Abstract
Incomplete cervical lesion is the most common type of human spinal cord injury (SCI) and causes permanent paresis of arm muscles, a phenomenon still incompletely understood in physiopathological and neuroanatomical terms. We performed spinal cord hemisection in adult rats at the caudal part of the segment C6, just rostral to the bulk of triceps brachii motoneurons, and analyzed the forces and kinematics of locomotion up to 4 months postlesion to determine the nature of motor function loss and recovery. A dramatic (50%), immediate and permanent loss of extensor force occurred in the forelimb but not in the hind limb of the injured side, accompanied by elbow and wrist kinematic impairments and early adaptations of whole-body movements that initially compensated the balance but changed continuously over the follow-up period to allow effective locomotion. Overuse of both contralateral legs and ipsilateral hind leg was evidenced since 5 days postlesion. Ipsilateral foreleg deficits resulted mainly from interruption of axons that innervate the spinal cord segments caudal to the lesion, because chronic loss (about 35%) of synapses was detected at C7 while only 14% of triceps braquii motoneurons died, as assessed by synaptophysin immunohistochemistry and retrograde neural tracing, respectively. We also found a large pool of propriospinal neurons projecting from C2–C5 to C7 in normal rats, with topographical features similar to the propriospinal premotoneuronal system of cats and primates. Thus, concurrent axotomy at C6 of brain descending axons and cervical propriospinal axons likely hampered spontaneous recovery of the focal neurological impairments.
Key words: cervical propriospinal premotoneuronal system; cervical spinal cord injury; forelimb force, kinematics, triceps brachii
Introduction
Approximately 55% of human traumatic spinal cord injury (SCI) occurs at cervical segments, causing incomplete lesions in about 70% of cases.1 Cervical SCI produces segmental neuronal death that contributes to chronic upper limb motor deficits in humans2,3 or forelimb in rats.4 Nevertheless, white matter destruction has greater clinical importance because it interrupts the synaptic inputs from brain centers to all the spinal cord segments caudal to the lesion site. Accordingly, thoracic SCI leads to chronic locomotor impairments that correlate with the extent of axonal damage and disconnection of the lumbar spinal cord from supraspinal nuclei.5–8 Moreover, the interneurons (INs) and motoneurons (MNs) close to the SCI site are expected to be more denervated than those located at distant segments, because the farther the segment is, the larger the number of propriospinal neurons (PNs) that remain connected with it. This may explain why human stepping-like movements are easier to induce aftercervical than thoracic SCI,9 and also the chronic weakness detected in human arm muscles that receive innervation from spinal levels just caudal to the lesion site.10
The loss and recovery of forelimb motor function has been studied in several models of cervical SCI in rats,4,11–27 cats,28–30 and primates.31–35 All those studies demonstrated some extent of spontaneous motor function recovery, albeit with persisting deficits as a consequence of interruption of descending axonal tracts or segmental neuronal death. Despite these advances, there is still a need of cervical SCI models in which motor performance is comprehensively assessed in the long term to determine the motor components that show recovery and to link the chronic deficits with the neuroanatomical damage. Moreover, motor compensations always coexist with associated impairments in limb synergies,4,5,8,16,21,36 complicating the interpretation and quantification of spontaneous and treatment-induced functional recovery and demanding the combined use of kinetic, kinematical, electrophysiological, and anatomical techniques for appropriate assessment.
The topographical organization of MNs in the cervical enlargement37 offers the possibility of developing lesion paradigms that cause death of selective MN pools,4,11 which are of interest for investigating strategies for MN protection and/or replacement.38–42 It is likewise possible to produce segmental motor deficits by performing restricted white matter lesions just rostral to specific groups of MNs and INs, which would become denervated as a consequence of the interruption of axonal tracts originating in brain and cervical propriospinal nuclei.43,44 These models will make easier to verify the functional impact of axonal regeneration across the lesion site, because the axons will have a good chance to achieve functional reconnection with the target MNs and INs in the adjacent segment.
In this context, the present work addresses the functional and anatomical consequences of C6 spinal cord hemisection using kinetic and kinematic analysis of locomotion, immunohistochemistry, and retrograde neural tracing. The lesion was aimed at interrupting the axons that originate in brain nuclei and C3/C4 propriospinal premotor neurons to innervate the segments C7/C8,44 which contain the spinal motor nuclei controlling the triceps brachii (TB) and some forepaw muscles4,37 and their segmental INs. This would cause major deficits for elbow extension and wrist joint movements in the ipsilateral forelimb that could be detected during locomotion.
We have previously reported the analysis of about 20 kinematic parameters for assessing rat locomotion after thoracic SCI.8 The present work presents a comprehensive quantification of primary motor deficits and compensations after C6 hemisection, based on the analysis of approximately 100 kinetic and kinematic variables. Part of this research was presented in abstract form.45
Methods
Animals
The experimental protocols adhered to the recommendations of the European Commission and the Spanish regulations for the protection of experimental animals (86/609/CEE, 32/2007, and 223/1988) and were approved by the Ethical Committee for Animal Research of the Hospital Nacional de Parapléjicos (Toledo, Spain). Adult male Wistar rats (n=98, 16–24 weeks old, 380–500 g) raised at the animal facility of the Hospital Nacional de Parapléjicos were used in this study. Rats were housed in groups of two at a 12 hours light/dark cycle with food and water available ad libitum. Eight normal rats served to standardize the surgical procedures of SCI and application of neural tracers, 46 animals were used for biomechanical analyses of locomotion and TB motoneuron counts after C6 hemisection, and 44 animals were used to study the changes in synaptophysin immunoreactivity after SCI and to quantify the cervical propriospinal neurons that project to C7.
All surgical procedures were performed under general anesthesia with intraperitoneally applied sodium pentobarbital (55 mg/kg) mixed with atropine (0.02–0.2 mg/kg) and xylazine (10 mg/kg), administering a supplemental 30% of these drugs 90 min after the initial dose. An unguent was applied to the eyes to prevent corneal abrasion and infection. Animals were kept at 37°C with the aid of a thermal pad. Antibiotics (enrofloxacine, 0.1 mL/kg) and analgesics (meloxicam, 2–5 mg/kg) were administrated subcutaneously immediately after surgery and at 8 and 16 hours later.
Spinal cord hemisection
The back and neck of the anesthetized animals were shaved and disinfected with povidone iodine. A dorsal midline incision was made in the skin and superficial muscles of the neck region, and blunt dissection was carefully performed to expose the cervical spine. The vertebra C5, which contains the spinal cord segment C6, was identified by counting from the vertebrae C2 and T2. The spinous apophysis and dorsal laminae of C5 were removed without applying pressure on the underlying spinal cord.
Subsequently, a transverse incision was performed in the dura mater, the right C6 dorsal root was identified, the visible dorsal and dorsolateral blood vessels were thermocauterized, and the right side of the spinal cord was cut at the caudal part of C6 with straight microscissors. The muscle planes were separately sutured and, finally, a subcutaneous suture was used for the skin, because our preliminary studies showed that the use of metallic suture clips causes discomfort to the animal and reduces the range of forelimb motion at 5 days postlesion. The animals were kept at 37°C until fully recovered from anesthesia. Sham-operated controls received only C5 laminectomy without opening of the dura mater or spinal cord hemisection.
Ground reaction forces (GRFs) and kinematics of locomotion
Before the lesion, rats were trained to walk spontaneously on a flat surface (120 cm long, 20 cm wide) limited by transparent walls and ending in a dark box. Two multicomponent force plates 40 cm long, 20 cm wide (9286A, Kistler Instrument Corp. Winterthur, Switzerland) that record forces in the three Cartesian axes were interspersed at the center of the runway. The three Cartesian components of GRFs represent specific actions: the vertical component indicates antigravity force that elevates the body or prevents falling; the fore-aft component describes braking and propulsion forces in the direction of locomotion; and the transverse components can be referred to as lateral stabilization forces because they normally have the same modulus but opposite directions for the right and left legs.
A key aspect of overground, legged locomotion is that the vertical forces generated for body support are always considerably larger than the forces used for forward propulsion or lateral stabilization, because gravity acts only in the vertical direction and friction forces opposing to forward movements are negligible. Hence, the motor impairments associated with weakness of antigravity limb extensors (i.e., the TB) are most likely to manifest in the vertical forces during the stance phase, while other force components or the unloaded extension of the leg during the swing phase may approach normality, particularly at spontaneous walking speeds that do not demand the maximum physiological motor output.
Animals were weighted each day of locomotor assessment, and the forces were expressed as percentage of the body weight. Locomotor force recordings were performed at 200 Hz and synchronized with animal motion images captured at 125 Hz by three high-speed video cameras (MotionScope, Redlake MASD Inc, San Diego, CA). Two cameras obtained an orthogonal view of animal locomotion from both sides of the body whereas the third camera showed an oblique, top view that allowed a precise visualization of the paws on each force plate. Points corresponding to hip, knee, ankle, metatarsus, shoulder, elbow, wrist, and metacarpus were marked on the shaved rat skin for kinematic analyses. Preinjury motor measurements were taken after at least 10 trials of spontaneous locomotion, when the gait of the animals was continuous and non-exploratory. A total of 46 animals were randomly assigned to one of the following groups: (a) right C6 hemisection (n=24), (b) sham-operated animals (n=11), and (c) normal controls (n=11). Locomotion was assessed pre-surgery and at 5, 10, 20, 45, and 120 days postinjury (DPI).
Retrograde TB MN tracing
It was necessary to investigate whether significant MN death occurred in the whole TB motor nucleus or in the subnucleus of the lateral fascicle, which is predominantly formed by large motor units and muscle fibers,46 because either of those phenomena will produce a substantial reduction of elbow extensor force after C6 hemisection. At the end of the follow-up, animals received neuronal tracers in the right foreleg to identify the TB MNs in the injured side of the spinal cord. Intramuscular injections of aminostilbamidine methanesulfonate (Molecular Probes; 4% w/v in saline solution) were performed in all fascicles of half of the animals for a global quantification of TB MNs. In the other half, independent labeling of the TB subnuclei was performed with dextran tetramethylrhodamine (3000 MW, Molecular Probes, 10% w/v in 0.1 M phosphate buffered saline [PBS], pH 7.35), fast blue (Polysciences, 2% in 0.9 saline) or dextran alexa 488 (Molecular Probes, 5% in 0.1 M PBS) applied separately to the cut nerve stumps at the very entrance of each TB fascicle. The surgical procedures for both types of neural tracing have been described in detail elsewhere.46
The animals were sacrificed 3.5 days later for histological processing. An additional group of eight animals with hemisection at the middle part of C6 (1 mm rostral to the usual lesion site) was used to investigate if nerve axotomy or the longitudinal extent of the lesion influenced the MN counts. Those animals sustained the triple-tracing paradigm bilaterally and were sacrificed at 45 DPI. MN counts of the injured side were expressed as percentage of counts of the contralateral side in each animal to reduce errors from interanimal variability in cell labeling and histological procedures, and were also compared with normal values.
Histological procedures and analyses
Anesthetized animals were transcardially perfused with isotonic saline followed by 4% paraformaldehyde in 0.1 M, pH 7.35 phosphate buffer (0.8 mL of perfusion solution per gram of body weight). The spinal cord was removed, immersed in 30% sucrose for 48 hours, cut serially into 60-μm sections, and mounted for visualization of fluorescent-labeled MNs. We have previously compared three different methods (namely simple, absolute, and stereological) for counting the MNs in each of the TB fascicles.46 The simple method consisted in counting all labeled cell profiles with identifiable nucleus or at least three dendrites in every tissue section. We found that this method was more efficient and accurate than the stereological estimation for this purpose and, hence, in the present study, we chose the simple counting to compare the relative numbers of MNs in the different experimental conditions.
After MN counting, the tissue was processed for hematoxylin and eosin (H&E) staining to determine lesion extent. It was necessary to include a defatting step with chloroform to achieve H&E staining in 60-μm slices. The maximal longitudinal extent of the lesion was defined as the distance between the most rostral and the most caudal transverse tissue sections in which evidence of tissue damage appeared with the H&E staining, excluding from consideration pathological changes attributable to wallerian degeneration. In addition, the spinal cord sections were photographed, and the amount of neural tissue present at the site of maximal transverse damage was quantified using the ImageJ software (1.39u, National Institutes of Health, Bethesda, MD), and subsequently the data from the injured side were expressed as percentage of the uninjured side in each animal. Only the animals that showed essentially unilateral and complete spinal cord hemisection (<5% of remaining tissue) were included in the analyses.
Synaptophysin immunohistochemistry and quantification
Synaptophysin is a synaptic vesicle protein useful for assessing the loss and remodeling of synapses after brain or spinal cord damage.47,48 We performed a systematic analysis of the immunostaining for synaptophysin in the cervical spinal cord (C4–C8) as a first step in characterizing the synaptic changes that occur in the partially denervated segments. Our objective was to investigate the possibility that synaptic loss persisted chronically in the spinal cord segments adjacent to the lesion site and, hence, we quantified the intensity of staining in a group of normal rats (n=8) and two groups of rats with C6 hemisection, at 30 (n=12) or 90 (n=12) DPI. Again, only those animals in which the lesion was a complete hemisection restricted to the right side of the spinal cord were analyzed.
For this, transverse sections of 30 μm were taken at intervals of 1 mm in the rostral and caudal direction from the lesion site, treated for 30 min with PBS containing 0.5% triton and 5% normal goat serum, rinsed three times with PBS, and incubated overnight at 4°C with the primary antibody (Sigma-Aldrich S5768; 1:500). Then, the sections were washed and incubated for 2 h at room temperature with Alexa 594 goat antimouse IgG (Molecular Probes, 1:500), washed, and mounted for fluorescence microscopy. The tissue sections were visualized and automatically photographed using a video time-lapse microscope (Leica DMI 6000 B, equipped with a camera DFC 350 FX; Leica Microsystems CMS GmbH). High-resolution (6959×5199 pixels) photographs were obtained with a 10× objective and converted to superimages with the Leica LAS AF Lite software.
The mean fluorescence intensity of the grey matter, the immunostained area, and the integrated fluorescence density were measured in both sides of the spinal cord with the ImageJ software. Data from the injured side were expressed as percentage of the non-injured side in each animal to reduce bias caused by variability in animal size, perfusion, and histological procedures.
Retrograde tracing of cervical PNs projecting to C7
To investigate the existence of a C3/C4 propriospinal system projecting to the segments containing the TB MNs in rats, uninjured animals (n=18) were anesthetized and the spinal cord segment C7 was exposed by dorsal laminectomy of the vertebra C6. The animals were placed in a stereotaxic frame, the spine was fixed by clamps attached to the vertebrae C2 and T2, and aminostilbamidine (4% w/v in saline) was injected into the right side of the spinal cord. Two injections of the tracer (0.2 μl each, 0.5 mm spaced) were carefully made into the right gray matter, 1 mm in depth at 0.8 mm from the midline, using a Hamilton syringe equipped with special needles (120 μm outer diameter). The needle was let in place for 10 min to prevent reflux of the injected solution.
Initially, six animals were sacrificed at 6, 24, or 72 h post-injection to determine the appropriate survival time for labeling PNs. Twenty-four hours was optimal and, hence, two additional groups of six animals were perfused with paraformaldehyde at this time and used for neuron quantification. The cervical spinal cord was transversally cut at 50 μm, mounted for fluorescence visualization, and photographed at 2776×2074 pixels with a digital image microscope system (Olympus DP50).
Every stained cell profile with identifiable nucleus or with two or more dendrites was counted in alternate tissue sections. In the first group, PNs were counted in the segments C1–C4, differentiating those located in the dorsal (laminae I to V), intermediate (laminae VI and VII), or ventral (laminae VIII and IX) regions of the gray matter. In the second group, only the PNs of laminae VII and VIII were counted separately. The drawings by Paxinos and Watson49 were used to delineate the laminae of the gray matter in both experiments. The segments C6–C8 were also sliced to verify the injection site, and 3 of 12 animals were excluded because the injections were made outside the C7 gray matter.
Collection and analysis of biomechanical parameters
BioWare software V. 3.24 (Kistler Instrument Corp) was used for the acquisition, smoothing (moving mean, window size 3), normalization, and analysis of force recordings. Automatic motion analysis software (WINanalyze V. 2.2, Mikromak GmbH, Münster, Germany) was used to digitize the movement of the skin markers and obtain body displacements, velocities, and joint angles. Data were exported to SigmaPlot software (V. 9.0, SPSS Inc, Chicago, IL), smoothed (Loess, sampling proportion 0.2, polynomial degree 3), and normalized to the duration of the forelimb or hind limb stride, averaging six walking cycles per animal and day of assessment. The cycles in which the animals did not contact the ground or reduced their velocity to explore the environment were excluded from kinetic and kinematical analyses.
For statistical analyses, data from the whole group of animals in the intact state were pooled and compared with pooled data from the whole group of animals at different DPI. Most biomechanical parameters were expressed as dimensionless numbers dividing by their values at the beginning of the stance phase, which allowed removing subtle sources of variability because of animal size and placement and movement of the skin markers. This procedure also produced a better normal distribution of the data (as assessed by the Kolmogorov-Smirnov test) in the great majority of cases. Usually, one-way, two-way, and repeated measure analysis of variance (ANOVA) and Holm Sidak (HS) post-test were used to compare the average, maximum, minimum, and range of biomechanical parameters, considering either the complete walking cycle or its phases as indicated in the Results section. A few variables (for instance, comparison of MNs counts or body weights between lesion groups to intact controls) required the use of the unpaired Student t test, whereas the chi square test (χ2) was used to compare the proportion of walking cycles with regularity index different to 1. Regression analyses were performed with SigmaPlot. All values, unless otherwise stated, are means±standard error of the mean (SEM). All results were considered statistically significant at p<0.05.
Results
General clinical outcome and leg use
Animals were capable of standing on their hind legs and left foreleg as early as 3 DPI, although at that time they still had severe problems for walking because of the general weakness and incapability to stand on the right foreleg. Nevertheless, this condition improved quickly, and it was possible to start the analysis of locomotion at 5 DPI if subcutaneous suture instead of surgical clips was used to close the neck skin. Four animals showed late (20–120 DPI) signs of allodynia in the hind paw contralateral (left) to the lesion that manifested as pain to touch, progressive reluctance to support on that hind limb, and finally autotomy of the hind paw. Those animals were sacrificed and excluded from all analyses. Sham-operated animals were capable of standing on their four legs, walking, eating, and drinking approximately 12 hours after surgery when fully recovered from anesthesia. Five days later, the kinetics and kinematics of their locomotion was completely normal. SCI animals lost approximately 5% of body weight by 10 DPI (one-way repeated measures ANOVA p<0.001, HS post-test p<0.01), recovered the original weight between 20 and 45 DPI, and ended the follow-up period weighting 6% more than the pre-injury values, similar to uninjured and sham-operated animals.
Before performing detailed biomechanical analyses of locomotion, we quantified the general use of each leg and its use regularity. Uninjured animals stepped only once with each leg during the walking cycle using a stereotyped sequence that commenced with the right foreleg (RF) and was quickly followed by the left hind leg (LH)—diagonal pair RF/LH. The stance phase of the contralateral pair of legs (left foreleg [LF]/right hind leg [RH]) started near the end of the stance of the former, existing little overlapping between both pairs. The locomotor assessment started at 5 DPI, when the gait was still very disorganized and variable and showed a reduction of 20% in the number of RF steps compared with the other legs (two-way repeated measures ANOVA p<0.001, HS post-test p<0.05). This abnormality rapidly disappeared, and all animals stepped with the four legs by 10 DPI, but the right fist was frequently closed, the RF collapsed during the stance phase and sometimes stepped twice per cycle while other times it did not step. At 20 DPI, however, the locomotion was very effective and fast.
Because the average number of steps was similar for the four legs since 10 DPI, we calculated a regularity index for the use of diagonal pairs by adding the steps of the pair RF/LH and dividing by the steps of the pair LF/RH. Although the mean values of this index were similar before and after injury, postlesion data always deviated from 1 (χ2, p<0.001) showing a small but systematic variability that did not exist in normal conditions and that reflected the need of corrections in the step sequence. The number of walking cycles with diagonal regularity ≠1 oscillated from 20–35% along the follow-up. Calculating the index as the ratio between the steps of the forelegs divided by those of the hind legs (fore/hind regularity) yielded similar results.
GRFs of locomotion
The typical vertical force patterns for each leg before and subsequent to C6 hemisection are exemplified in Fig. 1A. The normal peak vertical forces were about 20% higher in the hind legs compared with the forelegs (one way ANOVA p<0.05, HS post-test p<0.05), because the rat center of mass is located slightly caudal to the geometric middle of the body.8 In addition, the peak force timing occurred later in the stance of the forelegs (Fig. 1A), thus resembling the skewed force curves that allow optimal balance to quadrupeds walking at slow velocities.50
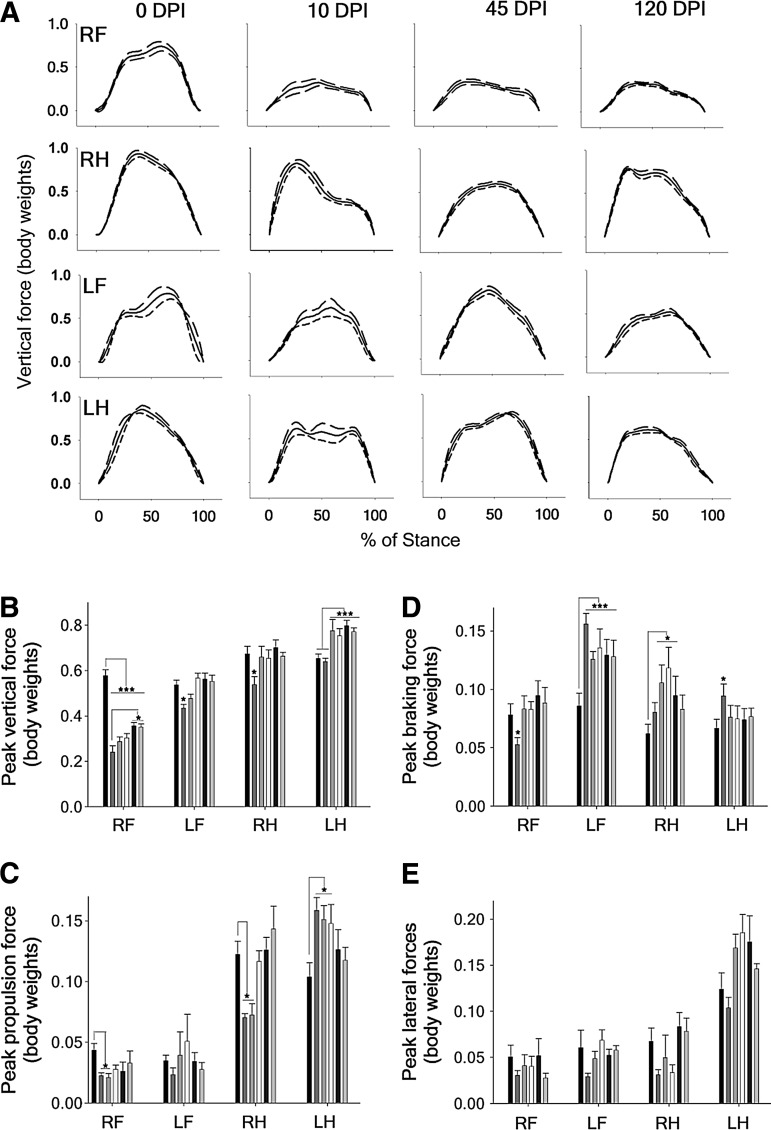
FIG. 1.
Ground reaction forces (GRFs) of locomotion. (A) Representative example of the vertical GRFs recorded after C6 hemisection. The data show the mean (solid lines) of six steps±standard error of the mean (SEM) (dash lines) of the same animal, normalized to the stance duration. (B) Mean peak vertical forces for the complete group of animals. (C, D, E) Mean peak propulsion, braking, and lateral forces for the complete group of animals. The data (mean±SEM) are presented separately for each leg: RF, right forelimb; RH, right hind limb; LF, left forelimb; LH, left hind limb; and the columns show the pre-injury measurements and those at 5, 10, 20, 45, and 120 DPI in order from left to right, respectively. *p<0.05. *** p<0.001.
Spinal cord hemisection completely disrupted the vertical force patterns. All animals showed a permanent and selective loss of more than 50% of support force in the RF, while the other three legs adapted the timing and magnitude of applied forces to compensate for the RF impairment. The compensations constantly changed during the follow-up period, in contrast to the fixed force pattern developed by the RF (Fig. 1A). This dynamic nature of adaptations was a general phenomenon detected in many kinetic and kinematic parameters of locomotion after SCI. The analysis of the averaged peak vertical forces for the whole group of hemisected animals confirmed the dramatic loss of support force that occurred in the RF immediately after the hemisection (one-way ANOVA p<0.001, HS post-test p<0.05), with only a transient reduction of 19% in the support forces of the contralateral foreleg (LF) and the ipsilateral hind leg (RH) at 5 DPI (one-way ANOVA p<0.01, HS post-test p<0.05) that disappeared afterwards (Fig. 1B).
The peak force of the RF showed an uptrend along the follow-up, reaching at 120 DPI only 70% of its pre-injury value but showing a statistically significant improvement in comparison with 5 DPI (one-way ANOVA p<0.05, HS post-test p<0.05). LH peak support forces were unaffected at 5 and 10 DPI and surpassed by 35% the normal value thereafter (one-way ANOVA p<0.001, HS post-test p<0.05). Intact animals showed peak breaking forces similar for all legs but peak propulsive and lateral stabilization forces of the hind legs that doubled those of the forelegs (one-way ANOVA p<0.001, HS post-test p<0.05). After C6 hemisection, the peak propulsion forces generated by the RF and RH decreased significantly at 5 and 10 DPI, while those of the LH increased at 5, 10, and 20 DPI (one way ANOVA p<0.05, HS post-test p<0.05) (Fig. 1C).
The braking forces transiently decreased at 5 DPI for the RF (one-way ANOVA p<0.05, HS post-test p<0.05), increased permanently more than 30% for the LF (one-way ANOVA p<0.001, HS post-test p<0.05), and increased around 20% between 10 to 20 DPI for the RH (one-way ANOVA p<0.05, HS post-test p<0 .05), returning to pre-injury values in the chronic stage (Fig. 1D).
Finally, lateral forces were adjusted continuously (Fig. 1E). Numerical integration of force with respect to time (i.e., calculation of the impulse, I) was necessary to determine the total contribution of a particular leg to accelerate the body. Remarkably, the vertical impulse evidenced overfunctioning at 5 DPI of all the legs except the RF (Fig. 2A). The vertical impulse produced by the RH at 5 DPI doubled the normal value (one-way ANOVA p<0.001, HS post-test p<0.05), strongly suggesting that the lesion interrupted the main axonal tracts that allow recruiting the RF elbow extensors while let intact some neural connections critical for driving the RH extensors.
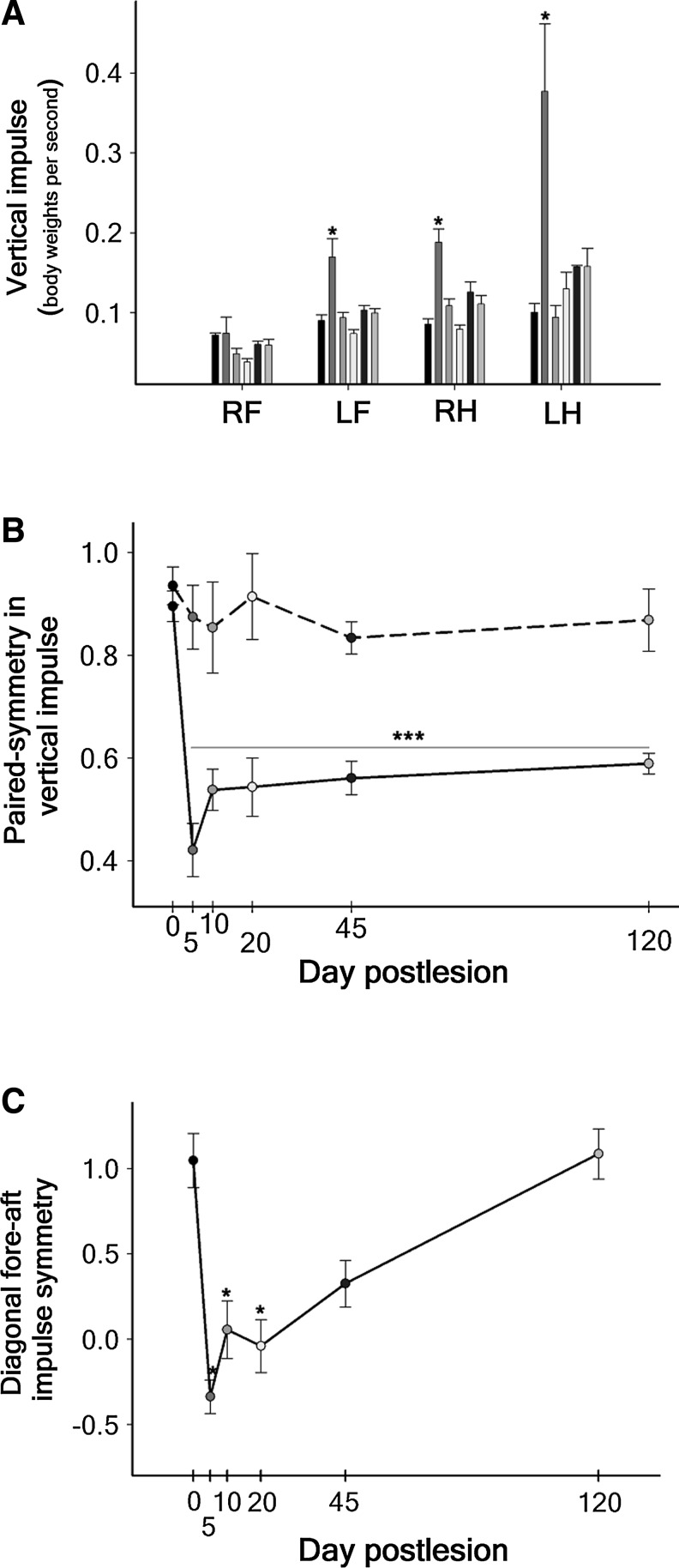
FIG. 2.
Averaged impulses calculated from the ground reaction forces during locomotion for the complete group of animals. (A) Vertical impulse. The data (mean±standard error of the mean) are presented separately for each leg, and the columns show the pre-injury measurements and those at 5, 10, 20, 45, and 120 DPI in order from left to right, respectively. (B) Paired-symmetry in vertical impulse calculated for the forelegs/hind legs (solid line) or for the diagonal leg pairs (dash line). (C) Diagonal fore-aft impulse symmetry. The pre-injury measurements are represented by day 0 in B and C. RF, right forelimb; LF, left forelimb; RH, right hind limb; LH, left hind limb. *p<0.05. *** p<0.001.
Nevertheless, the greatest increase in vertical impulse occurred in the LH (Fig. 2A), which normally forms pair with the RF, indicating that the injured animals attempted to normalize both balance and gait style by generating similar vertical impulses with alternating diagonal pairs. This possibility was substantiated by calculating the symmetry of the vertical impulses produced by pairs of legs, which showed that the forelegs/hind legs symmetry ([RF+ILF]/[IRH+ILH]) permanently changed from 0.90 to around 0.55 (one-way ANOVA p<0.001, HS post-test p<0.001), whereas the diagonal pairs symmetry ([IRF+ILH]/[ILF+IRH]) did not significantly deviate from the normal value (0.94; Fig. 2B). Interestingly, the fore-aft impulse symmetry of the diagonal pairs ([IRF+ILH)]/[ILF+IRH]) was dramatically lost after the hemisection but progressively recovered up to the normal value at 120 DPI (Fig. 2C).
Kinematics of locomotion
SCI animals markedly adapted the spatiotemporal structure of the walking cycle to compensate the RF force deficits. They transiently prolonged the stance phase of all legs after the hemisection, albeit in the RF it was only significant at 5 DPI (one-way ANOVA p<0.05, HS post-test p<0.05) while large increments occurred in the other legs over the complete follow-up period (one-way ANOVA p<0.001, HS post-test p<0.05) (Fig. 3A). Prolonged swing phases were detected in the RF at 20 DPI (one-way ANOVA p<0.001, HS post-test p<0.05), whereas this phase significantly shortened in the hind legs (one way ANOVA p<0.001, HS post-test p<0.05) (Fig. 3B).
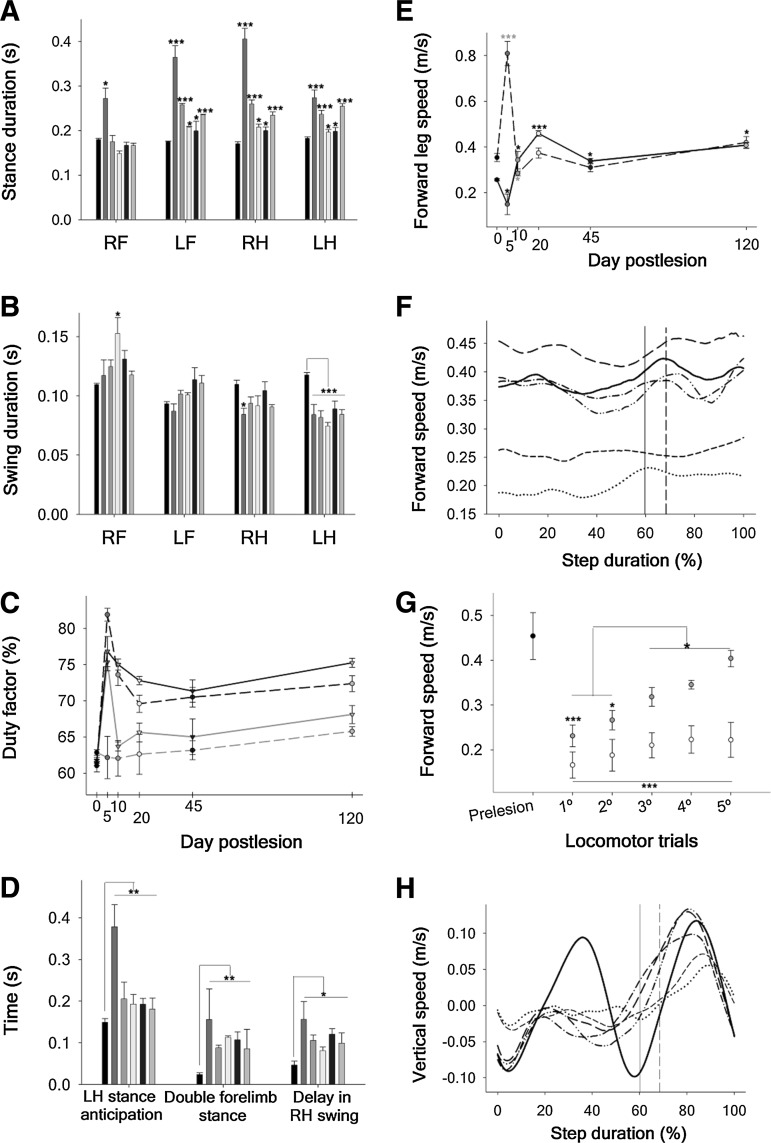
FIG. 3.
Kinematic analysis of the rat walking cycle in normal condition and after C6-hemisection. (A) Mean stance phase duration: RF, right forelimb; LF, left forelimb; RH, right hind limb; LH, left hind limb. (B) Mean swing phase duration. (C) Duty factor of each rat leg: (RF, dash gray line; LF, solid gray line; RH, dash black line; LH, solid black line). (D) LH stance anticipation, and LF and RH overlapping with respect to the RF. (E) Mean relative forward speed of the RF (dash line) and RH (solid line). In A, B and D, the data (mean±standard error of the mean) are presented separately for each leg, and the columns show the pre-injury measurements and those at 5, 10, 20, 45, and 120 DPI in order from left to right, respectively. In C and E, day 0 represents the pre-injury values. (F) Forward eye velocity normalized to the RF step cycle. For simplicity, only the mean curve of the complete group of animals is represented (pre-injury, solid black; 5 DPI, dotted black; 10 DPI, shorted dash black; 20 DPI, long dash black; 45 DPI, dash dotted black; and 120 DPI, dotted-dotted black line). A vertical line signals the transition from stance to swing phase in normal (solid) and C6 hemisected rats (dash). (G) Changes in the mean forward velocity occurring within the first five trials of locomotor assessment at 5 DPI (white circles) and 10 DPI (gray circles). The solid black circle on the left represents the normal values. (H) Mean vertical velocity normalized to the RF step cycle. Symbols are the same as in part F. *p<0.05. **p<0.01. ***p<0.001.
From the durations of the stance and swing phases, we obtained the duty factor (the percentage of the duration of the stride for which the foot is on the ground), which is one of the main parameters that defines the gait style.51 The normal duty factors were around 60–65% (characteristic of walking) and increased up to 75% at 5 DPI (one-way ANOVA p<0.001, HS post-test p<0.001) for all legs except the RF (Fig. 3C). They partially normalized at 20–45 DPI and increased again in the long term, in agreement with the continuous adaptations aforementioned for the GRFs. Those duty factor values imply that the gait style was preserved although with greater overlapping of the legs during stance, a compensation that allows body stabilization after cervical or thoracic SCI at the cost of reducing forward velocity.4,8
Leg stance overlapping after C6 hemisection was specifically aimed at assisting the RF during body weight transfer, as reflected by the fact the LH and the LF significantly anticipated their stance phases with respect to the RF (Fig. 3D) over the complete follow-up period (one way ANOVA p<0.01, HS post-test p<0.05), whereas the RH delayed its lift off (one-way ANOVA p<0.05, HS post-test p<0.05). LF stance anticipation led to large periods of double foreleg support (Fig. 3D) that prevented the complete collapse of the RF and allowed progression of the walking cycle. These compensatory mechanisms concomitantly require developing higher leg velocities during the swing phase.
At 5 DPI, the average speed of the RF tripled the normal value (one-way ANOVA p<0.001, HS post-test p<0.05) and returned to normal values afterward. On the contrary, the mean forward speed of the RH was reduced at 5 DPI but significantly surpassed the preinjury values thereafter (Fig. 3E). Fig. 3F shows animal forward velocity in normal conditions and after SCI. For simplification, each curve represents the average of the whole group at each follow-up day. In agreement with the prolongation of stance durations and duty factors at 5 DPI, the average forward speed decreased by greater amounts at this day (one-way ANOVA p<0.001, HS post-test p<0.05). Most importantly, it diminished 33% at 10 DPI but fully recovered the normal value at 20 DPI (Fig. 3F).
In fact, we observed that at 10 DPI, the animals tried different leg sequences that finally enabled them to increase significantly the speed from the first to the last locomotor trial of the same day, but this “self-training” effect was not obtained at 5 DPI (Fig. 3G). In sharp contrast with the efficiency of kinetic and kinematic compensations to increase forward speed, the peak of vertical speed corresponding to the RF/LH pair was permanently lost because of the paresis of the RF, and the vertical velocity was only modestly compensated by the LF/RH pair (Fig. 3H).
Balance means keeping the body elevated.50,52 To study rat balance during locomotion, we measured the eye, shoulder, and hip height, and compared the real data and also their dimensionless numbers. Normally, eye dimensionless height dropped to ≈0.92 in the first half of the RF stance phase, rising later to a peak of ≈1.02, and dropping again before the forepaw lifts off (Fig. 4A). In the swing phase of the pair RF/LH, the body weight is transferred to the pair LF/RH that repeats the sequence producing the second peak of eye height during a walking cycle. After hemisection, the rise of the eye permanently disappeared during the RF stance as a consequence of the force deficit; conversely, a continued drop occurred to minimum values that varied from 0.94 at 5 DPI to 0.82 at 120 DPI (Fig. 4A), and the lost height was always recovered by the other legs during the swing phase of the RF.
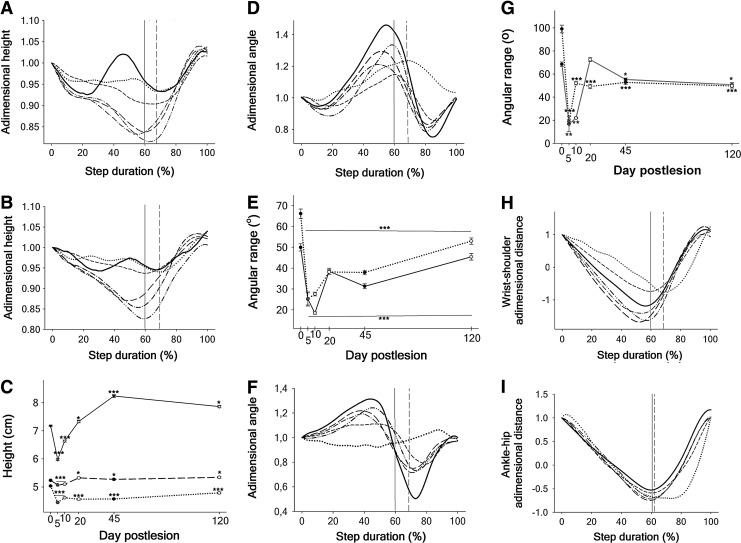
FIG. 4.
Geometrical parameters of the rat walking cycle in the normal condition and after C6 hemisection. (A, B) Adimensional eye and shoulder heights during the complete stride, respectively. For simplicity, only the mean curve of the complete group of animals is represented (pre-injury, solid black; 5 DPI, dotted black; 10 DPI, shorted dash black; 20 DPI, long dash black; 45 DPI, dash dotted black; and 120 DPI, dotted-dotted black line). A vertical line signals the transition from stance to swing phase in normal (solid) and injured rats (dash). (C) Real height of the eye (short dash line), shoulder (medium dash line) and hip (solid line) averaged for the complete walking cycle. The standard error of the mean is usually smaller than the data symbols. (D, F) Adimensional joint angles of the right elbow and wrist, respectively. Increase in the values means joint extension and decrease, flexion. (E, G) Joint angle ranges for the right elbow and wrist, respectively, presented separately for the stance phase (solid lines) and swing phase (dotted lines). (H) Adimensional, relative displacement of the right forepaw in the fore-aft direction during the step cycle. (I) Adimensional, relative displacement of the right hind paw in the fore-aft direction during the step cycle. Symbols are the same as in part A and B in D, F, H and I. In C, E and G, day 0 represents the pre-injury values. *p<0.05. **p<0.01. ***p<0.001.
The curve of RF shoulder dimensionless height (Fig. 4B) showed parallel changes to those observed in the eye, providing direct evidence that the RF paresis accounted for the loss of balance. The hip dimensionless height significantly decreased at 5 and 10 DPI (one-way ANOVA p<0.001, HS post-test p<0.05) but from 20–120 DPI showed increased oscillation dropping to minimal dimensionless values around 0.85 at midstance. In contrast to the paresis of RF, the RH was capable of raising again the hip in the second half of the stance phase, indicating that the increased oscillation was a compensatory mechanism. After 20 DPI the average real values of shoulder and hip height (Fig. 4C) increased with the greater use of the hind limbs for support, leading to greater body oscillations in presence of the paretic RF.
Fig. 4D shows the right elbow dimensionless angles. The RF elbow extension was dramatically reduced at 5 and 10 DPI, but partially recovered thereafter. Increased flexion was likewise observed at 120 DPI in the initial part of the stance phase. For this reason, we considered it more appropriate to analyze separately the elbow joint ranges of the stance and swing phases for statistical comparisons (Fig. 4E), which represent the complete extension and flexion of the joint, respectively.
At 5 DPI, the elbow extensor and flexor ranges dropped to about 50% and 62% of the normal value, respectively (two-way ANOVA p<0.001, HS post-test p<0.05), but while at 10 DPI, the extensor range dropped 14% more, the flexor range began to rise gradually. The reduction in the latter was for the most part secondary to the loss of extension (Fig. 4D) and, hence, it must not be interpreted as severe paresis of the RF elbow flexors. The range of elbow extension recovered up to 84% of the normal value at 120 DPI, although the difference to the control animals was still statistically significant (one-way ANOVA p<0.001, HS post-test p<0.05).
The normal RF wrist dimensionless angle is shown in Fig. 4F. At 5 DPI, the wrist was essentially paralyzed, showing a flat joint angle curve both in the stance and the swing phase. The wrist extensor range, however, completely recovered from 20 DPI onward whereas the flexor range was permanently reduced by 50% (two-way ANOVA p<0.001, HS post-test p<0.05) (Fig. 4G). This chronic loss of wrist flexion was a primary deficit because the extension was not reduced at the beginning of the swing phase. In contrast to the elbow, the hind leg joints did not loss extensor, anti-gravity function after C6 hemisection. In fact, the RH knee transiently increased its extension during the stance phase at 5 and 10 DPI (one-way ANOVA p<0.001, HS post-test p<0.05), with normalization at 20 DPI. Greater ranges of hip flexion and extension were detected thereafter, in agreement with the oscillations aforementioned for hip height.
Measurement of the rostral (protraction) and caudal (retraction) displacements of the wrist and ankle relative to the proximal joints (glenohumeral and iliofemoral joints, respectively) provides information regarding the configuration of the legs during locomotion, the torques acting on the legs, and the contribution of the proximal joints to step length in intact animals53 and after SCI.8,27 As shown in Figs. 4H and 4I, the dimensionless retraction is normally higher for the RF than for the RH, as explained by the important extension of the elbow and barely any of the knee in the second half of the stance phase. Also in agreement with the joint angles, significant less retraction (one-way ANOVA p<0.01, HS post-test p<0.05) was observed in the RF but not in the RH at 5 DPI. The retraction of both legs tended to increase from 20 DPI onward without statistical significance.
Integrative remarks: Relationship between RF support force and body balance
The data presented here so far indicate that the permanent loss of elbow extensor force drives the compensations for locomotion after C6 spinal cord hemisection. The most prominent kinematic consequence of that force deficit is the failure of the RF to support and elevate the body during the stride. The RF collapses under the body load and demands the support with other legs, the animal modifying the timing of the stance phases and force patterns and finally disrupting the progression of the walking cycle (Fig. 5). However, it seemed paradoxical that the RF peak support force showed a small but significant increment from the acute to the chronic postlesion phase (Fig. 1B) while body balance declined, as indicated by the progressively larger fall of eye and shoulder in the chronic phase (Fig. 4A,B). Two possibilities that are not mutually exclusive may explain this phenomenon—namely, that the animals attempted to normalize their forward speed while tolerating additional loss of body balance as unavoidable consequence, or that the increased vertical oscillation of the body could likewise compensate to some extent the functional loss.
FIG. 5.
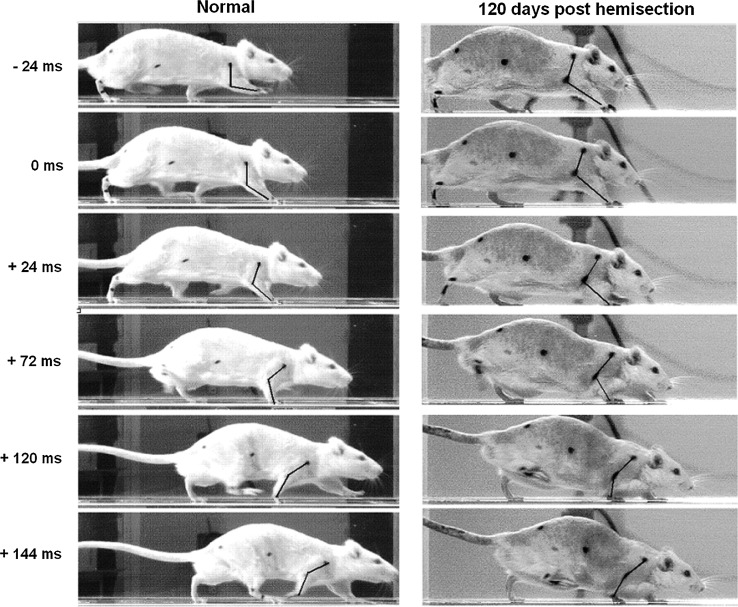
Video frames illustrate the manner in which a representative animal with right C6 spinal cord hemisection moved before the lesion and at 120 days post-lesion. Time=0 corresponds to the moment at which the right forepaw touches the ground. Note the collapse of the RF under the body weight and the assistance of the LF for supporting and raising the body.
A typical feature of locomotion was that the eye and shoulder dropped less than 9% and were essentially uncoupled during the RF stance in normal animals, whereas they coupled and dropped with an almost perfect linear interdependence during the stance phase after the lesion (Fig. 6A). This pathological, linear relationship had slope close to 1 and was maintained over the complete follow-up period (Table 1). The increased oscillation of the body together with greater in phase movement of the right and left legs did not simply represent a compensatory change of the locomotor style to galloping, because the duty factor (Fig. 3C) and its inverse dependence on speed (Fig. 6B) were characteristic of walking.51
FIG. 6.
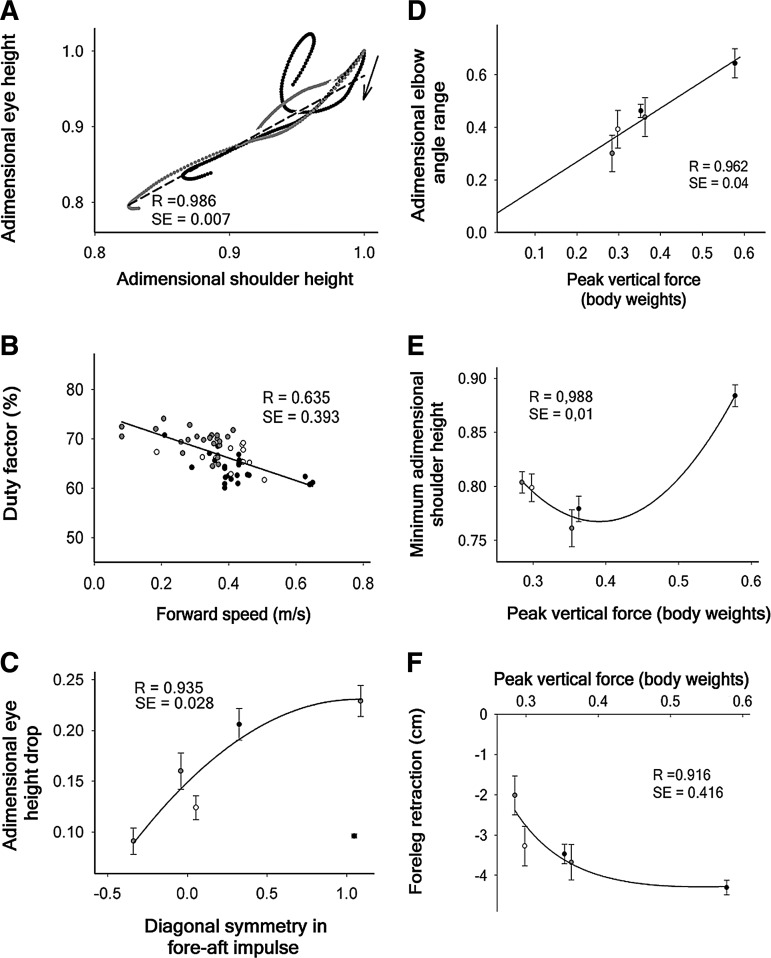
Correlations between kinematic and kinetic parameters of locomotion. (A) Dependence of the eye height on the shoulder height along the step cycle. The data represent mean values of 12 rats in the normal condition (black circles) and at different times after C6 hemisection. For simplification, a single regression line (dash) is shown for the average of all the post-lesion data, and the parameters of the regression lines corresponding to each time are detailed in Table 1. Animal movement starts at top-right as signaled by the arrow. (B) Relationship between animal's forward speed and duty factor averaged for the four legs. (C) Relationship between the diagonal symmetry in the fore-aft impulse and the eye height drop (n=12). The data show the mean±standard error of the mean (SE) at pre-injury (black circle at bottom-right) and at 5, 10, 20, 45, and 120 DPI (in order from left to right, respectively). The regression adjusted to a polynomial quadratic function. (D) Relationship between the adimensional RF elbow angle range and the mean peak vertical force. The slope of the line was 1.731. (E) Evolution of the right shoulder height drop as a function of the mean peak vertical RF force. (F) Evolution of the RF protraction as a function of the mean peak vertical RF force. In A and B, the black symbols correspond to the pre-injury values, and the grey ones shows the post-injury values. Data in D, E and F show the mean±SE of 10, 20, 45, and 120 DPI and the pre-injury values in order from left to right, respectively.
Table 1.
Parameters of the Regression Lines Obtained from the Correlation of Shoulder and Eye Adimensional Heights at Different Times Postlesion
| Day postlesion | Coefficient | SE | t | p |
|---|---|---|---|---|
| Pre-injury | R=0.331 | 0.039 | ||
| y0 = | 0.024 | 0.117 | 0.202 | 0.841 |
| a = | 0.980 | 0.121 | 8.089 | <0.001 |
| 5 DPI | R=0.921 | 0.006 | ||
| y0 = | 0.123 | 0.117 | 3.019 | 0.0351 |
| a = | 0.864 | 0.121 | 20.427 | <0.0001 |
| 10 DPI | R=0.991 | 0.005 | ||
| y0 = | −0.091 | 0.014 | −6.318 | <0.0001 |
| a = | 1.085 | 0.015 | 71.695 | <0.0001 |
| 20 DPI | R=0.946 | 0.004 | ||
| y0 = | −0.259 | 0.01 | −25.104 | <0.0001 |
| a = | 1.250 | 0.011 | 111.533 | <0.0001 |
| 45 DPI | R=0.972 | 0.012 | ||
| y0 = | −0.134 | 0.024 | −5.508 | <0.0001 |
| a = | 1.1 | 0.027 | 41.329 | <0.0001 |
| 120 DPI | R=0.969 | 0.017 | ||
| y0 = | −0.107 | 0.025 | −4.386 | <0.0035 |
| a = | 1.060 | 0.027 | 38.854 | <0.0001 |
The data correspond to the average values of 12 animals that had unilateral and complete right C6 hemisection. The p is the p value calculated for t; p<0.05 indicates a great probability that the coefficient is not zero. SE, standard error; DPI. Days post-injury.
One may ask why the body continues falling after the LF lands on the ground and provides double foreleg support with the RF (Fig. 5). The reason is that the LF is protracted at the beginning of the stance and, hence, if it produced the strong vertical force necessary to stop the body fall, it would simultaneously generate a large fore-aft breaking force, consequently impeding locomotion and altering the symmetry of the fore-aft impulse between diagonal pairs. In other words, partial loss of balance may be a tolerable secondary impairment that permits the progression of locomotion with symmetry in the fore-aft impulse of diagonal pairs, an idea supported by the correlation existing between both parameters (Fig. 6C).
In addition, our results suggest the possibility that the drop of the body on the RF, albeit representing a direct consequence of the paresis of the TB, paradoxically could contribute to increase its peak force by triggering the stretch reflex and facilitating the development of eccentric force. The postlesion increment in RF elbow flexion at the beginning of the stance phase (Fig. 4D), together with the directly proportional linear correlation existing between the peak vertical force and the elbow angle range (Fig. 6D) and the likely quadratic function relating RF peak vertical force and shoulder height (Fig. 6E) provide compelling evidence to support this interpretation.
Although the parabola represented in Fig. 6E is speculative, given that only one normal value appears on the right side of the vertex, the kinetics and kinematics of locomotion (Figs. 1–6) clearly indicate that in normal conditions, the TB makes positive work that raises the body, and this is no longer possible after SCI. Finally, it is relevant to mention that after the hemisection, the RF is retracted to almost normal values with small increments in the peak vertical force (Fig. 6F), suggesting that the RF mostly rotates about the pitch axis of the body providing little body support.
Lesion extent and TB MN death after C6 hemisection
The maximal longitudinal extent of the lesion was 1.11±0.25 mm on average. In normal animals, the white and gray matter occupied 67.6±1.79% and 32.4±0.04% of the total transverse area, respectively, without significant differences between right and left sides. This symmetry enabled us to obtain an approximate reconstruction of the injured side to know the proportion of spinal cord tissue damaged by the hemisection. Although the animals with partial preservation of white matter in the injured side might provide some keys to understand the involvement of different axonal tracts in the functional impairments, the size and location of the spared region in those animals was variable, thus precluding the gathering of consistent information. Consequently, 7 of 24 animals were excluded from functional and anatomical analyses, because either more than 5% of the targeted spinal cord side remained intact or more than 5% of the contralateral side was injured, and the data presented here correspond to 17 animals that had a complete or essentially complete unilateral hemisection (Fig. 7A).
FIG. 7.
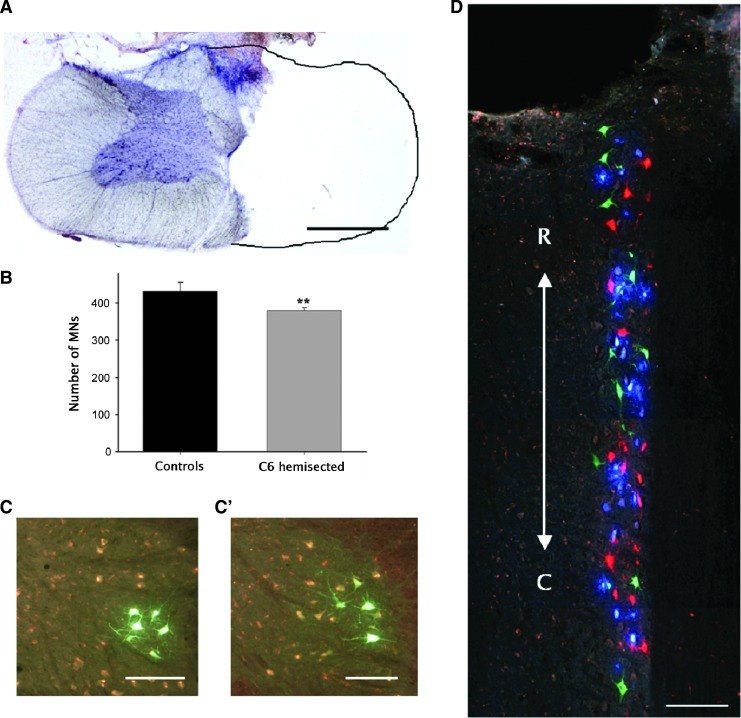
Histological analysis of the spinal cord lesion and triceps brachii (TB) motoneurons (MNs). (A) Microphotograph of 60-μ transverse section taken from the site of maximum damage at the caudal part of C6 at 120 days post-injury (DPI) and stained with hematoxylin and eosin. (B) Average number of TB MNs found in normal animals (black column) and C6-hemisected animals after 120 DPI (gray column). (C, C’) Transverse spinal cord sections of the right side of C7 showing TB MNs retrogradely labeled by intramuscular injection of aminostilbamidine methanesulfonate exemplify the appearance of normal and after 120 DPI, respectively. (D) TB MNs as observed in a horizontal spinal cord section of the C7 segment when the TB subnuclei were independently labeled with dextran tetramethylrhodamine (red, long fascicle), dextran alexa 488 (green, lateral fascicle), and fast blue (blue, medial fascicle) applied separately to the cut nerve stumps. R, rostral; C, caudal. **p<0.01.***p<0.001. Scale bar in A, 1 mm; in C, C’, and D, 200 μm.
No statistically significant differences in MN counts were found between global muscle injection of aminostilbamidine and the triple labeling paradigm by nerve axotomy (Student unpaired t test p=0.443) and, hence, data from both tracing methods were pooled. Retrograde neural tracing showed a small (14%) but significant reduction in the number of TB MNs ipsilateral to the lesion site compared with the uninjured side or to normal controls (Student unpaired t test p<0.01) (Fig 7B). Segmental comparisons established that neuronal loss occurred very close to the lesion, rostral to the middle part of C7 (Student unpaired t test p<0.01) instead than in the caudal part of the TB MN column. An additional group of animals was used to clarify the cause of neuronal death and to evaluate if the method of tracing likewise influenced the results.
Here, the hemisection was performed 1 mm more rostral to avoid a direct damage of the bulk of TB MNs, and the MNs were labeled using the triple tracing paradigm. As a result, a non-statistically significant reduction (Student unpaired t test p=0.37) of 9% was found in the number of ipsilateral TB MNs compared with the contralateral side and to uninjured controls. Importantly, differences were not found between injured and normal animals for MN counts in any of the three subnuclei, ruling out the possibility that C6-hemisection caused selective loss of large motor units in the TB.
It is worth mentioning that TB MN atrophy was not evident in chronic stages posthemisection (Figs. 7C, C’) and that the three MN subnuclei preserved the topographical distribution described for normal animals.46 Furthermore, double-labeled neurons were not found (Fig. 7D), indicating that each fascicle maintained its independent innervation.
In summary, hemisection at the distal part of C6 produced only a small loss of TB MNs of approximately 14%, which should not be sufficient to cause persistent loss of elbow extension force, an idea that was supported by the absence of correlation between MN counts and vertical GRFs of the ipsilateral forelimb (R=0.096, SE=0.045). Thus, the chronic deficits detected were mainly a consequence of the interruption of axons at the white matter of C6.
Neurological basis of the chronic forelimb extensor force deficit
With the aim of providing further insights into the mechanisms responsible for the permanent deficits, we performed synaptophysin immunohistochemistry to investigate if C6 hemisection causes chronic denervation of C7/C8. Because experiments in cats and monkeys have shown that spinal cord lesions at C6 interrupt not only axons descending from the brain but also from the cervical propriospinal premotoneuronal system (CPPS),44 we also performed neural tracing studies to confirm the existence of a CPPS projecting to C7 in the rat, as the first step to investigate its participation in the production of the motor impairments.
Assessment of synapse loss by synaptophysin immunoreactivity
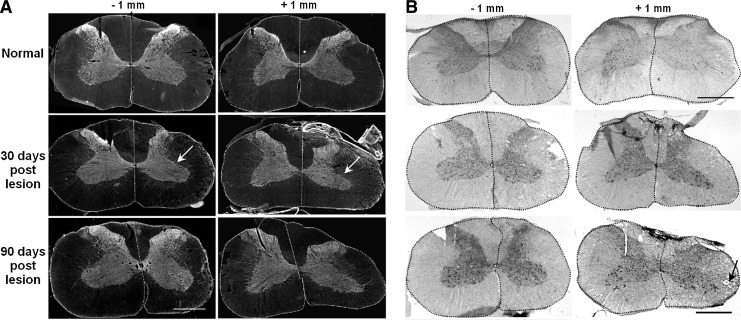
No significant differences were found in the global, mean fluorescence intensity of the injured side at 30 or 90 DPI compared with the contralateral side or normal controls. At 30 DPI, however, the area immunoreactive for synaptophysin was significantly reduced in the injured side at −1 mm rostral and particularly at +1 and +2 mm caudal to the lesion compared either with the contralateral side or with normal controls (two-way ANOVA p<0.001, HS post-test p<0.05). Subtle reductions that did not reach statistical significance were also noted at coordinates −2, +3, and +4 mm, while completely normal immunoreactivity was found rostral to −2 and caudal to +4 mm. At 90 DPI, the immunoreactive area was normal at the coordinate +2 but was still reduced at −1 and +1 (Fig. 8A; HS post-test p<0.05).
FIG. 8.
(A) Synaptophysin immunoreactivity in the spinal cord, rostral (-1 mm) or caudal (+1 mm) to the C6 hemisection site in normal conditions or at 30 and 90 days post-injury. The side ipsilateral to the lesion (right) is always on the right side of the microphotographs. The white arrow signals the reduced area of synaptophysin staining with atrophy of the gray matter. Each side of the spinal cord has been delineated for a better identification. (B) Spinal cord sections adjacent to those shown in A, stained with hematoxylin and eosin. The black arrow signals vacuoles in the white matter as a result of axonal wallerian degeneration. The scale bars represent 1 mm.
From these results, we judged that the integrated fluorescence density is the most appropriate parameter to express the loss of immunoreactivity. This parameter remained diminished by 35% at the coordinate +1 at 90 DPI, indicating that the synaptic pool of the segment C7 was chronically reduced after the hemisection. Tissue sections adjacent to those used for immunohistochemistry were stained with H&E (Fig. 8B) for a general confirmation of the aforementioned findings. At 30 DPI, the ipsilateral gray matter immediately rostral or caudal to the lesion site occupied a visibly smaller area but contained neurons of normal appearance. The white matter showed signs of wallerian degeneration both rostral and caudal to the lesion as a result of the interruption of ascending and descending axonal tracts, respectively.
Cervical propriospinal neurons that project to C7
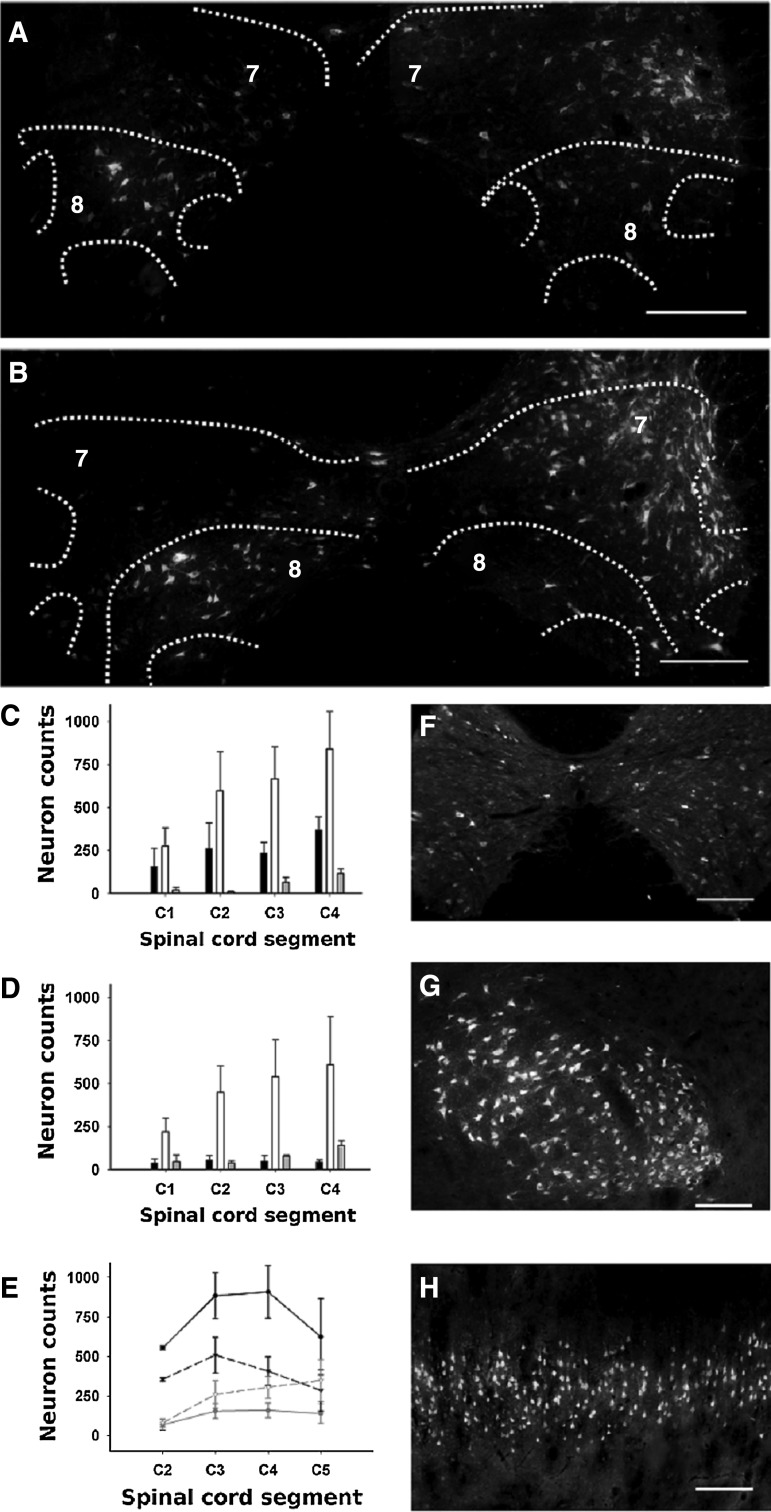
Concurrent damage of brain-descending axons and CPPS axons may explain the severe and chronic functional deficits caused by C6 spinal cord hemisection; however, anatomical data regarding the CPPS in the rat are presently unavailable in the literature. Therefore, we present information regarding the localization and number of cervical PNs that project to C7 in normal adult rats. At 24 h after the injection of aminostilbamidine in C7, the tracer had labeled neurons in all cervical segments (Fig. 9 A, B), the thoracic (Fig. 9F) and lumbar spinal cord, and the brainstem, particularly in the raphe, reticular nuclei, vestibular nuclei, and left red nucleus. At 72 h the intensity of labeling increased in the brainstem (Fig. 9G), and likewise appeared in some hypothalamic nuclei and the cerebral cortex (Fig. 9H), but some diffusion occurred to the contralateral spinal cord.
FIG. 9.
Cervical propriospinal neurons retrogradely labeled after aminostilbamidine injection at the right side of C7 in normal rats. The microphotographs and cell counts presented in A to F were obtained from animals processed at 24 h post injection, while those presented in G and H were obtained at 72 h post-injection. (A) Neurons at C3. (B) Neurons at C4. The right side of the spinal cord is on the right side of the microphotographs. (C, D) Quantification of labeled cells in the different regions of the ipsilateral and contralateral spinal cord segments, respectively. The black, white, and grey columns show the counts of the dorsal (laminae I–V), intermediate (laminae VI–VII), and ventral (laminae VIII–IX) regions, respectively. (E) Segmental distribution of neurons from C2 to C5 in the ipsilateral (solid lines) and contralateral (dash lines) sides of the spinal cord. Only the cell counts of laminae VII (black lines) and VIII (gray lines) are presented. The spinal cord laminae were delineated using the drawings of Paxinos and Watson.49 Statistical comparisons are presented in Results. (F, G, H) Examples of neuronal labeling in the thoracic spinal cord, the contralateral red nucleus, and the contralateral cerebral cortex, respectively. Scale bars in A and B, 500 μm; and in F, G, and H, 200 μm.
We concluded that 24 h was appropriate for labeling PNs and processed other two groups of animals at this survival time for cell counting. In the first group, we counted approximately 7500 neuronal profiles in alternate spinal cord sections from C1 to C4, with 56±8% of them located ipsilateral to the injection and 42±8% contralateral, and 2±0.4% located in lamina X. The number of neurons labeled in each side of the spinal cord, distributed per segments and regions of the gray matter is shown in Fig. 9C. The three-way ANOVA detected statistical differences (p<0.001) regarding those factors, the most relevant being the paucity of neurons in C1 (HS post-test p<0.05), the greatest number of neurons in the intermediate region (laminae VI and VII) in all segments (HS post-test p<0.05), and the existence of fourfold more neurons in the ipsilateral dorsal gray matter than in the same contralateral region (HS post-test p<0.05). In the second group, we included C5 in the counts and focused in the neurons located in laminae VII and VIII, which contain most of the last order spinal neurons controlling the forelimbs.54–56
This experiment confirmed the existence in the rat of a large pool of cervical PNs with a distribution that matches that described for the CPPS in cats and that project to C7. More neurons were globally labeled in the ipsilateral than in the contralateral side (3490±549 versus 2545±458; Student paired t test p<0.05). Ipsilateral neurons were always more numerous in laminae VII (two-way ANOVA p<0.001, HS post-test p<0.01) and were located laterally, whereas those of the contralateral lamina VII were located medially. Importantly, the number of neurons in the ipsilateral lamina VII doubled that of the contralateral side (HS post-test p<0.05), whereas neuronal counts in the contralateral lamina VIII doubled those of the ipsilateral side (Fig. 9A, B; HS post-test p<0.05).
Discussion
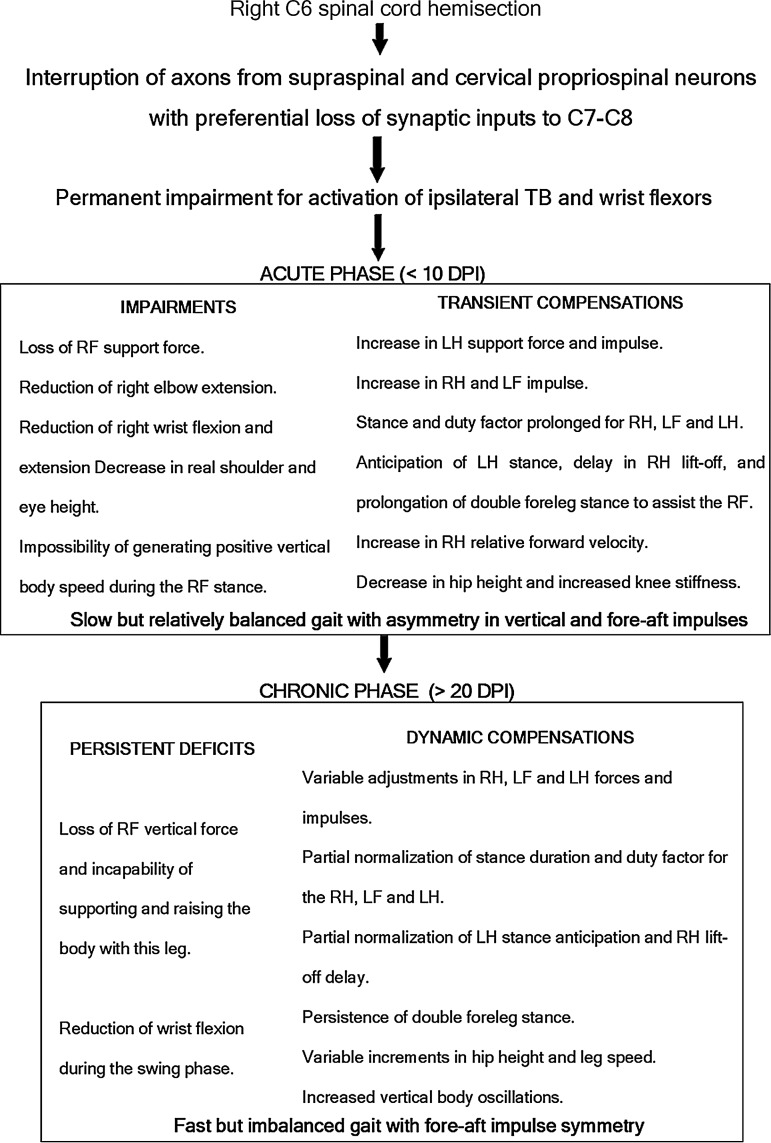
The present work combined GRFs and kinematic analysis of rat locomotion to achieve a comprehensive quantitative description of motor deficits and compensations from the acute to the late chronic phase after C6 spinal cord hemisection. Permanent impairments were detected in the motor output of the spinal cord segments located immediately caudal to the lesion site, associated with chronic denervation of these segments. This led to focal foreleg dysfunctions and drove the adaptation of whole-body movements to produce effective locomotion, including overuse of the contralateral legs and the ipsilateral hind leg since the very early days post-lesion. On this basis, we hypothesized that C6-hemisection interrupted both supraspinal and propriospinal axons that innervate the segments C7–C8, thus producing severe focal motor deficits and limiting the possibility of spontaneous recovery. On the contrary, the distant lumbar segments likely preserved sufficient propriospinal connectivity (from neurons located at C7–C8 and thoracic segments) allowing efficient overground locomotion and motor compensations. Additional neuroanatomical studies were undertaken to substantiate this hypothesis, finding a large pool of neurons that normally projects from C2–C5 to C7 with distribution broadly comparable to the CPPS of cats and primates. In the light of the information provided by our functional and anatomical data, it is possible to categorize the motor changes as primary deficits or compensations and likewise define the phases of SCI according to the functional outcome after the hemisection as shown in Fig. 10.
FIG. 10.
Synopsis and categorization of the main primary deficits and compensations detected in rat overground locomotion after right C6 spinal cord hemisection. Arrows signal a presumptive pathophysiological sequence. TB, triceps brachii; RF, right forelimb; LH, left hind limb; RH, right hind limb; LF, left forelimb.
Permanent, segmental deficits after SCI
Although the concept of focal damage and resulting focal deficit is commonly used when referring to diseases affecting the brain,57 it has been barely applied to SCI. The topographical organization of spinal MNs and segmental interneurons, however, makes it reasonable to extend that concept to SCI. In this case, there are two distinct neuropathological entities that cause focal deficits—viz. focal neuronal death and focal neuronal denervation. The case of focal MN death is more obvious, because it leads to paresis or paralysis of selective muscles. For instance, humans with SCI at the cervical enlargement show MN loss that contributes to paresis of the intrinsic hand muscles,2 or elbow extensors.3 Similarly, moderate contusions at the cervical or lumbar enlargements of the rat spinal cord partially damage specific MN nuclei thus contributing to the production of chronic, segmental motor impairments.4
In this report, we present experimental evidence for the second and less obvious phenomenon; namely, that the preferential, focal denervation of the cervical spinal cord segments immediately caudal to the lesion site causes permanent paresis of their myotomes, as it occurs in the human TB after cervical SCI.3,10,58 We chose C6 hemisection as an optimal model for testing our hypothesis, because this lesion spares the supraspinal and cervical propriospinal innervation of the spinal MN nuclei controlling elbow flexors and shoulder flexors and extensors,37 thus allowing sufficient foreleg protraction during the stride swing phase and providing proximal mechanical stability for body support and foreleg retraction during the stance phase. Consequently, the rat actively used the foreleg and manifested deficits related to inactivation of elbow extensors and wrist flexors. Importantly, the loss of function was surely underestimated, because walking does not demand the maximum forelimb motor output that rats can generate.
In quadruped animal locomotion, the body weight is almost equally distributed over the forelimbs and hind limbs,59 demanding relatively strong force development to the TB. Each of the TB fascicles is an independent neuromuscular compartment with different number, size, and type of motor units and muscle fibers.46 The segment C7 contains approximately 60% of TB MNs, and C7 together with C8 account for 90% of the total MN pool. The three MN subnuclei intermingle in C7, which contains approximately 90% of the MNs that innervate the lateral and medial fascicles and about 56% of the MNs of the long head. Moreover, the lateral and long fascicles have the largest muscle fibers and motor units (179 and 99 fibers/MN in average, respectively), and electromyographic recordings during locomotion indicated that the long head is activated shortly before ground contact of the forepaw while the lateral head is more active in the stance phase in correlation with elbow torques.60–62 Consequently, denervation of C7 was the most likely responsible for the profound impairment of TB function and body weight support.
Our neural tracing studies showed only a subtle loss of TB MNs after C6 hemisection that was unlikely to account for the focal force deficits and that might be compensated by peripheral motor nerve sprouting.63 Moreover, neuronal death was not significant in the lateral fascicle, ruling out the possibility that preferential death of large MNs caused disproportionate loss of elbow extensor force.
Dynamic, whole-body compensations that optimize the residual motor function
In agreement with previous studies of quadrupeds with partial SCI,4,5,8,16,36 whole-body compensations developed in rats with C6 hemisection that contributed to maintaining balance and/or to improve the global locomotor performance (Fig. 10). Our results show remarkable changes in the compensations during the follow-up period that should be taken into account when studying spontaneous and treatment-induced functional recovery. The reason for the continuous locomotor adjustments remains unknown, although it is possible that the animals attempted to restore the symmetry of movement and to reduce energy expenditure. In addition, the small but progressive increase in body weight likely played a role by generating higher torques on the legs.
In our analyses, we considered balance as the capacity of keeping the body elevated50 during the RF step, so that the less the body descended (relative to its initial height) during the RF stance, the better the balance was. The animals were weak during the first DPI, as expected because of the spinal shock and transient biochemical changes at the lesion site. But despite the weakness, animals walked with better balance in this acute phase simply because they used in greater extent the three less impaired legs to assist the RF for body support. By anticipating the stance or delaying the lift-off of those legs, they finally increased the stance phase duration and consequently reduced forward velocity. Furthermore, the absolute body height decreased, thus showing less oscillation when stepping on the RF.
In contrast, in the chronic phase, although the animals still needed the less affected legs to assist the RF for body support, they seemed more confident on their residual capabilities and tried to achieve faster locomotion. As a result, they partially normalized the stance phase durations and reduced leg stance overlapping, a mechanism allowing gaining speed at the cost of worsening body balance. At this stage, animals also showed hip and shoulder heights above the normal, a factor itself that contributed to increased body oscillations.
Whereas fall of the body on the RF was an undesired consequence of the primary RF extensor force deficit, it is important to remark that rats likely took some advantage of it and attempted achieving new optima for locomotion in the pathological state. In normal walking at intermediate velocities, the alternate transfer between gravitational-potential energy and kinetic energy within each stride reduces the need of energy supply by muscles for up 70%.64 Trotting Long-Evans rats did not save energy by this mechanism in the normal condition, although they did it during parts of the stride after spinal cord hemisection at C3.16
The extent to which energy transfer operates at walking velocities in our Wistar rats is uncertain, because we did not study the general movement of the center of mass. In the present and previous work,8 however, (Collazos-Castro et al., 2006) the eye's velocity apparently represented that of the center of mass, oscillating twice per cycle and mostly out of phase in the vertical and horizontal directions. Thus, animals seemed to save energy during normal walking and, if some energy transfer still occurred during body oscillations after the hemisection, it simply ameliorated the increased expenditure of energy in pathological locomotion.
We as well think that the vertical oscillation of the body, which increased from the acute to the chronic period after C6 hemisection, may account for the small but progressive increment in the vertical GRF of the RF by triggering the stretch reflex of the TB at the beginning of the RF stance. If occurring, this would represent a mechanism of peripheral neuromuscular compensation instead of true neurological recovery. This hypothetical effect could be implemented via the lateral TB fascicle, which is specialized in phasic, powerful movements given its content of large muscle fibers and MUs,46 and has a biphasic pattern of active stretch followed by active shortening during stance.65
Such a mechanism cannot compensate force generation when positive work is needed to raise the body in the second third of the stance, a functional component that is permanently lost after the C6 hemisection because it probably requires activation of the long and lateral TB spinal circuits by supraspinal commands. Clearly, there is a need of combined kinetic, kinematical, and electrophysiological studies to approach the complexity of quadrupedal locomotion in normal conditions and after SCI.
Axonal tracts involved in the production of permanent motor deficits
Although the present work was not designed to determine the role of specific axonal tracts on the capability of the rat foreleg to sustain locomotion, our results together with other works provide clues into the mechanisms responsible for the locomotor impairments after C6 hemisection. The finding of such severe and permanent impairments was unexpected, because the lesion was unilateral and also preserved the ipsilateral long propriospinal axons that, originating from C7 to T1, are responsible to coordinate quadrupedal rat locomotion,66,67 and because several studies have established that plasticity of spinal circuits enables substantial functional recovery after SCI.68–71
The long propriospinal axons ascending from lumbar regions to innervate the segments C7 to T1 and presumably participating in quadrupedal locomotion72–75 were also spared from lesion, ruling out that damage of the intrinsic spinal networks for interlimb coordination was responsible for the permanent functional loss. Still, a possible explanation for the permanent deficits is that the lesion interrupted both—the major descending axonal tracts that allow recruitment of C7/C8 MN pools and segmental interneurons for locomotion, and the axons of the propriospinal circuitry that works in parallel and as relay for descending tracts and may serve as substrate for plastic changes.
This situation might be somewhat comparable to that occurring in the lumbar region, where the short propriospinal pathways provide a pre-MN circuit that can be accessed by supraspinal descending pathways and is capable of generating locomotion. When those propriospinal pathways are interrupted by sectioning their axons close to the hind leg MN pools in chronic spinal cats, the MNs can no longer be recruited for treadmill locomotion.76
The integrated fluorescence density of synaptophysin immunostaining chronically decreased in the spinal cord segments adjacent to the lesion, particularly in C7. Although this marker provided only a general estimation of the synaptic pool, the most likely interpretation for its persistent reduction is that C7 was massively denervated from descending supraspinal and cervical propriospinal axons, thus precluding a more complete process of reactive synaptogenesis and hampering motor recovery. The possibility exists, however, that atrophy or death of denervated segmental MNs and INs contributed to reduce the synaptic pool and additional neuroanatomical studies are needed to clarify this point.
Nevertheless, given that C6 hemisection interrupted thousands of brain-descending and propriospinal axons whereas little neuronal death occurred in the TB motor nuclei, it is reasonable to assume that axotomy was the main responsible for the chronic synapse loss. In support of this interpretation, we found that retrograde neural tracing from C7 labeled numerous PNs in the rostral cervical segments of normal rats. Processing only alternate spinal cord sections and restricting the counts to laminae VII and VIII from C2–C5, we counted in average about 6000 PNs that project to C7, thus showing that the cervical propriospinal system is anatomically relevant in the rat.
The anatomophysiology of the CPPS has been studied in detail in cats and monkeys.54–56,77,78 It comprises different types of neurons mainly located at laminae VII and VIII in C3–C4, with axons that descend through the ventrolateral funiculi and innervate forelimb MNs and segmental INs from C6 to T1. They receive convergent inputs from the corticospinal tract and the rubro, reticulo, and tectospinal tracts, and play an important role in the control of voluntary foreleg movements.
The foreleg motoneuronal excitation evoked by cortical stimulation in rats, however, is mediated either via the reticuloespinal tract or segmental INs.43 Although C3/C4 PNs have been electrophysiologically identified in rats, they do not seem to participate in forepaw voluntary control, and their functional role remains unknown. This system may be important to generate force or to execute tasks that do not depend on corticospinal control in rats, such as overground locomotion. It must be emphasized, however, that the single damage of the CPPS is not expected to produce permanent locomotor impairments, because the takeover of foreleg motor function by other tracts is common after selective cervical spinal cord lesions.17,32,79 Hence, the permanent deficits were very likely caused by damage of brain descending axons together with damage of axons of the CPPS.
In cats, reticulospinal, vestibulospinal, and/or rubrospinal neurons are very active during walking on a level surface.80,81 The discharge of vestibulospinal neurons is highly correlated with the activity of the TB and the hind leg extensors during the stance phase, whereas the discharge of reticulospinal and rubrospinal neurons correlates with the activity of either flexors or extensors.
A unilateral lesion of the ventrolateral tracts at the spinal segment C3 in rats caused unilateral, transient reduction (∼25%) of the foreleg extensor force during overground locomotion that fully recovered at 1.5 weeks later.17 Unilateral lesions of the rubrospinal tract or the dorsal columns, however, caused bilateral and more persistent deficits, the foreleg vertical forces being apparently reduced by about 15% at 6 weeks post-lesion.79 On the other hand, rats with complete spinal cord hemisection at C4/C5 showed ipsilateral foreleg kinematic impairments for locomotion through shallow water,27 with limited recovery at the end of the follow-up (28 days postlesion).
Axonal tract lesions at different cervical segments have distinct functional outcomes because they eliminate synaptic inputs to different groups of MNs, INs, and PNs, but the aforementioned studies jointly support our interpretation that the interruption of the CPPS together with other lateral axonal tracts accounted for the severe and permanent foreleg motor deficits after C6-hemisection.
Conclusion
Our data demonstrate that cervical spinal cord hemisection causes focal foreleg impairments attributable to axonal damage with ensuing focal denervation of the immediately caudal spinal cord segments. They also raise the possibility that the CPPS is involved in the production/chronification of the foreleg deficits, a hypothesis that, if proven correct, will have important implications for the design of treatments for cervical SCI. They also show that after recovering from the spinal shock, rats display only small functional gain but instead develop dynamic and complex neuromechanical compensations, which include adjustments in the timing of the step cycle, overuse of the less affected legs, and optimization of the residual motor functions.
Finally, the present work emphasizes the need for using careful functional and anatomical studies to differentiate real functional recovery from sensorimotor compensations after SCI, providing a comprehensive, long-term kinematic and kinetic baseline of locomotor recovery in a rat model of incomplete cervical SCI, which will be useful to evaluate therapeutic strategies aimed at promoting neural plasticity and repair.
Acknowledgments
We thank Enrique Páramo Rosell for help with veterinary care of animals. This work was sponsored by the Consejería de Sanidad de Castilla La Mancha (Grant PN 04021).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.NSCISC. Spinal Cord Injury Facts and Figures at a Glance. 2011. www.nscisc.uab.edu. www.nscisc.uab.edu [DOI] [PMC free article] [PubMed]
- 2.Curt A. Dietz V. Neurographic assessment of intramedullary motoneuron lesions in cervical spinal cord injury: consequences for hand function. Spinal Cord. 1996;34:326–332. doi: 10.1038/sc.1996.60. [DOI] [PubMed] [Google Scholar]
- 3.Thomas C.K. Zaidner E.Y. Calancie B. Broton J.G. Bigland-Ritchie B.R. Muscle weakness, paralysis, and atrophy after human cervical spinal cord injury. Exp. Neurol. 1997;148:414–423. doi: 10.1006/exnr.1997.6690. [DOI] [PubMed] [Google Scholar]
- 4.Collazos-Castro J.E. Soto V.M. Gutierrez-Davila M. Nieto-Sampedro M. Motoneuron loss associated with chronic locomotion impairments after spinal cord contusion in the rat. J. Neurotrauma. 2005;22:544–558. doi: 10.1089/neu.2005.22.544. [DOI] [PubMed] [Google Scholar]
- 5.Brustein E. Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. Deficits and adaptive mechanisms. J. Neurophysiol. 1998;80:1245–1267. doi: 10.1152/jn.1998.80.3.1245. [DOI] [PubMed] [Google Scholar]
- 6.Magnuson D.S. Trinder T.C. Zhang Y.P. Burke D. Morassutti D.J. Shields C.B. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp. Neurol. 1999;156:191–204. doi: 10.1006/exnr.1999.7016. [DOI] [PubMed] [Google Scholar]
- 7.Metz G.A. Curt A. Van de Meent H. Klusman I. Schwab M.E. Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J. Neurotrauma. 2000;17:1–17. doi: 10.1089/neu.2000.17.1. [DOI] [PubMed] [Google Scholar]
- 8.Collazos-Castro J.E. López-Dolado E. Nieto-Sampedro M. Locomotor deficits and adaptive mechanisms after thoracic spinal cord contusion in the adult rat. J. Neurotrauma. 2006;23:1–17. doi: 10.1089/neu.2006.23.1. [DOI] [PubMed] [Google Scholar]
- 9.Dietz V. Nakazawa K. Wirz M. Erni T. Level of spinal cord lesion determines locomotor activity in spinal man. Exp. Brain Res. 1999;128:405–409. doi: 10.1007/s002210050861. [DOI] [PubMed] [Google Scholar]
- 10.Thomas C.K. Broton J.G. Calancie B. Motor unit forces and recruitment patterns after cervical spinal cord injury. Muscle Nerve. 1997;20:212–220. doi: 10.1002/(sici)1097-4598(199702)20:2<212::aid-mus12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Pisharodi M. Nauta H.J. An animal model for neuron-specific spinal cord lesions by the microinjection of N-methylaspartate, kainic acid, and quisqualic acid. Appl. Neurophysiol. 1985;48:226–233. doi: 10.1159/000101132. [DOI] [PubMed] [Google Scholar]
- 12.Schrimsher G. Reier P. Forelimb motor performance following cervical spinal cord contusion injury in the rat. Exp. Neurol. 1992;117:287–298. doi: 10.1016/0014-4886(92)90138-g. [DOI] [PubMed] [Google Scholar]
- 13.Schrimsher G.W. Reier P.J. Forelimb motor performance following dorsal column, dorsolateral funiculi, or ventrolateral funiculi lesions of the cervical spinal cord in the rat. Exp. Neurol. 1993;120:264–276. doi: 10.1006/exnr.1993.1060. [DOI] [PubMed] [Google Scholar]
- 14.Onifer S.M. Rodríguez J.F. Santiago D.I. Benitez J.C. Kim D.T. Brunschwig J.P. Pacheco J.T. Perrone J.V. Llorente O. Hesse D.H. Martinez-Arizala A. Cervical spinal cord injury in the adult rat: assessment of forelimb dysfunction. Restor. Neurol. Neurosci. 1997;11:211–223. doi: 10.3233/RNN-1997-11405. [DOI] [PubMed] [Google Scholar]
- 15.Soblosky J.S. Song J.H. Dinh D.H. Graded unilateral cervical spinal cord injury in the rat: evaluation of forelimb recovery and histological effects. Behav. Brain Res. 2001;119:1–13. doi: 10.1016/s0166-4328(00)00328-4. [DOI] [PubMed] [Google Scholar]
- 16.Webb A.A. Muir G.D. Compensatory locomotor adjustments of rats with cervical or thoracic spinal cord hemisections. J. Neurotrauma. 2002;19:239–256. doi: 10.1089/08977150252806983. [DOI] [PubMed] [Google Scholar]
- 17.Webb A.A. Muir G.D. Course of motor recovery following ventrolateral spinal cord injury in the rat. Behav. Brain Res. 2004;155:55–65. doi: 10.1016/j.bbr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Pearse D.D. Lo T.P., Jr. Cho K.S. Lynch M.P. Garg M.S. Marcillo A.E. Sanchez A.R. Cruz Y. Dietrich W.D. Histopathological and behavioral characterization of a novel cervical spinal cord displacement contusion injury in the rat. J. Neurotrauma. 2005;22:680–702. doi: 10.1089/neu.2005.22.680. [DOI] [PubMed] [Google Scholar]
- 19.Anderson K.D. Gunawan A. Steward O. Quantitative assessment of forepaw motor function after cervical spinal cord injury in rats: relationship to corticospinal tract. Exp. Neurol. 2005;194:161–174. doi: 10.1016/j.expneurol.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Anderson K.D. Gunawan A. Steward O. Spinal pathways involved in the control of forelimb motor function in rats. Exp. Neurol. 2007;206:318–331. doi: 10.1016/j.expneurol.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Anderson K.D. Sharp K. G. Hofstadter M. Irvin K.A. Murray M. Steward O. Forelimb locomotor assessment scale (FLAS): Novel assessment of forelimb dysfunction after cervical spinal cord injury. Exp. Neurol. 2009;220:23–33. doi: 10.1016/j.expneurol.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onifer S.M. Zhang Y.P. Burke D.A. Brooks D.L. Decker J.A. McClure N.J. Floyd A.R. Hall J. Proffitt B.L. Shields C.B. Magnuson D.S. Adult rat forelimb dysfunction after dorsal cervical spinal cord injury. Exp. Neurol. 2005;192:25–38. doi: 10.1016/j.expneurol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Gensel J.C. Tovar C.A. Hamers F.P. Deibert R.J. Beattie M.S. Bresnahan J.C. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J. Neurotrauma. 2006;23:36–54. doi: 10.1089/neu.2006.23.36. [DOI] [PubMed] [Google Scholar]
- 24.Kanagal S.G. Muir G.D. The differential effects of cervical and thoracic dorsal funiculus lesions in rats. Behav. Brain Res. 2008;187:379–386. doi: 10.1016/j.bbr.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 25.Kanagal S.G. Muir G.D. Task-dependent compensation after pyramidal tract and dorsolateral spinal lesions in rats. Exp. Neurol. 2009;216:193–206. doi: 10.1016/j.expneurol.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh A. Sydekum E. Haiss F. Peduzzi S. Zörner B. Schneider R. Baltes C. Rudin M. Weber B. Schwab M.E. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J. Neurosci. 2009;29:12210–12219. doi: 10.1523/JNEUROSCI.1828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filli L. Zörner B. Weinmann O. Schwab M.E. Motor deficits and recovery in rats with unilateral spinal cord hemisection mimic the Brown-Sequard syndrome. Brain. 2011;134:2261–2273. doi: 10.1093/brain/awr167. [DOI] [PubMed] [Google Scholar]
- 28.Alstermark B. Lundberg A. Norrsell U. Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. Differential behavioural defects after spinal cord lesions interrupting defined pathways from higher centres to motoneurons. Exp. Brain Res. 1981;42:299–318. doi: 10.1007/BF00237496. [DOI] [PubMed] [Google Scholar]
- 29.Pettersson L.G. Perfiliev S. Descending pathways controlling visually guided updating of reaching in cats. Eur. J. Neurosci. 2002;16:1349–1360. doi: 10.1046/j.1460-9568.2002.02203.x. [DOI] [PubMed] [Google Scholar]
- 30.Fenrich K.K. Rose P.K. Spinal interneuron axons spontaneously regenerate after spinal cord injury in the adult feline. J. Neurosci. 2009;29:12145–12158. doi: 10.1523/JNEUROSCI.0897-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galea M.P. Darian-Smith I. Manual dexterity and corticospinal connectivity following unilateral section of the cervical spinal cord in the macaque monkey. J. Comp. Neurol. 1997;381:307–319. [PubMed] [Google Scholar]
- 32.Pettersson L.G. Alstermark B. Blagovechtchenski E. Isa T. Sasaski S. Skilled digit movements in feline and primate—recovery after selective spinal cord lesions. Acta Physiol. (Oxf.) 2007;189:141–154. doi: 10.1111/j.1748-1716.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- 33.Freund P. Schmidlin E. Wannier T. Bloch J. Mir A. Schwab M.E. Rouiller E.M. Anti-Nogo-A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates—re-examination and extension of behavioral data. Eur. J. Neurosci. 2009;29:983–996. doi: 10.1111/j.1460-9568.2009.06642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura Y. Morichika Y. Isa T. A subcortical oscillatory network contributes to recovery of hand dexterity after spinal cord injury. Brain. 2009;132:709–721. doi: 10.1093/brain/awn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenzweig E.S. Courtine G. Jindrich D.L. Brock J.H. Ferguson A.R. Strand S.C. Nout Y.S. Roy R.R. Miller D.M. Beattie M.S. Havton L.A. Bresnahan J.C. Edgerton V.R. Tuszynski M.H. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat. Neurosci. 2010;13:1505–1510. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKenna J.E. Whishaw I.Q. Complete compensation in skilled reaching success with associated impairments in limb synergies, after dorsal column lesion in the rat. J. Neurosci. 1999;19:1885–1894. doi: 10.1523/JNEUROSCI.19-05-01885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenna J.E. Prusky G.T. Whishaw I.Q. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J. Comp. Neurol. 2000;419:286–296. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 38.Clowry G. Sieradzan K. Vrbová G. Transplants of embryonic motoneurones to adult spinal cord: survival and innervation abilities. Trends Neurosci. 1991;14:355–357. doi: 10.1016/0166-2236(91)90162-n. [DOI] [PubMed] [Google Scholar]
- 39.Nakajima H. Uchida K. Kobayashi S. Inukai T. Horiuchi Y. Yayama T. Sato R. Baba H. Rescue of rat anterior horn neurons after spinal cord injury by retrograde transfection of adenovirus vector carrying brain-derived neurotrophic factor gene. J. Neurotrauma. 2007;24:703–712. doi: 10.1089/neu.2006.0004. [DOI] [PubMed] [Google Scholar]
- 40.Grumbles R.M. Sesodia S. Wood P.M. Thomas C.K. Neurotrophic factors improve motoneuron survival and function of muscle reinnervated by embryonic neurons. J. Neuropathol. Exp. Neurol. 2009;68:736–746. doi: 10.1097/NEN.0b013e3181a9360f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pintér S. Gloviczki B. Szabó A. Márton G. Nógrádi A. Increased survival and reinnervation of cervical motoneurons by riluzole after avulsion of the C7 ventral root. J. Neurotrauma. 2010;27:2273–2282. doi: 10.1089/neu.2010.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nógrádi A. Pajer K. Márton G. The role of embryonic motoneuron transplants to restore the lost motor function of the injured spinal cord. Ann. Anat. 2011;193:362–370. doi: 10.1016/j.aanat.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Alstermark B. Ogawa J. Isa T. Lack of monosynaptic corticomotoneuronal EPSPs in rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J. Neurophysiol. 2004;91:1832–1839. doi: 10.1152/jn.00820.2003. [DOI] [PubMed] [Google Scholar]
- 44.Alstermark B. Isa T. Pettersson L.G. Sasaki S. The C3–C4 propriospinal system in the cat and monkey: a spinal pre-motoneuronal centre for voluntary motor control. Acta Physiol. (Oxf ) 2007;189:123–140. doi: 10.1111/j.1748-1716.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- 45.Collazos-Castro J.E. López-Dolado E. Presented at the Society for Neuroscience. Atlanta: 2006. Early gait adaptations with permanent impairments in forelimb function after cervical spinal cord hemisection in the adult rat. [Google Scholar]
- 46.Lucas-Osma A. Collazos-Castro J.E. Compartmentalization in the triceps brachii motoneuron nucleus and its relation to muscle architecture. J. Comp. Neurol. 2009;516:226–239. doi: 10.1002/cne.22123. [DOI] [PubMed] [Google Scholar]
- 47.Masliah E. Fagan A.M. Terry R.D. De Teresa R. Mallory M. Gage F.H. Reactive synaptogenesis assessed by synaptophysin immunoreactivity is associated with GAP-43 in the dentate gyrus of the adult rat. Exp. Neurol. 1991;113:131–142. doi: 10.1016/0014-4886(91)90169-d. [DOI] [PubMed] [Google Scholar]
- 48.Nacimiento W. Sappok T. Brook G. Tóth L. Schoen S. Noth J. Kreutzberg G. Structural changes of anterior horn neurons and their synaptic input caudal to a low thoracic spinal cord hemisection in the adult rat: a light and electron microscopic study. Acta Neuropathol. 1995;90:552–564. doi: 10.1007/BF00318567. [DOI] [PubMed] [Google Scholar]
- 49.Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press; Amsterdam: 2005. [Google Scholar]
- 50.Jayes A.S. Alexander R.McN. The gaits of chelonians: walking techniques for very low speeds. J. Zool. Lond. 1980;191:353–378. [Google Scholar]
- 51.Alexander R.McN. Principles of Animal Locomotion. Princeton UP; New Jersey: 2002. [Google Scholar]
- 52.Lee D.V. Bertram J.E. Todhunter R.J. Acceleration and balance in trotting dogs. J. Exp. Biol. 1999;202:3565–3573. doi: 10.1242/jeb.202.24.3565. [DOI] [PubMed] [Google Scholar]
- 53.Fischer M.S. Schilling N. Schmidt M. Haarhaus D. Witte H. Basic limb kinematics of small therian mammals. J. Exp. Biol. 2002;205:1315–1338. doi: 10.1242/jeb.205.9.1315. [DOI] [PubMed] [Google Scholar]
- 54.Illert M. Lundberg A. Padel Y. Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. Properties of and monosynaptic excitatory convergence on C3–C4 propriospinal neurons. Exp. Brain Res. 1978;33:101–130. doi: 10.1007/BF00238798. [DOI] [PubMed] [Google Scholar]
- 55.Alstermark B. Kümmel H. Transneuronal transport of wheat germ agglutinin conjugated horseradish peroxidase into last order spinal interneurones projecting to acromio- and spinodeltoideus motoneurons in the cat. Location of labelled interneurones and influence of synaptic activity on the transneuronal transport. Exp. Brain Res. 1990;80:83–95. doi: 10.1007/BF00228850. [DOI] [PubMed] [Google Scholar]
- 56.Alstermark B. Isa T. Tantisira B. Integration in descending motor pathways controlling the forelimb in the cat. Morphology, axonal projection and termination of collaterals from C3–C4 propriospinal neurones in the segment of origin. Exp. Brain Res. 1991;84:561–568. [PubMed] [Google Scholar]
- 57.Love S. Louis D.N. Ellison D.W. 8th. Edward Arnold (Publishers) Ltd; London: 2008. Greenfield's Neuropathology. [Google Scholar]
- 58.Bryden A.M. Kilgore K.L. Lind B.B. Yu D.T. Triceps denervation as a predictor of elbow flexion contractures in C5 and C6 tetraplegia. Arch. Phys. Med. Rehabil. 2004;85:1880–1885. doi: 10.1016/j.apmr.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 59.Muir G.D. Whishaw I. Complete locomotor recovery following corticospinal tract lesions: measurement of ground reaction forces during overground locomotion in rats. Behav. Brain Res. 1999;103:45–53. doi: 10.1016/s0166-4328(99)00018-2. [DOI] [PubMed] [Google Scholar]
- 60.Fischer M.S. Kinematics, EMG, and inverse dynamics of the therian forelimb—a synthetic approach. Zool. Anz. 1999;238:41–54. [Google Scholar]
- 61.Scholle H.C. Schumann N.P. Biedermann F. Stegeman D.F. Grassme R. Roeleveld K. Schilling N. Fischer M.S. Spatiotemporal surface EMG characteristics from rat triceps brachii muscle during treadmill locomotion indicate selective recruitment of functionally distinct muscle regions. Exp. Brain Res. 2001;138:26–36. doi: 10.1007/s002210100685. [DOI] [PubMed] [Google Scholar]
- 62.Schumann N.P. Biedermann F.H. Kleine B.U. Stegeman D.F. Roeleveld K. Hackert R. Scholle H.Ch. Multi-channel EMG of the M triceps brachii in rats during treadmill locomotion. Clin. Neurophysiol. 2002;113:1142–1151. doi: 10.1016/s1388-2457(02)00143-8. [DOI] [PubMed] [Google Scholar]
- 63.Rafuse V.F. Gordon T. Orozco R. Proportional enlargement of motor units after partial denervation of cat triceps surae muscles. J. Neurophysiol. 1992;68:1261–1276. doi: 10.1152/jn.1992.68.4.1261. [DOI] [PubMed] [Google Scholar]
- 64.Cavagna G.A. Heglund N.C. Taylor C.R. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am. J. Physiol. 1977;233:R243–R261. doi: 10.1152/ajpregu.1977.233.5.R243. [DOI] [PubMed] [Google Scholar]
- 65.Carroll A.M. Biewener A.A. Mono- versus biarticular muscle function in relation to speed and gait changes: in vivo analysis of the goat triceps brachii. J. Exp. Biol. 2009;212:3349–3360. doi: 10.1242/jeb.033639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Juvin L. Simmers J. Morin D. Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J. Neurosci. 2005;25:6025–6035. doi: 10.1523/JNEUROSCI.0696-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cowley K.C. Zaporozhets E. Schmidt B.J. Propriospinal neurons are sufficient for bulbospinal transmission of the locomotor command signal in the neonatal rat spinal cord. J. Physiol. 2008;586:1623–1635. doi: 10.1113/jphysiol.2007.148361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kato M. Murakami S. Hirayama H. Hikino K. Recovery of postural control following chronic bilateral hemisections at different spinal cord levels in adult cats. Exp. Neurol. 1985;90:350–364. doi: 10.1016/0014-4886(85)90024-x. [DOI] [PubMed] [Google Scholar]
- 69.Bareyre F.M. Kerschensteiner M. Raineteau O. Mettenleiter T.C. Weinmann O. Schwab M.E. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 70.Barrière G. Leblond H. Provencher J. Rossignol S. Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J. Neurosci. 2008;28:3976–3987. doi: 10.1523/JNEUROSCI.5692-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Courtine G. Song B. Roy R.R. Zhong H. Herrmann J.E. Ao Y. Qi J. Edgerton V.R. Sofroniew M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller S. van der Burg J. van der Meché F. Coordination of movements of the hindlimbs and forelimbs in different forms of locomotion in normal and decerebrate cats. Brain Res. 1975;91:217–237. doi: 10.1016/0006-8993(75)90544-2. [DOI] [PubMed] [Google Scholar]
- 73.Dietz V. Fouad K. Bastiaanse C.M. Neuronal coordination of arm and leg movements during human locomotion. Eur. J. Neurosci. 2001;14:1906–1914. doi: 10.1046/j.0953-816x.2001.01813.x. [DOI] [PubMed] [Google Scholar]
- 74.Beaumont E. Onifer SM. Reed WR. Magnuson DS. Magnetically evoked inter-enlargement response: an assessment of ascending propriospinal fibers following spinal cord injury. Exp. Neurol. 2006;201:428–440. doi: 10.1016/j.expneurol.2006.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reed W.R. Shum-Siu A. Onifer S.M. Magnuson D.S. Inter-enlargement pathways in the ventrolateral funiculus of the adult rat spinal cord. Neuroscience. 2006;142:1195–1207. doi: 10.1016/j.neuroscience.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Langlet C. Leblond H. Rossignol S. Mid-lumbar segments are needed for the expression of locomotion in chronic spinal cats. J. Neurophysiol. 2005;93:2474–2488. doi: 10.1152/jn.00909.2004. [DOI] [PubMed] [Google Scholar]
- 77.Alstermark B. Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. Comparison of the projection from excitatory C3–C4 propriospinal neurones to different species of forelimb motoneurones. Exp. Brain Res. 1986;63:543–556. doi: 10.1007/BF00237477. [DOI] [PubMed] [Google Scholar]
- 78.Alstermark B. Isa T. Ohki Y. Saito Y. Disynaptic pyramidal excitation in forelimb motoneurons mediated via C(3)-C(4) propriospinal neurons in the Macaca fuscata. J. Neurophysiol. 1999;82:3580–3585. doi: 10.1152/jn.1999.82.6.3580. [DOI] [PubMed] [Google Scholar]
- 79.Webb A.A. Muir G.D. Unilateral dorsal column and rubrospinal tract injuries affect overground locomotion in the unrestrained rat. Eur. J. Neurosci. 2003;18:412–422. doi: 10.1046/j.1460-9568.2003.02768.x. [DOI] [PubMed] [Google Scholar]
- 80.Matsuyama K. Drew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. I. Walking on a level surface. J. Neurophysiol. 2000;84:2237–2256. doi: 10.1152/jn.2000.84.5.2237. [DOI] [PubMed] [Google Scholar]
- 81.Lavoie S. Drew T. Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. J. Neurophysiol. 2002;88:1791–1814. doi: 10.1152/jn.2002.88.4.1791. [DOI] [PubMed] [Google Scholar]