Abstract
Purpose
To compare mortality among patients with selected autoimmune diseases treated with anti-tumor necrosis factor alpha (TNF-α) agents with similar patients treated with non-biologic therapies.
Methods
Cohort study set within several large health care programs, 1998–2007. Autoimmune disease patients were identified using diagnoses from computerized healthcare data. Use of anti-TNF-α agents and comparison non-biologic therapies were identified from pharmacy data and mortality was identified from vital records and other sources. We compared new users of anti-TNF-α agents to new users of non-biologic therapies using propensity scores and Cox proportional hazards analysis to adjust for baseline differences. We also made head-to-head comparisons among anti-TNF-α agents.
Results
Among the 46,424 persons included in the analysis, 2,924 (6.3%) had died by the end of follow-up, including 1,754 (6.1%) of the 28,941 with a dispensing of anti-TNF-α agent and 1,170 (6.7%) of the 17,483 who used non-biologic treatment alone. Compared to use of non-biologic therapies, use of anti-TNF-α therapy was not associated with an increased mortality in patients with rheumatoid arthritis (adjusted hazard ratio [aHR] 0.93 with 95% CI 0.85–1.03); psoriasis, psoriatic arthritis, or ankylosing spondylitis (combined aHR 0.81 with CI 0.61–1.06; or inflammatory bowel disease (aHR 1.12 with CI 0.85–1.46). Mortality rates did not differ to an important degree between patients treated with etanercept, adalimumab, or infliximab.
Conclusion
Anti-TNF-α therapy was not associated with increased mortality among patients with autoimmune diseases.
Keywords: Rheumatoid arthritis, psoriatic arthritis, psoriasis, Crohn’s Disease, ulcerative colitis, inflammatory bowel disease, pharmacoepidemiology, drug safety, drug toxicity, adverse events, cohort studies, propensity scores, automated healthcare data, mortality
INTRODUCTION
Most autoimmune diseases are treated with immunosuppressive medications that could pose unintended consequences. Some potential consequences could be deleterious, such as increasing the risk of serious infections and malignancies (1, 2). Others could be beneficial, such as reducing the incidence of myocardial infarction (3–6). While studies of selected outcomes lend important insight, they do not inform the trade-off of potential benefits and harms across competing outcomes. In contrast, mortality is a composite outcome that encompasses the trade-off of potential benefits and harms.
To date, studies of the potential risks of anti-tumor necrosis factor (TNF)-α therapy on mortality have provided conflicting results. (4, 5, 7–10). Thus, uncertainty about the impact of anti-TNF-α therapy on mortality remains. As part of the federally-funded study “Safety Assessment of Biologic Therapy” (11, 12), we conducted a cohort study to evaluate the association of anti-TNF-α therapy with adverse events among persons across a range of autoimmune diseases. We previously reported on associations with infection risk (1); this report presents our findings for mortality.
METHODS
Design Overview
To protect personal health information and optimize study efficiency while preserving the flexibility needed to analyze a variety of adverse events, we used common data formats to aggregate data across data systems and create files that could be shared across workgroups. We pooled individual-level data containing a limited number of class variables while masking more detailed covariate information through the use of data system-specific propensity scores (11, 12). Institutional Review Board approval was obtained at each of the study centers.
Data Systems
The study included information from the following data systems that are described in detail in our earlier publications (1, 11, 12): Centers for Medicare and Medicaid Services (2000–2006) (13), TennCare (1998–2005) (14), pharmacy benefits programs for low-income elderly in New Jersey and Pennsylvania (1998–2006) (16), and Kaiser Permanente Medical Care Program, Northern California, (1998–2007) (17). The populations included in these data sources include the national census of Medicaid and dual-eligible (Medicaid-Medicare) beneficiaries, less those from Tennessee; all beneficiaries of TennCare; all beneficiaries of the New Jersey and Pennsylvania program; and all members of Kaiser Permanente Northern California.
Study Population
Patients were eligible if (1) they met an operational definition for autoimmune disease (11), (2) had at least one dispensing of a biologic agent or comparison non-biologic regimen relevant to their autoimmune disease, and (3) had an eligible baseline period, defined as a period 12 months of observation in the data system preceding their first eligible dispensing of biologic agent or comparison non-biologic regimen (Figure 1) (11). Only those persons with eligible treatment episodes (defined below) were retained in the study.
Figure 1.
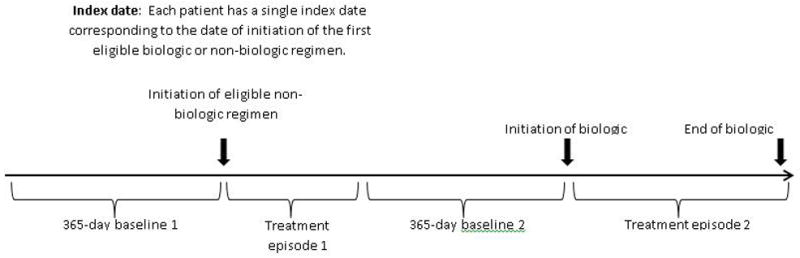
Concepts used to define treatment episodes for a hypothetical patient. For primary analyses, the patient was observed from the initial cohort entry date to the date of death, disenrollment, or the closing date of the data system.
The operational definitions of autoimmune arthritides were age >16 years, and ≥ 1 relevant inpatient or outpatient physician visit codes per the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM)[11] of rheumatoid arthritis (RA) (714.x but not 714.3), psoriatic arthritis (PsA) (696.0), and ankylosing spondylitis (AS) (696.0). Psoriasis (PsO) and inflammatory bowel disease (IBD) were defined without regard to age, with the relevant codes for PsO being 696.1; and for IBD, 555 and 556. In most circumstances, a single diagnosis code was accepted because the therapies under study are used for patients with active and relatively severe disease, and because use of the therapies has strong face validity for ascertaining autoimmune disease. The diagnosis code may have been present at any position on the claim or clinical record from an inpatient or outpatient physician evaluation or management visit, and it may have been recorded in the computerized data at any time during the 12 months preceding the treatment episode start date.
Patients who had human immunodeficiency virus (HIV) infection, solid organ transplantation, advanced kidney or liver disease, a cancer diagnosis, or who were treated with cyclosporine or tacrolimus during the 12-month period preceding study entry were excluded because these persons were few in number, the conditions often represent a contraindication for biologic therapy, and the conditions have strong associations with mortality.
Exposure Assessment
We evaluated three anti-TNF-α drugs (etanercept, infliximab, and adalimumab). Comparison non-biologic therapy was defined specifically for each autoimmune disease under study. For RA, the comparison therapy was intensifying therapy with methotrexate (MTX) by adding or switching to hydrochloroquine, sulfasalazine, or leflunomide. We refer to this regimen as “MTX step-up”. For PsA and AS, the comparison was initiation of MTX or sulfasalazine; for PsO, initiation of MTX; and for IBD, initiation of azathioprine or 6-mercaptopurine. The start date of the first eligible treatment episode (biologic or comparison) served as the index date. Consistent with an incident user design, patients were required to have a look-back period of 12 months of enrollment without an eligible treatment episode before their index date. The earliest possible start date was January 1, 1998. The patient’s baseline morbidity and health care utilization were coded using information recorded during the 12-month look-back period.
Outcome Assessment
Deaths among persons in the Medicaid Analytic eXtract files, Medicare and the two pharmaceutical assistance programs were identified by linkage to the Social Security Administration’s Death Master File. Deaths in TennCare were identified by linkage with State of Tennessee vital records and by an indication of death in the TennCare files. Deaths in the Kaiser Permanente data were ascertained by matching computerized health plan membership data to the California Death Master File, which includes both in-state and out-of-state deaths reported to the National Center for Health Statistics National Death Index; these were further supplemented by deaths documented directly in the computerized medical record.
Covariates
Study covariates were ascertained during the 365-day baseline period preceding each treatment episode.
Some covariates were shared across the collaboration; we refer to these as “shared covariates”. They were used as stratifying variables for subgroup and sensitivity analyses to assess study validity and facilitate analysis of vulnerable populations (selected by the funding agency and defined as non-white, aged ≥75 years, ≥2 comorbidities, or rural). Shared covariates included data system identifier, calendar year of cohort entry, age (5-year categories), sex, race/ethnicity, urban/rural residence (census block), smoking status, elements of the Charlson-Deyo comorbidity index, and oral glucocorticoid exposure. For oral glucocorticoids, we calculated the average daily dose and the cumulative prednisone-equivalent dose over each 6-month interval in the baseline period and following the initial cohort entry date. Thus, oral glucocorticoid treatment was not mutually exclusive with other non-biologic regimens or with biologic treatments.
Other covariates could not be shared across the collaboration because of data use agreements and were incorporated into a propensity score such that their values were known only to those who had custodial responsibility for the data (11, 12) (see supplemental material).
Data Analysis
Propensity score methods
The propensity score was computed separately for each database and disease group, using >100 variables recorded during the 12-month baseline period (1, 11, 12). Logistic regression models were used to estimate the propensity score summarizing the probability of receiving anti-TNF-α therapy versus nonbiologic regimen or, for head-to-head comparisons, alternative anti-TNF-α therapy. Propensity scores were computed at the index date and again if a patient switched from the non-biologic comparator drug to an anti-TNF-α therapy. Following computation of propensity scores, we excluded patients who were in the tails of the distribution for which the exposure groups had no overlapping propensity scores. In the primary analysis, we adjusted for the propensity score decile. In secondary analyses, we matched patients on their propensity scores using a 5-to-1 greedy matching algorithm and conducted a matched analysis (19).
Calculation of follow-up time
For each analysis, patients entered follow-up on their index date. We continued to follow the patients after they stopped therapy, and censored them on the earliest of the death date, disenrollment, their 90th birthday, or the end of the study (December 31, 2005, 2006, or 2007, depending on the dataset).
To describe the patterns of medication use after the index date, we categorized all follow-up time into mutually exclusive episodes defined by the biologic and comparison therapies under study, with some follow-up time being categorized as exposed to neither. The latter may have involved no treatment or treatment with a non-biologic drug that was outside the operational definition for the comparison therapy (e.g., a non-steroidal anti-inflammatory agent alone).
To estimate the mortality rate and the association of anti-TNF-α therapy with mortality, we categorized follow-up time differently. Patients who initiated an anti-TNF-α agent on the index date were coded as anti-TNF-α exposed to the end of follow-up even if they switched from anti-TNF-α therapy to a non-biologic comparison therapy. In contrast, patients who initiated a comparison therapy on the index date were coded as such only until they switched to anti-TNF-α therapy. Thereafter they contributed person-time to the anti-TNF-α group, provided they had a second 365-day baseline period without exposure to a biologic preceding the start of the anti-TNF-α agent. If they did not switch to anti-TNF-α therapy, they contributed deaths and person-time to the non-biologic comparison group until the end of follow-up. Patients who switched from one anti-TNF-α drug to another (e.g., etanercept to infliximab), contributed deaths and person-time to the first agent until they switched, whereupon they contributed person-time to the second agent, through the end of follow-up.
Estimated mortality rates
The 2000 Census data were used as a reference population to compute age and sex standardized mortality rates using the direct method with 5-year age groups. Ninety-five percent confidence intervals (CI) were calculated for the rates, assuming a Poisson distribution (20).
Association of anti-TNF-α therapy with mortality
The adjusted hazard ratio (aHR) for the association of anti-TNF-α therapy with mortality was estimated using Cox proportional hazards modeling. The number of days from the index date, i.e., the date the patient initiated their first eligible treatment episode, was used as the time axis. We hypothesized that mortality was greater following initiation of anti-TNF-α therapy versus non-biologic comparisons therapies. In addition, in head-to-head comparisons, we hypothesized that mortality was greater for one anti-TNF-α drug than another. Before starting the data analysis, we determined that the Cox models employed for the study would be stratified by data system and would include as independent variables (i) exposure to anti-TNF-α or comparison therapy, (ii) the propensity score decile, (iii) the average daily dose of oral glucocorticoid, averaged across and updated every 6 months, and (iv) the shared covariates (calendar year of cohort entry, age, sex, race/ethnicity, urban/rural, smoking status, elements of the Charlson co-morbidity index, and baseline dose of steroid during the 12-month look-back period). Models including multiple autoimmune disease patients were further adjusted for autoimmune disease. The proportionality assumption was assessed through visual inspection of the survival function. Because patients could contribute multiple treatment episodes, we used the Huber–White sandwich variance estimator (21).
The anti-TNF-α agents include soluble TNF receptor-Fc fusion proteins (etanercept) and anti-TNF monoclonal antibodies (adalimumab and infliximab). We therefore considered adalimumab and infliximab as a single exposure category in several analyses. Subgroup analyses were conducted in pre-specified vulnerable populations (non-white, aged ≥75 years, ≥2 comorbidities, rural) selected by the funding agency. All statistical procedures were performed using SAS software version 9.13 (SAS Institute Inc, Cary, NC).
RESULTS
Characteristics of the Study Population
Before computing propensity scores, the number of eligible persons identified for the study was 48,127 of which 29,367 (61%) initiated an anti-TNF-α therapy and 18,760 (39%) did not. A total of 426 (1%) anti-TNF-α treated patients and 1277 (4%) non-biologic comparator treated patients were excluded due to non-overlapping propensity scores, leaving 46,424 patients for the primary analysis. The average length of follow-up was 2.48 years (standard deviation, 1.32) with median 1.69 years. Characteristics of the study population are shown in Table 1. Patients initiating anti-TNF-α differed from those initiating non-biologic comparison therapy in several ways. They entered the study later, were older on the index date, were more likely to have exposure to steroid during baseline, had greater co-morbidity, and had lower estimated annual income. Because of the large number of patients available for analysis, even small differences were statistically significant.
Table 1.
Characteristics of the Study Population (N=46,424)*, %
| All patients | Patients with overlapping propensity scores | ||||
|---|---|---|---|---|---|
|
| |||||
| Characteristic* | Anti-TNF-α** N=29,299 |
Non-biologic comparison *** N=18,656 |
Anti-TNF-α**
N=28,941 |
Non-biologic comparison*** N=17,483 |
|
| Year at index | 1998–1999 | 2 | 7 | 2 | 4 |
| 2000–2001 | 12 | 17 | 12 | 17 | |
| 2002–2003 | 32 | 29 | 32 | 30 | |
| 2004–2005 | 40 | 32 | 40 | 34 | |
| 2006–2007 | 14 | 14 | 14 | 14 | |
| Sex | Female | 79 | 72 | 79 | 72 |
| Age at index, years | 0–39 | 18 | 24 | 18 | 25 |
| 40–49 | 21 | 19 | 21 | 19 | |
| 50–59 | 24 | 21 | 24 | 21 | |
| 60–69 | 19 | 17 | 19 | 17 | |
| 70–90 | 19 | 19 | 19 | 18 | |
| Race/ethnicity | African-American | 15 | 13 | 15 | 13 |
| Asian | 4 | 5 | 4 | 5 | |
| Hispanic | 11 | 8 | 11 | 8 | |
| Native American | 2 | 1 | 2 | 1 | |
| White | 66 | 68 | 66 | 67 | |
| Other | 4 | 5 | 4 | 5 | |
| Steroid exposure during 12-month baseline | Yes | 62 | 55 | 62 | 56 |
| Smoking | Unrecorded or never | 90 | 90 | 90 | 89 |
| Former | 2 | 2 | 2 | 3 | |
| Current | 8 | 8 | 8 | 8 | |
| Charlson comorbidity index | 0 | 18 | 38 | 18 | 38 |
| 1 | 49 | 38 | 49 | 38 | |
| 2+ | 33 | 24 | 33 | 24 | |
| Chronic pulmonary disease | Yes | 19 | 19 | 19 | 19 |
| Median household income, mean (SD)§ | $40,663 ($17,130) | $43,256 ($19,423) | $40,663 ($17,098) | $42,906 ($19,160) | |
Restricted to patients with propensity scores in the overlapping region.
Each patient is shown in the table only once. The 5,064 patients contributing person-years to both the anti-TNF-α group and the non-biologic comparison group are shown with the anti-TNF-α group.
Among PS overlapping patients, all variables except former smoking status and chronic obstructive pulmonary disease are statistically significant at p<0.01 in logistic regression models that contain all variables in this table.
SD: standard deviation
Among the 28,941 patients who initiated anti-TNF-α therapy and had propensity scores in the overlapping region, the average time under observation was 2.37 years, with 1.39 years (59%) on anti-TNF-α therapy; 0.14 years on non-biologic comparison therapy (6%); and 0.84 years (35%) on neither (Table 2). Of the 28,941 patients who initiated anti-TNF-α therapy, 5,064 subsequently switched to a non-biologic regimen; their time on non-biologic average 0.78 years, while their time on anti-TNF-α was 1.37 years. Among the 17,483 propensity-score matched patients who initiated comparison non-biologic therapy and never used anti-TNF-α therapy, the average time on non-biologic comparison therapy was 1.06 years and average time not on a study therapy was 1.57 years.
Table 2.
Length of Treatment Episodes in Relation to Therapeutic Regimen, Years*
| Disease and therapy** | N | Average duration of follow-up, years
|
|||
|---|---|---|---|---|---|
| Duration on anti-TNF-α therapy | Duration on nonbiologic comparison | Duration on neither non-biologic comparison nor anti-TNF-α therapy | Total duration of follow-up (exit minus entry) | ||
| RA | |||||
| Initiated anti-TNF-α | 21,260 | 1.48 | 0.12 | 0.84 | 2.44 |
| Did not switch | 18,177 | 1.49 | 0 | 0.75 | 2.24 |
| Switched to non-biologic | 3,083 | 1.44 | 0.83 | 1.35 | 3.63 |
| Did not initiate anti-TNF-α | 10,651 | 0 | 1.08 | 1.61 | 2.68 |
| PsO, PsA, AS | |||||
| Initiated anti-TNF-α | 4,368 | 1.21 | 0.18 | 0.65 | 2.04 |
| Did not switch | 3,431 | 1.19 | 0 | 0.53 | 1.72 |
| Switched to non-biologic | 937 | 1.27 | 0.80 | 1.13 | 3.20 |
| Did not initiate anti-TNF-α | 5,836 | 0 | 0.97 | 1.61 | 2.58 |
| IBD | |||||
| Initiated anti-TNF-α | 3,329 | 0.96 | 0.24 | 1.23 | 2.43 |
| Did not switch | 2,163 | 0.95 | 0 | 1.03 | 1.98 |
| Switched to non-biologic | 1,166 | 0.97 | 0.68 | 1.63 | 3.28 |
| Did not initiate anti-TNF-α | 6,078 | 0 | 1.07 | 1.44 | 2.51 |
| Total*** | |||||
| Initiated anti-TNF-α | 28,941 | 1.39 | 0.14 | 0.84 | 2.37 |
| Did not switch | 23,877 | 1.40 | 0 | 0.14 | 2.14 |
| Switched to non-biologic | 5,064 | 1,37 | 0.78 | 1.37 | 3.43 |
| Did not initiate anti-TNF-α | 17,483 | 0.00 | 1.06 | 1.57 | 2.63 |
Restricted to patients with propensity scores in the overlapping region.
Patients with multiple autoimmune diseases are shown in multiple disease categories.
Under Total, each patient is shown in the table only once. The 5,064 patients contributing person-years to both the anti-TNF-α group and the non-biologic comparison group are shown with the anti-TNF-α group.
By the end of follow-up, 2,924 (6.3%) of the subjects included in the analysis had died, including 1,754 (6.1%) of patients initiating anti-TNF-α therapy and 1,170 (6.7%) of patients who did not. For anti-TNF-α and non-biologic comparison therapy, the standardized mortality rate, in deaths per 100 person-years, was 2.09 (95% CI 1.94–2.24) and 1.94 (95% CI 1.74–2.14) in RA; 0.97 (95% CI 0.77–1.18) and 1.49 (95% CI, 1.12–1.85) in PsA/PsO/AS; and 2.14 (95% 1.71–2.58) and 1.92 (95% 1.60–2.23) in IBD (Table 3).
Table 3.
Standardized Mortality Rate (SMR) and Adjusted Hazard Ratio (aHR) with 95% Confidence Intervals (CI) for the Association of Initiating Use of Anti-TNF-α with Mortality among 46,424 Persons with Autoimmune Diseases,* of Whom 2,924 Died
| Autoimmune disease | Anti-TNF-α | Comparison | aHR** | 95% CI | ||
|---|---|---|---|---|---|---|
|
| ||||||
| No. of deaths/no. of patients | SMR per 100 person-years | No. of deaths/no. of patients | SMR per 100 person-years | |||
| Rheumatoid arthritis | ||||||
| Anti-TNF-α compared with non-biologic comparison therapy | 1,511/21,260 | 2.09 (1.94–2.24) | 703/10,651 | 1.94 (1.74–2.14) | 0.93 | 0.85–1.03 |
| INF compared with ETA | 676/8,699 | 2.21 (1.90–2.52) | 600 /10,522 | 2.25 (1.94–2.55) | 0.90 | 0.80–1.02 |
| ADA compared with ETA | 359/8,627 | 2.01 (1.70–2.33) | 345/8,528 | 2.78 (1.75–3.82) | 0.95 | 0.81–1.10 |
| ADA or INF compared with ETA | 955/15,369 | 2.08 (1.88–2.29) | 565/10,568 | 2.23 (1.93–2.52) | 0.91 | 0.82–1.02 |
| ADA compared with INF | 368/8,584 | 2.00 (1.68–2.31) | 242/5,178 | 2.03 (1.67–2.39) | 1.06 | 0.89–1.26 |
| Psoriatic disease and ankylosing spondylitis | ||||||
| Anti-TNF-α compared with non-biologic comparison therapy | 111/4,368 | 0.97 (0.77–1.18) | 266/5,836 | 1.49 (1.12–1.85) | 0.81 | 0.61–1.06 |
| Inflammatory bowel disease*** | ||||||
| Anti-TNF-α compared with non-biologic comparison therapy | 134/3,329 | 2.14 (1.71–2.58) | 203/6,078 | 1.92 (1.60–2.23) | 1.12 | 0.85–1.46 |
INF, infliximab; ETA, etanercept; ADA, adalimumab.
Restricted to patients with propensity scores in the overlapping region. Patients are shown multiple times in the table if they had multiple autoimmune diseases. The 11% of patients contributing person-years to both the anti-TNF-α cohort and the nonbiologic comparison cohort are shown in both denominators.
In addition to propensity score decile, the analysis controlled for baseline dose of steroid during the 12-month look-back period, data system, calendar year of cohort entry, age, sex, race/ethnicity, urban/rural, smoking status, chronic pulmonary disease, Charlson co-morbidity index, and daily dose of steroid following index date as a time-dependent variable.
Etanercept is not indicated for IBD.
The mortality rate with anti-TNF-α did not differ from the comparator therapies in any of the disease groups [RA, aHR 0.93 (95% CI 0.85–1.03); PsA/PsO/AS, aHR 0.81 (95% CI, 0.61–1.06); IBD, aHR 1.12 (95% CI 0.85–1.46)].
In sensitivity analyses, we further evaluated the comparative mortality of any anti-TNF-α in RA patients. To assess the importance of switching therapy, we censored follow-up at the time of the switch, but the aHR did not change (aHR, 0.94 with 95% CI 0.85–1.03). Matching on the propensity score substantially reduced the number of subjects included in the analysis without changing the result to an important degree (aHR, 0.91 with 95% CI 0.76–1.10).
In head-to-head comparisons among RA patients, no statistically significant differences in risk were observed across anti-TNF-α agents (Table 3). The numbers of patients with PsA, PsO, or AS using anti-TNF-α drugs other than etanercept and the number with IBD using anti-TNF-α drugs other than infliximab was too small to allow meaningful comparisons of specific anti-TNF-α agent.
Results for vulnerable populations with RA are presented in Table 4. Anti-TNF-α therapy was associated with a reduction in mortality among RA patients with ≥2 co-morbid conditions (aHR, 0.87; 95% CI 0.77–0.99). Similar results for those aged ≥75 years (aHR, 0.89; 95% CI 0.76–1.03), or living in rural areas (aHR 0.87, 95% CI 0.72–1.05) were not statistically significant. The aHR did not differ appreciably for non-whites compared with the overall population.
Table 4.
Rheumatoid arthritis only: Adjusted Hazard Ratio (aHR) and 95% Confidence Interval (CI) for the Association of Anti-TNF-α Compared with Non-biologic Comparison Therapy with Mortality *
| Subgroup | Anti-TNF-α (N) | Non-biologic comparison (N) | aHR** | 95% CI |
|---|---|---|---|---|
| Overall | 21,260 | 10,651 | 0.94 | 0.86–1.04 |
| Non-white | 8,018 | 4,116 | 0.94 | 0.77–1.14 |
| Aged ≥75 years | 2,742 | 1,469 | 0.89 | 0.76–1.03 |
| ≥2 Comorbidities | 8,655 | 4,321 | 0.87 | 0.77–0.99 |
| Rural | 5,087 | 2,562 | 0.87 | 0.72–1.05 |
Restricted to patients with propensity scores in the overlapping region. Patients are shown multiple times in the table if they had multiple autoimmune diseases. The 11% of patients contributing person-years to both the anti-TNF-α cohort and the nonbiologic comparison cohort are shown in both denominators.
In addition to the propensity score, the analysis controlled for baseline dose of steroid during the 12-month look-back period, data system, calendar year, race, gender, age group, smoking status, chronic pulmonary disease, Charlson co-morbidity index, and daily dose of steroid following index date as a time-dependent variable.
DISCUSSION
We evaluated the association between anti-TNF-α therapy and mortality in a cohort of persons with autoimmune disease. The number of deaths among persons with prior anti-TNF-α use was 1,754, enabling detailed estimation of the association. Compared with RA patients with step-up from MTX therapy, we observed no association of anti-TNF-α use with mortality (HR 0.93, 95% CI 0.85–1.03) and lack of evidence of an increased risk associated with for anti-TNF-α use in other autoimmune diseases, although the HR for inflammatory bowel disease was 1.12 (95% CI, 0.85–1.03). In pre-specified subgroup analyses restricted to RA patients, the anti-TNF-α therapy was associated with reduced mortality among those with ≥2 co-morbidities. Mortality rates did not differ between the three anti-TNF-α drugs studied.
The design and results of the present study are broadly similar to those of a British cohort study, restricted to RA patients only, which also observed no association of anti-TNF-α therapy with mortality (8). The British study prospectively followed 12,672 patients starting anti-TNF-α therapy and 3,522 biologic-naive patients receiving disease modifying anti-rheumatic drugs (8). Similar to the present study, patients switching from the comparison cohort to the anti-TNF-α cohort contributed person-years to each, but the converse was not true. The study observed 856 deaths among the anti-TNF-treated patients during 4 years of follow-up. The HR for all-cause mortality was 0.86 (95% CI, 0.64–1.16) after accounting for baseline differences in age, sex, disease severity, disability, and comorbidity. Our study can also be compared with studies conducted in Sweden (4)and Spain (5) that reported a reduced mortality among RA patients treated with anti-TNF-α agents. In both studies, national registries of anti-TNF-α users were compared with geographically narrower cohorts of non-users that had been identified for separate studies, with the non-users quite possibly having less severe disease. The Swedish study reported an adjusted HR for death of 0.65 (95% CI 0.46 to 0.93) in those treated with anti-TNF-α versus those not treated, although the study controlled only for age, sex, disability and baseline co-morbidity. The Spanish study observed an standardized mortality ratio of 0.32 (0.20–0.53), with information on confounding factors being available for only 8% of patients and the anti-TNF-α cohort having lower smoking (9 vs. 31%) and possibly hypertension; therefore, these results may well have resulted from confounding. Our study has strengths relative to these earlier studies in terms of its sample size (1,754 deaths among anti-TNF-α users), use of comparison groups with active disease, use of propensity scores, evaluation of less common autoimmune diseases, and evaluation of vulnerable populations. However, despite the large study power, the study did not allow detailed assessment of mortality among those with less common autoimmune diseases.
Computerized data systems are essential for drug safety research and represent real-world experience; however, they have important limitations. Key limitations include (i) the lack of randomization of patients, (ii) incomplete information, and (iii) complicated patterns of use of drug therapies. We addressed confounding through use of propensity scores, restriction, adjustment, and careful selection of comparison groups. Use of comparison groups with severe autoimmune disease was intended to control for baseline risk of conditions that lead to death, such as cardiovascular disease. These diseases may be more common in RA and other autoimmune diseases. On the other hand, physicians may prescribe anti-TNF-α agents less frequently in patients with these conditions. Using comparison groups with similar severity of autoimmune disease was intended to minimize this bias. With respect to the length of observation and completeness of information, the data systems used for this study have relatively long follow-up compared with other data systems. Nonetheless, the length of observation in this analysis was quite short (mean, 2.48 years; median 1.69 years), so that it best portrays the experience in the few years immediately following the start of therapy for severe autoimmune disease. To assess the importance of drug switching and discontinuation, we conducted a sensitivity analysis, censoring follow-up at the time of the switch, but the aHR did not change. Death differs from other outcome variables, such as infection, in that it is the last event in the disease process, whereas for infection and most other outcomes, follow-up more typically ends at the diagnosis date. Biologic agents are expensive with high co-payments for some and may be discontinued at the end of life. Indeed, the number of people who died on therapy was only 36% of the total. For this reason, it was not practical for us to restrict the study to patients who remained on therapy at the time of death.
With respect to the study’s generalizability, the population studied here is comprised of patients with a chronic condition and over-represents those with Medicaid, many of whom have disabilities. The mortality rates observed in this study (ranging from 1 to 3 per 100 person-years) were higher than those in the general U.S. population (0.85 per 100 person-years) for 2002 (22), the midpoint of the observation period. In contrast, the adjusted mortality observed in the Rochester, Minnesota RA cohort during 1965–2005 was similar to the mortality rates we observed (2.4 per 100 person-years) (23). Although overall mortality in the Medicaid population has not been well described, 30-day mortality following hospitalization is elevated (24). Thus, the mortality rates we report are similar to or somewhat lower than expected.
In conclusion, this study found that anti-TNF-α therapy was not associated with higher mortality than control, non-biologic therapy in patients with a variety of autoimmune diseases. Similar results were observed in high risk patients such as the elderly and those with multiple co-morbidities and across all three anti-TNF-α drugs investigated. These results are reassuring, given that these therapies are highly effective at controlling symptoms and reducing disability and damage,.
Supplementary Material
Acknowledgments
On behalf of the SABER collaboration: Agency for Healthcare Research and Quality, Parivash Nourjah; Brigham and Women’s Hospital, Robert Glynn, Mary Kowal, Joyce Lii, Jeremy Rassen, Sebastian Schneeweiss; Food and Drug Administration, David Graham, Carolyn McCloskey, Rita Ouellet-Hellstrom, Kristin Phucas; University of Alabama at Birmingham, Elizabeth Delzell, Nivedita Patkar, Fenglong Xie; University of Pennsylvania, Kevin Haynes, Vanderbilt University, Carlos Grijalva, Ed Mitchel.
This work was supported by the Agency for Healthcare Research and Quality and the Food and Drug Administration, US Department of Health and Human Services as part of a grant (No. 1U18 HSO17919-0) administered through the Agency for Healthcare Research and Quality Centers for Education and Research in Therapeutics Program. Dr. Harrold receives support from the National Institutes for Health (AR053856). Dr. Curtis receives support from the National Institutes for Health (AR053351) and Agency for Healthcare Research and Quality (R01HS018517). The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by Agency for Healthcare Research and Quality, Food and Drug Administration, or Department for Health and Human Services.
We further acknowledge the Tennessee Bureau of TennCare of the Department of Finance and Administration and the Tennessee Department of Health, Office of Health Statistics, which provided the TennCare and vital status data.
References
- 1.Grijalva CG, Chen L, Delzell E, Baddley JW, Beukelman T, Griffin MR, Herrinton L, Liu L, Nourjah P, Ouellet-Hellstrom R, Patkar NM, Solomon DH, Winthrop KL, Lewis JD, Xie F, Saag KG, Curtis JR. Initiation of biologic DMARDs and the risk of hospitalization for infection in patients with autoimmune disease. JAMA. 2011;306:2331–9. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–85. doi: 10.1001/jama.295.19.2275. Review Erratum in: JAMA 2006 295 2482. [DOI] [PubMed] [Google Scholar]
- 3.Naranjo A, Sokka T, Descalzo MA, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther. 2008;10:R30. doi: 10.1186/ar2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobsson LT, Turesson C, Gulfe A, et al. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. The Journal of Rheumatology. 2005;32:1213–8. [PubMed] [Google Scholar]
- 5.Carmona L, Descalzo MA, Perez-Pampin E, Ruiz-Montesinos D, Erra A, Cobo T, Gómez-Reino JJ BIOBADASER and EMECAR Groups. All-cause and cause-specific mortality in rheumatoid arthritis are not greater than expected when treated with tumour necrosis factor antagonists. Ann Rheum Dis. 2007;66:880–5. doi: 10.1136/ard.2006.067660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576–82. doi: 10.1136/ard.2010.129916. [DOI] [PubMed] [Google Scholar]
- 7.Burmester GR, Mease PJ, Dijkmans BA, Gordon K, Lovell D, Panaccione R, Perez J, Pangan AL. Adalimumab safety and mortality rates from global clinical trials of six immune-mediated inflammatory diseases. Ann Rheum Dis. 2009;68:1863–9. doi: 10.1136/ard.2008.102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lunt M, Watson KD, Dixon WG, Symmons DP, Hyrich KL British Society for Rheumatology Biologics Register Control Centre Consortium, British Society for Rheumatology Biologics Register. No evidence of association between anti-tumor necrosis factor treatment and mortality in *patients* with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2010;62:3145–53. doi: 10.1002/art.27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidder H, Schnitzler F, Ferrante M, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501–8. doi: 10.1136/gut.2008.163642. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JD. Immortal time bias in estimates of mortality among infliximab-treated patients with Crohn’s disease. Gut. 2010;59:1586–7. doi: 10.1136/gut.2009.191015. [DOI] [PubMed] [Google Scholar]
- 11.Herrinton LJ, Curtis JR, Chen L, Liu L, Delzell E, Lewis JD, Solomon DH, Griffin MR, Quellet-Hellstom R, Beukelman T, Grijalva CG, Haynes K, Kuriya B, Lii J, Mitchel E, Patkar N, Rassen J, Winthrop KL, Nourjah P, Saag KG. Study Design for a Comprehensive Assessment of Biologic Safety Using Multiple Healthcare Data Systems. Pharmacoepidemiol Drug Saf. 2011;11:1199–209. doi: 10.1002/pds.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rassen JA, Solomon DH, Curtis JR, Herrinton L, Schneeweiss S. Privacy-maintaining propensity score-based pooling of multiple databases applied to a study of biologics. Med Care. 2010;48(6 Suppl):S83–9. doi: 10.1097/MLR.0b013e3181d59541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [Accessed February 15, 2011];Medicaid Analytic eXtract (MAX) General Information. http://www.cms.gov/MedicaidDataSourcesGenInfo/07_MAXGeneralInformation.asp.
- 14.Tennessee Government. [Accessed February 15, 2011];TennCare. http://www.tn.gov/tenncare/news.html.
- 15.State of New Jersey. Department of Health and Human Services. [Accessed February 15, 2011];Pharmaceutical Assistance to the Aged and Disabled (PAAD) http://www.state.nj.us/health/seniorbenefits/paad.shtml.
- 16.Pennsylvania Department of Aging. [Accessed February 15, 2011];Prescription Assistance (PACE) http://www.portal.state.pa.us/portal/server.pt/community/prescription_assistance/17942.
- 17.Kaiser Permanente Division of Research. [Accessed February 15, 2011];Member Health Survey. http://www.dor.kaiser.org/external/DORExternal/mhs/index.aspx.
- 18.Health and Human Services Dept., Centers for Disease Control and Prevention; and Centers for Medicare and Medicaid Services. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) 2010. [Google Scholar]
- 19.Sturmer T, Schneeweiss S, Brookhart MA, Rothman KJ, Avorn J, Glynn RJ. Analytic Strategies to Adjust Confounding using Exposure Propensity Scores and Disease Risk Scores: Nonsteroidal Antiinflammatory Drugs and Short-term Mortality in the Elderly. Am J Epidemiol. 2005;161:891–898. doi: 10.1093/aje/kwi106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garwood F. Fiducial limits for the Poisson distribution. Biometrika. 1936;28:437–442. [Google Scholar]
- 21.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–8. [Google Scholar]
- 22.Centers for Disease Control and Prevention. National Vital Statistics System. Worktable 23R. [Accessed July 14, 2011];Death Rates by 10-year age groups: United States and each State. 2002 http://www.cdc.gov/nchs/data/dvs/mortfinal2002_work23r.pdf.
- 23.Gonzalez A, Maradit Kremers H, Crowson CS, Nicola PJ, Davis JM, 3rd, Therneau TM, Roger VL, Gabriel SE. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;(56):3583–7. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 24.Lyon SM, Benson NM, Cooke CR, Iwashyna TJ, Ratcliffe SJ, Kahn JM. The Effect of Insurance Status on Mortality and Procedural Utilization in Critically Ill Patients. Am J Respir Crit Care Med. 2011;184:809–15. doi: 10.1164/rccm.201101-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


