Abstract
Objective: The purpose of this study was to analyze proliferation, inflammation, and osteogenic effects on periodontal ligament (PDL) cells after low-level laser therapy (LLLT) under simulated orthodontic tension conditions. Background data: Low-level lasers affect fibroblast proliferation and collagen synthesis and reduce inflammation. Few studies have focused on the LLLT changes in the PDL caused by moving teeth. Materials and methods: A human PDL cell line was cultured in a −100 kPa tension incubator. The PDL cells were treated with a 670 nm low-level diode laser, output power of 500 mW (continuous wave modus) for 2.5 or 5 sec, spot area 0.25 cm2, corresponding to 1.25 and 2.5 J at an energy density of 5 or 10 J/cm2, respectively. PDL cell viability was assayed by detecting the ability of the cells to cleave tetrazolium salt to formazan dye. Inflammation and osteogenic markers were analyzed by Western blot analysis. Results: PDL cell viablity increased in the experimental group, based on the ability of the cells to cleave tetrazolium salt at day 7 (p<0.05). The experimental group showed no difference in PDL cellular morphology compared with the control group. The inflammation markers inducible NO synthase (iNOS), cyclooxygenase (COX)-2 and interleukin (IL)-1 showed stronger expression in 5 and 10 J/cm2 therapy at days 1 and 5, but decreased in expression at day 7. The osteogenic marker osteocalcin (OC) expression level was significantly higher at day 7 (p<0.05) than in the control cells. Conclusions: LLLT significantly increased PDL cell proliferation, decreased PDL cell inflammation, and increased PDL OC activity under the tension conditions used in this study.
Introduction
Orthodontic tooth movement is a process that combines both pathologic and physiologic responses to externally applied forces. Recently, how to enhance the rate of tooth movement without causing harmful effects to periodontal tissue has become an issue of significant interest to patients as well as orthodontists. Efficient tooth movement may be accomplished by mechanical, biomechanical, or biostimulatory methods. The mechanic methods are focused on the bracket structure design, such as self-ligating brackets or materials involving metal or ceramic. The biomechanical methods involve medicine such as prostaglandin E2, a parathyroid hormone.1,2 Biostimulation involves the modification of the environment to stimulate existing bacteria capable of bioremediation. With the introduction of therapeutic lasers, biostimulation also refers to the application of photon energy to injured tissue, in order to achieve a stimulatory and regenerative effect at the molecular level. One biostimulatory method is performed using sub-500 mW ranges of low-level laser therapies (LLLTs).3
Previous studies have shown that low-level lasers affect fibroblast proliferation and collagen synthesis and reduce inflammation.4–6 LLLT irradiation facilitates the reorganization of the connective tissues during tooth movement in rats.7 In orthodontics, LLLTs induce an enhancement of rapid palatal expansion and rapid tooth movement.8,9
The purpose of the present study is to analyze the proliferation, inflammation, and osteogenic effects in periodontal ligament (PDL) cells after LLLT in simulated orthodontic tension conditions.
Materials and Methods
Periodontal ligament cell line culture
A PDL was obtained from the Bioresource Collection and Research Center of Taiwan (ATCC 33277; DSM 20709). PDL cells were cultivated in two different media. Medium A (MA) was composed of Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 mM HEPES. Medium B (MB), differentiation medium, is composed of DMEM medium supplemented with 10 μL/μL 10−5 M dexamethasone, 0.05 g/μL L-Ascorbic acid and 2.16 g/μL glycerol 2-phosphate disodium salt hydrate. All incubations were at 37°C, 95% humidity and 5% CO2.
Tension incubator and diode laser on PDL cells
The PDL cells were seeded at a density of 1×104 cells/well in 96 well plates. After 24 h of cultivation, the media were changed either MA or MB. The culture plates were transferred to a tension incubator (TI, Model. 3618P, Lab-Line Instrument, Thermolyne Co., IL) for 1 day under the following conditions: −100 kPa (1 Pa=1/100,000 kg/cm2, equal to a negative force of 101g/mm2), 370C and 5% CO2.
The PDL cells were irradiated with a 670 nm diode laser (Ga-Al-As, Arts-Laser Model 970, Arts International Biotechnology, Inc., Germany) using directly mounted fiberoptics at an output power of 500 mW (continuous wave modus) for 2.5 or 5 sec, spot area 0.25 cm2, corresponding to 1.25 and 2.5 J at an energy density of 5 or 10 J/cm,2 respectively.5 In the experimental groups, the laser beam was adjusted to exactly cover the bottom of one culture well. After laser treatment, the plates continued incubating in the TI for 1, 5, or 7 days. The experiment was performed in triplicate. The extracts of mineral trioxide aggregate (MTA), which have osteoinduction ability, were used as a positive control group.10 After treatment, cells were analyzed for their proliferation and expression of cellular proteins.
PDL cell viability assay
PDL cell viability was determined using the mitochondria tetrazolium bromide (MTT) colorimetric assay. Briefly, the control and experimental PDL cells were harvested after 1, 5, or 7 days in the TI. Then, 10 μL MTT (5 mg/mL in phosphate-buffered saline [PBS]) was added to each well, and the plate was incubated at 37°C for 4 h to produce formazan. The medium was then removed from the culture wells, and the cells were gently rinsed three times with PBS. Following this step, a 100:1 dilution of 0.04 N HCl (stop solution) was added to each well of the tissue culture plate to halt further reduction of MTT. This stop solution was removed, the cells were rinsed three times with PBS, and the formazan in the cells was extracted with isopropanol. The formazan extracts were transferred, and their absorbance was measured at a wavelength of 570–600 nm in a spectrophotometer (U 2000 type, Hitachi Co., Tokyo, Japan). The results were compared using analysis of variance (ANOVA). Differences in treatment means were analyzed using the Student–Newman–Keuls test and were considered to be significant at p<0.05.
Cellular protein expression analyzed by Western blot
According to our previously described Western blot analysis method,11 the harvested cells were lysed in 50 mL of lysis buffer (1% Triton X-100, 0.5% NP40, 10 mM ethylene glycol tetraacetic acid [EGTA], 0.2 mM Na3VO4, 0.2 mM NaF, and 0.2 mM phenylmethanesulfonylfluoride [PMSF]). Cell lysates were cleared at 15,000 rpm for 15 min at 40C. Twenty-five micrograms of protein from each sample was boiled for 5 min after adding 1 μL sodium dodecyl sulfate (SDS) gel-loading buffer (125 mM Tris, pH 6.8, 5% glycerol, 28 mM SDS, 1% beta-mercaptoethanol, and 0.006% brompophenol blue).
Proteins were separated by 12.5% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto polyvinylidend difluoride membranes. The membranes were blocked for 1 h at room temperature in 3% bovine serum albumin (BSA), 5% nonfat dried milk, 10 mM Tris, pH 7.5, 100 mM NaCl, and 0.1% Tween 20. To detect the proliferation, inflammation, and osteoblastic effects of the experimental PDL, the appropriate antibodies (inducible NO synthase [iNOS], interleukin [IL]-1, cyclooxygenase (COX)-2, matrix metalloproteinase (MMP)-3 alkaline phosphatase [ALP], osteocalcin [OC]) were used to immunoblot the membrane. After four washes, the membrane was overlaid with a secondary antibody for 1 h, then washed with TBS-T buffer for 20 min. The resultant films were scanned and quantified using a densitometer and the SCION image program.
Results
PDL cell viability and morphology
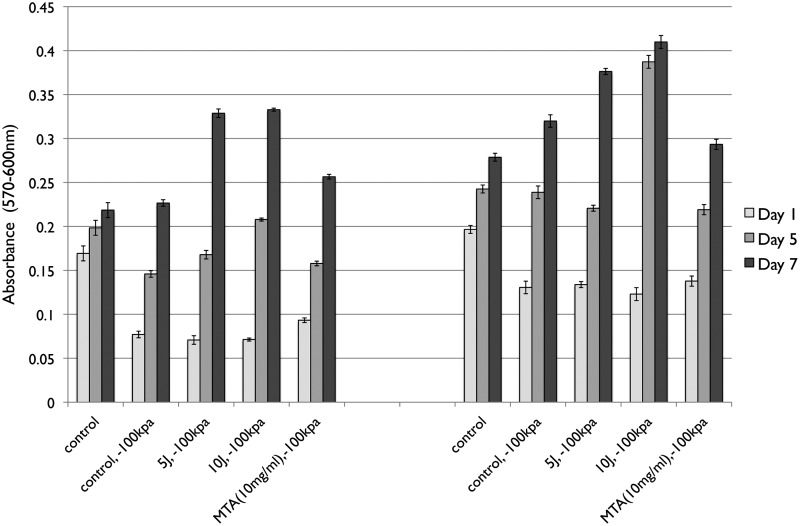
The LLLT-treated PDLs cultured in MA or MB and incubated under −100 kPa demonstrated similar trends in behavior. PDL cells under −100 kPa and LLLT showed a significantly lower viability rate than the control group at day 1 (p<0.05). PDL cell viability in MA under −100 kPa incubation was lower than in the control group at days 1 and 5 (p<0.05). However, in MB, the PDL cell viability was lower at day 1 only. There was no difference in PDL cell viability between the 5 and 10 J/cm2 laser in the MA group under −100 kPa incubation (p>0.05). PDL cell viability in the MB group at 10 J under −100 kPa incubation showed higher viability than the 5 J/cm2 condition at days 5 and 7 (p<0.05) (Fig. 1).
FIG. 1.
The periodontal ligament (PDL) cells cultured in Dulbecco's Modified Eagle Medium (DMEM) (MA) and differentiation medium (MB) in a −100 kPa tension incubator. The control group was PDL cells cultured normally, and the positive control was mineral trioxide aggregate extract (MTA)-treated PDL cells in a −100 kPa tension incubator without low-level laser therapy (LLLT). The experimental groups were PDL cultured in −100 kPa tension incubator with 5 or 10 J LLLT. The mitochondria tetrazolium bromide (MTT) colorimetric assay was performed to detect PDL cell viability. *The statistically significant difference of the highest value of all tested experiments (p<0.05).
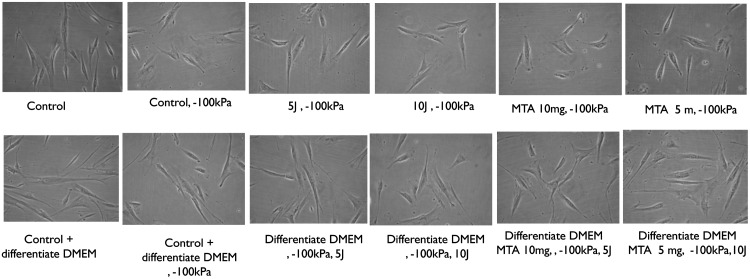
The PDL cell morphologies under the different treatment condition are shown in Fig. 2. Normal PDL cell morphology takes on a spindle shape, and this morphology was observed with no changes in all conditions tested.
FIG. 2.
Periodontal ligament (PDL) cellular morphologies under different culture conditions. PDL cells are spindle shaped. There is no evidence of PDL cell morphology change in response to changes in culture conditions.
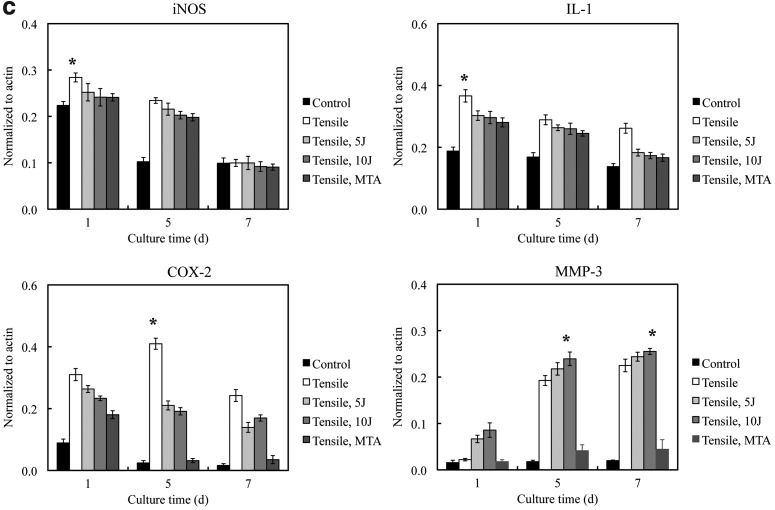
Inflammation expression
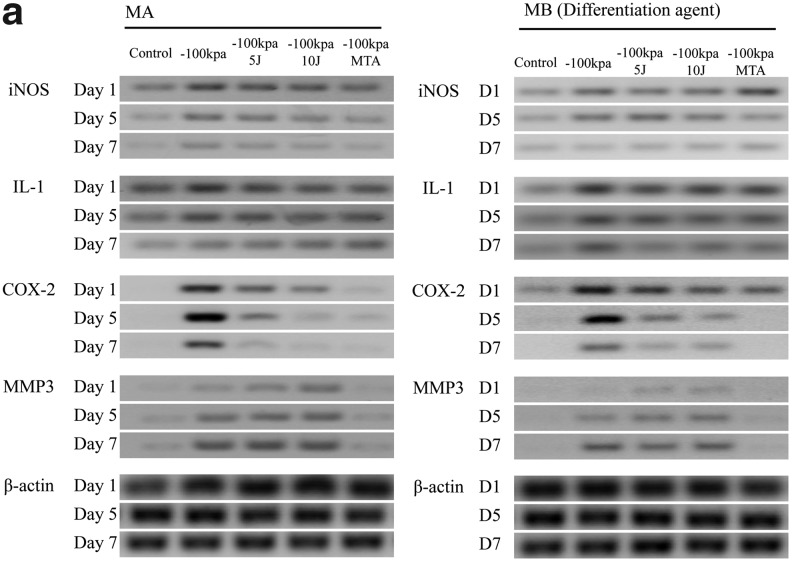
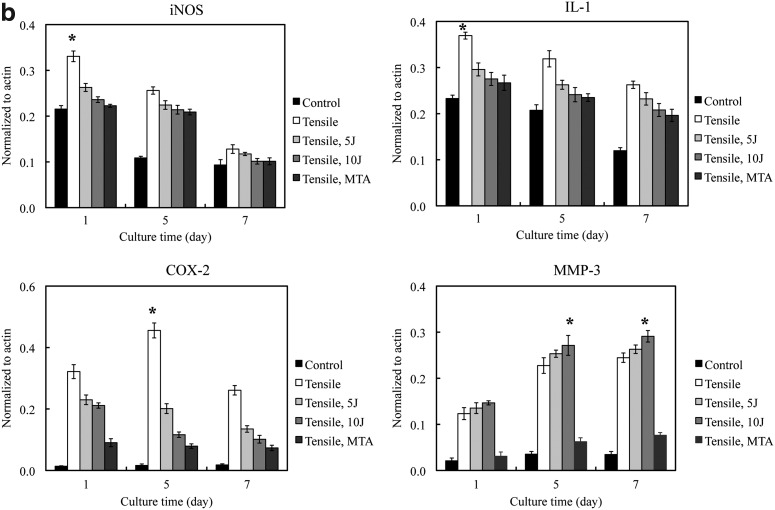
Inflammation markers in PDL cells cultured in MA and MB showed similar patterns of expression (Fig. 3). iNOS, COX-2, and IL-1 expression appeared highest at day 1 and decreased in expression by day 7 (p<0.05). MMP-3 expression increased from days 1 to 7 (p<0.05) (Fig. 3b, c). The PDL cells cultured under −100 kPa incubation with 10 J/cm2 showed the highest MMP-3 expression of all the treatment conditions (p<0.05).
FIG. 3.
(a) Periodontal ligament (PDL) inflammation marker expression assayed by western blot analysis. Inducible NO synthase (iNOS), interleukin (IL)-1 and cyclooxygenase (COX)-2 expression are prominent at day 1 and decrease by days 5 and 7. Matrix metalloproteinase (MMP)-3 expression showed a significant increase at day 7. (b) Quantification of PDL cell inflammation markers in the MA group under different experimental conditions. (c) Quantification of PDL inflammation markers in the MB group under different experimental conditions. *The statistically significant difference of the highest value of all tested experiments (p<0.05).
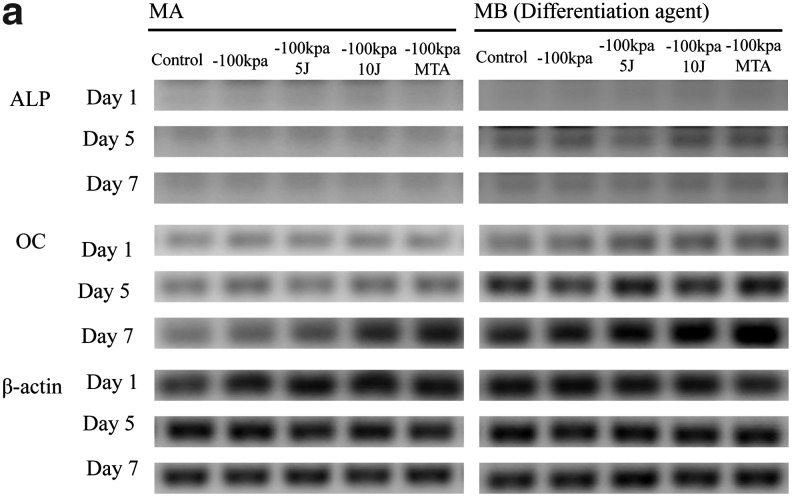
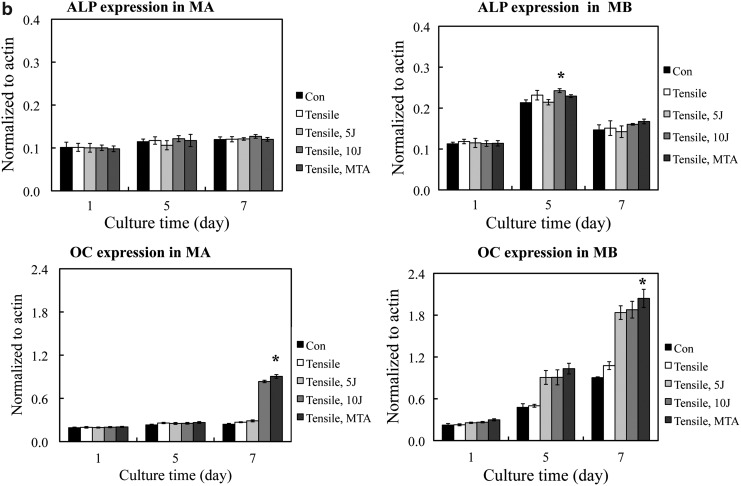
Osteogenic reaction
Osteogenic markers in PDL cells cultured in MA and MB showed similar patterns of expression (Fig. 4). In the Western blot analysis, the bands corresponding to ALP and OC in the MB group were stronger than those in the MA group. Expression of the bone formation marker ALP was highest in PDL cells cultured in MB at day 5 (p<0.05). The OC expressed in PDL cells cultured in MB was higher in the 10 J/cm2 treatment group than in the 5 J/cm2 group at day 7 (p<0.05). The PDL cells cultured in MB had higher ALP and OC expression than PDL cells cultured in MA (p<0.05) (Fig. 4).
FIG. 4.
(a) Periodontal ligament (PDL) osteogenic marker expression assayed by Western blot analysis. Alkaline phosphate (ALP) protein expression showed no significant difference between the control and experimental groups. Osteocalcin (OC) expression showed a significant increase on day 7 in all experimental groups. OC expression showed a time-dependent increase in this study. (b) Quantification of PDL osteogenic marker expression under different experimental conditions. *The statistically significant difference of the highest value of all tested experiments (p<0.05).
Discussion
Orthodontists are interesting in alveolar bone change under orthodontic force. To be able to fasten tooth movement at the orthodontic pressure side and increase the orthodontic tooth stability is still a work in progress. Until recently, the mechanism for osteogenesis at tension sites in tooth movement was not well understood, but reasonable inferences could be made from various mechanotransduction models.12 PDL cells respond to force by increasing cell viability and apoptosis. The relative extent to which these two competing processes occur controls the proportion of the various cell populations in the PDL and reflects the specific biomechanics of osteogenesis.13
The present in vitro study aimed to simulate the tension site of tooth movement and apply LLLT to PDL cells to evaluate whether this procedure could promote osteogenesis. The results of this study reveal that LLLT caused PDL cell viability to decrease at days 1 and 5 but increase at day 7 in both experimental groups (grown in either MA or MB) incubated under −100 kPa. After LLLT was applied, the PDL cells cultured in a tension incubator showed higher viability at both 5 and 10 J/cm2 than the control group at day 7 (Fig. 1). The inflammation markers iNOS, COX-2, MMP-3, and IL-1 showed higher expression in PDL cells cultured in the −100 kPa incubator than in control cells (Fig. 3). There was a statistically significant increase in the expression of these markers at days 1 and 5 but expression decreased at day 7. Similar studies have shown that inflammatory responses to tension might be strain dependent because low magnitude tensile strains are anti-inflammatory and induce magnitude-dependent anabolic signals in osteoblast-like periodontal ligament cells, culminating in the regulation of inflammatory gene transcription.14
PDL cell inflammatory markers decreased after LLLT at days 1 and 5 in MA or MB groups (Fig. 3b, c). This finding is similar to another study, which compared the tumor necrosis factor (TNF)-α and IL-1 expression on LLLT-induced inflammation in rats.15 The results showed that LLLT has anti-inflammatory activity.15 Similarly, LLLT reduced inflammation in our present study, which indicates that LLLT can be beneficial in overcoming cellular inflammation. After cellular inflammation subsided, initiation of the osteogenic effect could be observed. It is reasonable that the tension side of the alveolar bone has osteogenic capabilities.
The application of MTA in the present study was to provide osteoinduction material.10 Both the MTA-positive control group and the experimental laser treatment group under −100 kPa tension induced similar cellular reaction patterns. Morphologic evidence of PDL cell disruption at tension sites in tooth movement has also been described after only 5 min of tension loading, further suggesting the involvement of an inflammatory mechanism.16,17 In the present study, the lack of any PDL cell morphology changes may be the result of the 5 min delay between loading and imaging (Fig. 2).
In vivo, normal cells are in differentiation. To add differentiation medium in the test group was to promote that the cell was under differentiating. It provided more information on experimental conditions that were similar to in vivo conditions. ALP expression in the MA group showed no significant difference in growth at any time interval of culture (Fig. 4b). LLLT did not increase ALP expression in PDL cells grown in MA. In contrast, ALP expression in PDL cells was higher in the MB group at day 5 but showed no difference at days 1 and 7. Differentiation medium (MB), which has been optimized to promote preferential differentiation, seemed to enhance the ALP expression in the present study.
The OC expression in PDL cells was higher, compared with control cells, in the MA group treated with LLLT 10 J/cm2 at day 7, whereas the PDL cells in the MB group treated with LLLT had higher, compared with control cells, OC expression at days 5 and 7 (Fig. 4b). OC is known to express late in the process of bone formation. In the present study, the presence or absence of differentiation medium did not affect OC expression. These results indicate that the LLLT-applied tension in PDL cells can promote osteogenesis.
The clinical relevance of present study may be more important with regard to the stability of post-orthodontic tooth movement. It is because bone formation can be quickly initiated at the tension site of orthodontic tooth movement, thus increasing post-orthodontic stability. An animal model to set up and test with present experimental conditions will be the subject of a future study.
Conclusions
LLLT significantly increased cellular viability, decreased cellular inflammatory marker expression, and increased OC activity in PDL cells in the present study. Although not yet proven by our studies, LLLT may promote the tooth movement rate under clinical conditions.
Acknowledgments
This study was supported by the National Science Council grants (NSC 99-2314-B-040-014-MY3) of Taiwan.
Author Disclosure Statement
No competing financial interests exist.
Reference
- 1.Yamasaki K. Shibata Y. Fukuhara T. The effect of prostaglandins on experimental tooth movement in monkeys (Macaca fuscata) J. Dent. Res. 1982;61:1444–1446. doi: 10.1177/00220345820610121401. [DOI] [PubMed] [Google Scholar]
- 2.Soma S. Iwamoto M. Higuchi Y. Kurisu K. Effect of continuous infusion of PTH on experimental tooth movement. J. Bone. Miner. Res. 1999;14:546–554. doi: 10.1359/jbmr.1999.14.4.546. [DOI] [PubMed] [Google Scholar]
- 3.Mester E. Mester A.F. Mester A. The biomedical effects of laser application. Lasers Surg. Med. 1985;5:31–39. doi: 10.1002/lsm.1900050105. [DOI] [PubMed] [Google Scholar]
- 4.Marques M.M. Pereira A.N. Fujihara N.A. Nogueira F.N. Eduardo C.P. Effect of low-power laser irradiation on protein synthesis and ultrastructure of human gingival fibroblasts. Lasers Surg. Med. 2004;34:260–265. doi: 10.1002/lsm.20008. [DOI] [PubMed] [Google Scholar]
- 5.Kreisler M. Christoffers A.B. Willerstausen B. d'Hoedt B. Effect of low-level GaAlAs laser irradiation on the proliferation rate of human periodontal ligament fibroblasts: an in vitro study. J. Clin. Periodontol. 2003;30:353–358. doi: 10.1034/j.1600-051x.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- 6.Huang T.H. Lu Y.C. Kao C.T. Low-level diode laser therapy reduces lipopolysaccharide (LPS)-induced bone cell inflammation. Laser Med. Sci. 2012;27:621–627. doi: 10.1007/s10103-011-1006-y. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y.D. Kim S.S. Km S.J. Kwon D.W. Jeon E.S. Son W.S. Low level laser irradiation facilitates fibronectin and collagen type I turnover during tooth movment. Laser Med. Sci. 2010;25:25–31. doi: 10.1007/s10103-008-0585-8. [DOI] [PubMed] [Google Scholar]
- 8.Saito S. Shimizu N. Stimulatory effects of low-power laser irradiation on bone regeneration in mid-palatal suture during expansion in the rat. Am. J. Orthod. Dentofacial. Orthop. 1997;111:525–532. doi: 10.1016/s0889-5406(97)70152-5. [DOI] [PubMed] [Google Scholar]
- 9.Limpanichkul W. Godfrey K. Srisuk N. Rattanayatikul C. Effects of low-level laser therapy on the rate of orthodontic tooth movement. Orthod. Craniofac. Res. 2006;9:38–43. doi: 10.1111/j.1601-6343.2006.00338.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen C.L. Huang T.H. Shie M.Y. Ding S.J. Kao C.T. Comparison of calcium and silicate cement (CS) and mineral trioxide aggregate (MTA) biologic effects and bone markers expression in MG63 cells. J. Endod. 2009;35:681–685. doi: 10.1016/j.joen.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Huang T.H. Ding S.J. Hsu T.C. Kao C.T. Effects of mineral trioxide aggregate (MTA) extracts on mitogen-activated protein kinase activity in human osteosarcoma cell line (U2OS) Biomaterial. 2003;24:3909–3913. doi: 10.1016/s0142-9612(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 12.Wiass G.E. King G.J. Mechanisms of tooth eruption and orthodontic tooth movement. J. Dent. Res. 2008;87:414–434. doi: 10.1177/154405910808700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabuchi R. Matsuzaka K. Shimono M. Cell proliferation and cell death in periodontal ligaments during orthodontic tooth movement. J. Periodontal Res. 2002;37:118–124. doi: 10.1034/j.1600-0765.2001.10602.x. [DOI] [PubMed] [Google Scholar]
- 14.Long P. Hu J. Piesco N. Buckley M. Agarwal S. Low magnitude of tensile strain inhibits IL-1betadependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. J. Dent. Res. 2001;80:1416–1420. doi: 10.1177/00220345010800050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honmura A. Yanase M. Obata J. Haruki E. Therapeutic effect of Ga-Al-As diode laser irradiation on experimentally induced inflammation in rats. Lasers Surg. Med. 1992;12:441–449. doi: 10.1002/lsm.1900120414. [DOI] [PubMed] [Google Scholar]
- 16.Orellana M.F. Smith A.K. Waller J.L. DeLeon E. J. Borke J.L. Plasma membrane disruption in orthodontic tooth movement in rats. J. Dent. Res. 2002;81:43–47. doi: 10.1177/002203450208100110. [DOI] [PubMed] [Google Scholar]
- 17.Orellana-Lezcano M.F. Major P.W. McNeil P.L. Borke J.L. Temporary loss of plasma membrane integrity in orthodontic tooth movement. Orthod. Craniofac. Res. 2005;8:106–113. doi: 10.1111/j.1601-6343.2005.00306.x. [DOI] [PubMed] [Google Scholar]