi performed the data analysis, wrote the initial draft of the manuscript, and edited and approved the final manuscript. David Malebranche developed the conceptual framework for the parent study, trained the interviewers, conducted some of the qualitative interviews, and edited and approved the final manuscript. Lisa Bowleg and Thurka Sangaramoorthy contributed to development of the interview guide, and they reviewed and edited the manuscript.
Rupali Doshi received tuition support from the NIH/NCRR (UL RR025008). David Malebranche is the Principal Investigator for Project Adofo, which is funded by NIH/NINR (R01 NR011137-03). Lisa Bowleg is a Co-Investigator for Project Adofo. Thurka Sangaramoothy is a Consultant for Project Adofo. We thank the men who participated in the study to tell us their stories; Luella Rhodes and Columbus Wellness Center; Shannon Buckner and the Boyz Salon Barbershop; Audrey Dixon with Exceptional Transcription and Business Solutions; and January Smith, MPH, and Brenda Mims, RN, with the Valdosta Department of Health. Finally, we would like to thank the Project Adofo research team: Brandi Park, MPH, Alanna Stone, MD, MPH, Bernard Owens, Jeffery Roman, and Leonard Moore, MD.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Prejean J. Song R. Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. HIV/AIDS Statistics and Surveillance. 2010. http://www.cdc.gov/hiv/topics/surveillance/resources/slides/index.htm. [May 24;2012 ]. http://www.cdc.gov/hiv/topics/surveillance/resources/slides/index.htm
- 3.Campsmith ML. Rhodes PH. Hall HI. Green TA. Undiagnosed HIV prevalence among adults and adolescents in the United States at the end of 2006. J Acquir Immune Defic Syndr. 2010;53:619–624. doi: 10.1097/QAI.0b013e3181bf1c45. [DOI] [PubMed] [Google Scholar]
- 4.CDC. HIV Surveillance in Urban and Nonurban Areas. 2009. http://www.cdc.gov/hiv/topics/surveillance/resources/slides/urban-nonurban/index.htm. [Mar 13;2012 ]. http://www.cdc.gov/hiv/topics/surveillance/resources/slides/urban-nonurban/index.htm
- 5.CDC. Previous HIV testing among adults and adolescents newly diagnosed with HIV infection—National HIV Surveillance System, 18 jurisdictions, United States, 2006–2009. MMWR. 2012;61:441–445. [PubMed] [Google Scholar]
- 6.Granich RM. Gilks CF. Dye C. De Cock KM. Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: A mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 7.Montaner JS. Lima VD. Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: A population-based study. Lancet. 2010;376:532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das M. Chu PL. Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen MS. Chen YQ. McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New Eng J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC. Zidovudine for the prevention of HIV transmission from mother to infant. MMWR. 1994;43:285–287. [PubMed] [Google Scholar]
- 11.Gardner EM. McLees MP. Steiner JF. Del Rio C. Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Check Hayden E. 'Seek, test and treat' slows HIV. Nature. 2010;463:1006. doi: 10.1038/4631006a. [DOI] [PubMed] [Google Scholar]
- 13.Taege A. Seek and treat: HIV update 2011. Cleveland Clinic J Med. 2011;78:95–100. doi: 10.3949/ccjm.78gr.10003. [DOI] [PubMed] [Google Scholar]
- 14.Branson BM. Handsfield HH. Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR-CDC. 2006;55:1–17. quiz CE11–14. [PubMed] [Google Scholar]
- 15.CDC. Results of the Expanded HIV Testing Initiative–25 jurisdictions, United States, 2007–2010. MMWR. 2011;60:805–810. [PubMed] [Google Scholar]
- 16.CDC. Life expectancy at selected ages by race, Hispanic origin, race for non-Hispanic population, and sex: United States. 2009.
- 17.Downie DL. Schmid D. Plescia MG, et al. Racial disparities in blood pressure control and treatment differences in a Medicaid population, North Carolina, 2005–2006. Prevent Chronic Dis. 2011;8:A55. [PMC free article] [PubMed] [Google Scholar]
- 18.Kressin NR. Orner MB. Manze M. Glickman ME. Berlowitz D. Understanding contributors to racial disparities in blood pressure control. Circ Cardiovasc Qual Outcomes. 2010;3:173–180. doi: 10.1161/CIRCOUTCOMES.109.860841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manze M. Rose AJ. Orner MB. Berlowitz DR. Kressin NR. Understanding racial disparities in treatment intensification for hypertension management. J Gen Intern Med. 2010;25:819–825. doi: 10.1007/s11606-010-1342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ndumele CD. Shaykevich S. Williams D. Hicks LS. Disparities in adherence to hypertensive care in urban ambulatory settings. J Health Care Poor Underserved. 2010;21:132–143. doi: 10.1353/hpu.0.0259. [DOI] [PubMed] [Google Scholar]
- 21.Redmond N. Baer HJ. Hicks LS. Health behaviors and racial disparity in blood pressure control in the national health and nutrition examination survey. Hypertension. 2011;57:383–389. doi: 10.1161/HYPERTENSIONAHA.110.161950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter VL. Tippett F. Anderson DL. Tameru B. Increasing prostate cancer screening among African American men. J Health Care Poor Underserved. 2010;21:91–106. doi: 10.1353/hpu.0.0366. [DOI] [PubMed] [Google Scholar]
- 23.Cruz-Flores S. Rabinstein A. Biller J, et al. Racial-ethnic disparities in stroke care: The American experience: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2091–2116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
- 24.DeNavas-Walt C. Proctor BD. Smith JC. Bureau USC. Washington, DC: U.S. Government Printing Office; 2011. Income, Poverty, and Health Insurance Coverage in the United States: 2010; pp. 60–239. [Google Scholar]
- 25.AHRQ. National Healthcare Disparities Report. 2010. http://www.ahrq.gov/qual/qrdr10.htm. [May 25;2012 ]. http://www.ahrq.gov/qual/qrdr10.htm
- 26.Malebranche DJ. Peterson JL. Fullilove RE. Stackhouse RW. Race and sexual identity: perceptions about medical culture and healthcare among Black men who have sex with men. J Natl Med Assoc. 2004;96:97–107. [PMC free article] [PubMed] [Google Scholar]
- 27.Viera AJ. Thorpe JM. Garrett JM. Effects of sex, age, and visits on receipt of preventive healthcare services: A secondary analysis of national data. BMC Health Serv Res. 2006;6:15. doi: 10.1186/1472-6963-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green CA. Pope CR. Gender, psychosocial factors and the use of medical services: A longitudinal analysis. Soc Sci Med. 1999;48:1363–1372. doi: 10.1016/s0277-9536(98)00440-7. [DOI] [PubMed] [Google Scholar]
- 29.Sandman D. Simantov E. An C. Out of Touch: American Men and the Health Care System. Commonwealth Fund. 2000 [Google Scholar]
- 30.Neighbors HW. Howard CS. Sex differences in professional help seeking among adult black Americans. Am J Comm Psychol. 1987;15:403–417. doi: 10.1007/BF00915210. [DOI] [PubMed] [Google Scholar]
- 31.Hammond WP. Matthews D. Corbie-Smith G. Psychosocial factors associated with routine health examination scheduling and receipt among African American men. J Natl Med Assoc. 2010;102:276–289. doi: 10.1016/s0027-9684(15)30600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen RM. Revisiting the behavioral model and access to medical care: Does it matter? J Health Soc Behav. 1995;36:1–10. [PubMed] [Google Scholar]
- 33.Vyas A. Madhavan S. Lemasters T, et al. Factors influencing adherence to mammography screening guidelines in Appalachian women participating in a mobile mammography program. J Community Health. 2012;37:632–646. doi: 10.1007/s10900-011-9494-z. [DOI] [PubMed] [Google Scholar]
- 34.Guilcher SJ. Craven BC. McColl MA. Lemieux-Charles L. Casciaro T. Jaglal SB. Application of the Andersen's health care utilization framework to secondary complications of spinal cord injury: A scoping review. Disabil Rehabil. 2011 doi: 10.3109/09638288.2011.608150. [DOI] [PubMed] [Google Scholar]
- 35.Saint-Jean G. Metsch L. Gomez-Marin O, et al. Use of HIV primary care by HIV-positive Haitian immigrants in Miami, Florida. AIDS Care. 2011;23:486–493. doi: 10.1080/09540121.2010.516339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravenell JE. Johnson WE., Jr. Whitaker EE. African-American men's perceptions of health: A focus group study. J Natl Med Assoc. 2006;98:544–550. [PMC free article] [PubMed] [Google Scholar]
- 37.Hammond WP. Matthews D. Mohottige D. Agyemang A. Corbie-Smith G. Masculinity, medical mistrust, and preventive health services delays among community-dwelling African-American men. J Gen Int Med. 2010;25:1300–1308. doi: 10.1007/s11606-010-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vyavaharkar MV. Moneyham L. Corwin S. Health care utilization: The experiences of rural HIV-positive African American women. J Health Care Poor Underserved. 2008;19:294–306. doi: 10.1353/hpu.2008.0013. [DOI] [PubMed] [Google Scholar]
- 39.United States Census. 2010. http://www.factfinder.census.gov. [May 15;2012 ]. http://www.factfinder.census.gov
- 40.2010 Georgia HIV/AIDS Surveillance Summary. Section HAE. p. ed2012.
- 41.Malebranche DJ. Fields EL. Bryant LO. Harper SR. Masculine socialization and sexual risk behaviors among Black men who have sex with men. A qualitative exploration. Men Masc. 2009;12:90–112. [Google Scholar]
- 42.Patton MQ. Qualitative Research and Evaluation Methods. 3rd. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 43.Miles MB. Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. Thousand Oaks, CA: Sage; 1994. [Google Scholar]
- 44.Strauss AL. Corbin JM. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. Newbury Park, CA: Sage; 1990. [Google Scholar]
- 45.Corbin JM. Strauss AL. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. 3rd. Thousand Oaks, CA: Sage; 2008. [Google Scholar]
- 46.Huberman AM. Miles MB. Drawing valid meaning from qualitative data. Some techniques of data reduction and display. Qual Quant. 1983;17:281–339. [Google Scholar]
- 47.NCQA. Patient-Centered Medical Home. http://www.ncqa.org/tabid/631/default.aspx. [May 29;2012 ]. http://www.ncqa.org/tabid/631/default.aspx
- 48.Pathela P. Hajat A. Schillinger J. Blank S. Sell R. Mostashari F. Discordance between sexual behavior and self-reported sexual identity: A population-based survey of New York City men. Ann Intern Med. 2006;145:416–425. doi: 10.7326/0003-4819-145-6-200609190-00005. [DOI] [PubMed] [Google Scholar]
- 49.Baicker K. Chandra A. Skinner JS. Wennberg JE. Who you are and where you live: How race and geography affect the treatment of medicare beneficiaries. Health Affairs (Project Hope) 2004;(Suppl Variation) doi: 10.1377/hlthaff.var.33. VAR33-44. [DOI] [PubMed] [Google Scholar]
- 50.Chandra A. Who you are and where you live: Race and the geography of healthcare. Med Care. 2009;47:135–137. doi: 10.1097/MLR.0b013e31819a4c5e. [DOI] [PubMed] [Google Scholar]
- 51.Smedley BD. Stith AY. Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, D.C.: National Academy Press; 2003. Institute of Medicine (U.S.). Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. [PubMed] [Google Scholar]
- 52.Gamble VN. Under the shadow of Tuskegee: African Americans and health care. Am J Public Health. 1997;87:1773–1778. doi: 10.2105/ajph.87.11.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones CP. Levels of racism: A theoretic framework and a gardener's tale. Am J Public Health. 2000;90:1212–1215. doi: 10.2105/ajph.90.8.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bird ST. Bogart LM. Perceived race-based and socioeconomic status(SES)-based discrimination in interactions with health care providers. Ethn Dis. 2001;11:554–563. [PubMed] [Google Scholar]
- 55.Bogart LM. Bird ST. Exploring the relationship of conspiracy beliefs about HIV/AIDS to sexual behaviors and attitudes among African-American adults. J Natl Med Assoc. 2003;95:1057–1065. [PMC free article] [PubMed] [Google Scholar]
- 56.Bird ST. Bogart LM. Delahanty DL. Health-related correlates of perceived discrimination in HIV care. AIDS Patient Care STDS. 2004;18:19–26. doi: 10.1089/108729104322740884. [DOI] [PubMed] [Google Scholar]
- 57.Bogart LM. Bird ST. Walt LC. Delahanty DL. Figler JL. Association of stereotypes about physicians to health care satisfaction, help-seeking behavior, and adherence to treatment. Soc Sci Med. 2004;58:1049–1058. doi: 10.1016/s0277-9536(03)00277-6. [DOI] [PubMed] [Google Scholar]
- 58.Bird ST. Bogart LM. Conspiracy beliefs about HIV/AIDS and birth control among African Americans: Implications for the prevention of HIV, other STIs, and unintended pregnancy. J Soc Issues. 2005;61:109–126. doi: 10.1111/j.0022-4537.2005.00396.x. [DOI] [PubMed] [Google Scholar]
- 59.Thorburn S. Bogart LM. Conspiracy beliefs about birth control: Barriers to pregnancy prevention among African Americans of reproductive age. Health Educ Behav. 2005;32:474–487. doi: 10.1177/1090198105276220. [DOI] [PubMed] [Google Scholar]
- 60.Bogart LM. Thorburn S. Are HIV/AIDS conspiracy beliefs a barrier to HIV prevention among African Americans? J Acquir Immune Defic Syndr. 2005;38:213–218. doi: 10.1097/00126334-200502010-00014. [DOI] [PubMed] [Google Scholar]
- 61.Bogart LM. Thorburn S. Relationship of African Americans' sociodemographic characteristics to belief in conspiracies about HIV/AIDS and birth control. J Natl Med Assoc. 2006;98:1144–1150. [PMC free article] [PubMed] [Google Scholar]
- 62.Bogart LM. Kalichman SC. Simbayi LC. Endorsement of a genocidal HIV conspiracy as a barrier to HIV testing in South Africa. J Acquir Immune Defic Syndr. 2008;49:115–116. doi: 10.1097/QAI.0b013e318181b889. [DOI] [PubMed] [Google Scholar]
- 63.Whitehead TL. Urban low-income African American men, HIV/AIDS, and gender identity. Med Anthropol Quart. 1997;11:411–447. doi: 10.1525/maq.1997.11.4.411. [DOI] [PubMed] [Google Scholar]
- 64.Aronson RE. Whitehead TL. Baber WL. Challenges to masculine transformation among urban low-income African American males. Am J Public Health. 2003;93:732–741. doi: 10.2105/ajph.93.5.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolfe WA. Overlooked role of African-American males' hypermasculinity in the epidemic of unintended pregnancies and HIV/AIDS cases with young African-American women. J Natl Med Assoc. 2003;95:846–852. [PMC free article] [PubMed] [Google Scholar]
- 66.Hart T. Peterson JL Team CIYS. Predictors of risky sexual behavior among young African American men who have sex with men. Am J Public Health. 2004;94:1122–1123. doi: 10.2105/ajph.94.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Northington L. Chronic sorrow in caregivers of school age children with sickle cell disease: A grounded theory approach. Issues Comp Pediatr Nursing. 2000;23:141–154. doi: 10.1080/01460860050174693. [DOI] [PubMed] [Google Scholar]
- 68.Millett GA. Peterson JL. Wolitski RJ. Stall R. Greater risk for HIV infection of black men who have sex with men: A critical literature review. Am J Public Health. 2006;96:1007–1019. doi: 10.2105/AJPH.2005.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Millett GA. Flores SA. Peterson JL. Bakeman R. Explaining disparities in HIV infection among black and white men who have sex with men: A meta-analysis of HIV risk behaviors. AIDS. 2007;21:2083–2091. doi: 10.1097/QAD.0b013e3282e9a64b. [DOI] [PubMed] [Google Scholar]
- 70.Wimberly Y. Moore S. Sexual history taking should be taught in medical school. Am Family Phys. 2003;68:223. [PubMed] [Google Scholar]
- 71.Wimberly YH. Hogben M. Moore-Ruffin J. Moore SE. Fry-Johnson Y. Sexual history-taking among primary care physicians. J Natl Med Assoc. 2006;98:1924–1929. [PMC free article] [PubMed] [Google Scholar]
- 72.Ramsey PG. Curtis JR. Paauw DS. Carline JD. Wenrich MD. History-taking and preventive medicine skills among primary care physicians: An assessment using standardized patients. Am J Med. 1998;104:152–158. doi: 10.1016/s0002-9343(97)00310-0. [DOI] [PubMed] [Google Scholar]
- 73.Verhoeven V. Bovijn K. Helder A, et al. Discussing STIs: Doctors are from Mars, patients from Venus. Family Pract. 2003;20:11–15. doi: 10.1093/fampra/20.1.11. [DOI] [PubMed] [Google Scholar]
- 74.Johnson CV. Mimiaga MJ. Reisner SL. VanDerwarker R. Mayer KH. Barriers and facilitators to routine HIV testing: Perceptions from Massachusetts Community Health Center personnel. AIDS Patient Care STDS. 2011;25:647–655. doi: 10.1089/apc.2011.0180. [DOI] [PubMed] [Google Scholar]
- 75.Bogart LM. Howerton D. Lange J, et al. Provider-related barriers to rapid HIV testing in U.S. urban non-profit community clinics, community-based organizations (CBOs) and hospitals. AIDS Behav. 2010;14:697–707. doi: 10.1007/s10461-008-9456-3. [DOI] [PubMed] [Google Scholar]
- 76.Auerbach C. Beckerman NL. HIV/AIDS prevention in New York City: Identifying sociocultural needs of the community. Social Work Health Care. 2010;49:109–133. doi: 10.1080/00981380903158011. [DOI] [PubMed] [Google Scholar]
- 77.Aral SO. Adimora AA. Fenton KA. Understanding and responding to disparities in HIV and other sexually transmitted infections in African Americans. Lancet. 2008;26372:337–340. doi: 10.1016/S0140-6736(08)61118-6. [DOI] [PubMed] [Google Scholar]
- 78.USPSTF. Recommendations for adults. http://www.uspreventiveservicestaskforce.org/adultrec.htm. [Apr 3;2012 ]. http://www.uspreventiveservicestaskforce.org/adultrec.htm
- 79.Branson BM. Handsfield HH. Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR. Recommendations and reports CDC. 2006;55:1–17. quiz CE11–14. [PubMed] [Google Scholar]
- 80.Bogart LM. Wagner G. Galvan FH. Banks D. Conspiracy beliefs about HIV are related to antiretroviral treatment nonadherence among African American men with HIV. J Acquir Immune Defic Syndr. 2010;53:648–655. doi: 10.1097/QAI.0b013e3181c57dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bogart LM. Galvan FH. Wagner GJ. Klein DJ. Longitudinal association of HIV conspiracy beliefs with sexual risk among black males living with HIV. AIDS Behav. 2011;15:1180–1186. doi: 10.1007/s10461-010-9796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schneider J. Kaplan SH. Greenfield S. Li W. Wilson IB. Better physician-patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. J Gen Int Med. 2004;19:1096–1103. doi: 10.1111/j.1525-1497.2004.30418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Worthington C. Myers T. Factors underlying anxiety in HIV testing: Risk perceptions, stigma, and the patient-provider power dynamic. Qual Health Res. 2003;13:636–655. doi: 10.1177/1049732303013005004. [DOI] [PubMed] [Google Scholar]
- 84.Mimiaga MJ. Goldhammer H. Belanoff C. Tetu AM. Mayer KH. Men who have sex with men: perceptions about sexual risk, HIV and sexually transmitted disease testing, and provider communication. Sex Transm Dis. 2007;34:113–119. doi: 10.1097/01.olq.0000225327.13214.bf. [DOI] [PubMed] [Google Scholar]
- 85.Zuger A. A skeptic looks at "Test and Treat". J Watch AIDS Clin Care. 2012;24:45–46. [Google Scholar]