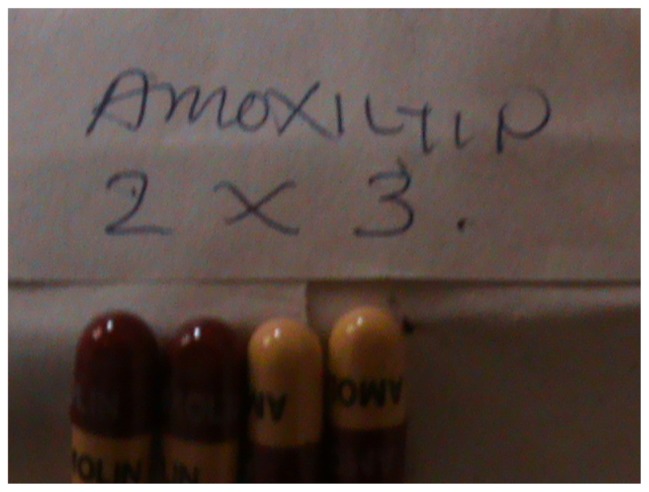Abstract
Background
Provision of pharmaceutical services in accredited drug-dispensing outlets (ADDOs) in Tanzania has not been reported. This study compared the antibiotics dispensing practice between ADDOs and part II shops, or duka la dawa baridi (DLDBs), in Tanzania.
Methodology
This was a cross-sectional study that was conducted in ADDOs and DLDBs. A simulated client method for data collection was used, and a total of 85 ADDOs, located in Mvomero, Kilombero, and Morogoro rural districts, were compared with 60 DLDBs located in Kibaha district. The research assistants posed as simulated clients and requested to buy antibiotics from ADDOs and DLDBs after presenting a case scenario or disease condition. Among the diseases presented were those requiring antibiotics and those usually managed only by oral rehydration salt or analgesics. The simulated clients wanted to know the antibiotics that were available at the shop. The posed questions set a convincing ground to the dispenser either to dispense the antibiotic directly, request a prescription, or refer the patient to a health facility. Proportions were used to summarize categorical variables between ADDOs and DLDBs, and the chi-square test was used to test for statistical difference between the two drug-outlet types in terms of antibiotic-dispensing practice.
Results
As many as 40% of trained ADDO dispensers no longer worked at the ADDO shops, so some of the shops employed untrained staff. A larger proportion of ADDOs than DLDBs dispensed antibiotics without prescriptions (P = 0.004). The overall results indicate that there was no difference between the two types of shops in terms of adhering to regulations for dispensing antibiotics. However, in some circumstances, eg, antibiotic sale without prescription and no referral made, for complicated cases, ADDOs performed worse than DLDBs. As many as 30% of DLDBs and 35% of ADDOs dispensed incomplete doses of antibiotics. In both ADDOs and DLDBs, fortified procaine penicillin powder was dispensed as topical application for injuries.
Conclusion
There was no statistical difference between ADDOs and DLDBs in the violation of dispensing practice and both ADDOs and DLDBs expressed poor knowledge of the basic pharmacology of antibiotics.
Keywords: antibiotic-dispensing practice, duka la dawa baridi, accredited drug-dispensing outlets
Introduction
Antibiotics are frontline weapons in the war against many infectious diseases. The success of antibiotics in combating diseases caused by microbes is a great achievement in modern medicine. During the past few decades, various medical practitioners have dramatically increased the habit of prescribing antibiotics for treatment of different infections. Overuse of antibiotics, and underuse such as failure to complete the treatment course, have led to the development and spread of antibiotic-resistant bacteria in the human environment.1–7 The current levels of resistance to antibiotics commonly used locally for empirical treatment are alarming. For instance, the emergence of strains producing extended spectrum beta lactamases and others exhibiting resistance to quinolones is on the increase and threatens the empirical use of cephalosporin and ciprofloxacin.8 Resistance of Vibrio cholerae to tetracycline was reported in Tanzania as early as 1980.9
Published reports indicate that irrational prescribing and dispensing of antibiotics in the developing world, particularly in Africa, is very common.10–16 Antibiotic-dispensing practices at various types of drug outlets in sub-Saharan countries have been reported as infringing a number of regulations. This includes misinformation on the use of these medicines, perhaps as a result of underqualified staff.17 In some medical practices, not prescribing antibiotics for a patient may negatively affect the clinician–patient relationship.18
The dispensing of antibiotics and other prescription-only medicines has been reported to be based on patient demand and not clinician’s assessment or prescription.19 The use of underqualified staff, illegal provision of certain drugs and services, and failure to meet quality standards and obtain official registration are among the common problems in many drug outlets in developing countries, including Tanzania.20–25
In Tanzania, there are three types of independent drug outlets in which medicines reach the consumer: part I shops (pharmacies); over-the-counter (OTC) or part II shops, known locally as duka la dawa baridi (DLDBs); and accredited drug-dispensing outlets (ADDOs). Part I pharmacies are supervised by registered pharmacists who are allowed to sell both prescription-only and OTC medicines. DLDBs are authorized to sell OTC drugs only, and, according to the regulations in Tanzania, are not authorized to sell prescription-only medicines, including antibiotics. However, the plan is to phase out this type of drug outlet by transforming all DLDBs to ADDO shops.
The quality of services provided by DLDB premises has been reported to be inadequate. The main challenges affecting the DLDB as a drug outlet include lack of qualified and trained personnel and lack of supervision leading to malpractice, such as selling prescription-only medicines.20–23 In an attempt to address these problems, the Tanzania Food and Drug Authority (TFDA), in 2003, embarked on the ADDO Program. Under this program, ADDOs were established to improve access to medicines and provision of quality medical service in rural areas. The program focused on improving the availability of essential medicines and the quality of services by working with independent shop owners and dispensing staff through provision of education, training and supervision, and commercial incentives combined with decentralized regulatory oversight.26 Under this program, a DLDB could be upgraded to an ADDO if it met specified quality criteria, including a training program of 26 days for drug sellers and 6 days for owners.26 After accreditation, the shop would then be allowed to sell a limited range of prescription-only essential medicines and receive additional support through regular supervision, refresher training, and commercial incentives, such as training in business skills and access to microfinance. A limited number of antibiotics are among the medicines allowed to be sold in an ADDO.
Based on the fact that the agency that established the ADDO is the TFDA, which is a national drug regulatory authority, a concern was raised about the effectiveness of this body in regulating the performance of drug outlets established by it.26 In this study, we compare the performance of ADDOs to DLDBs, from which the former evolved.
Methods
Study design
This was a cross-sectional explorative study conducted in ADDO and DLDB shops from January to March 2011. A simulated client (mystery shopper) method was used for data collection. Research assistants (RAs) used a checklist that was designed to assess dispensing practices of drug-dispensing personnel.
Study area
The study was conducted in the Mvomero, Kilosa, and Morogoro rural districts in the Morogoro Region, Tanzania. In addition, 60 DLDBs located in the rural district of Kibaha were studied for comparison. The choice of these districts was based on accessibility and availability of both ADDOs and DLDBs.
Data collection
A total of 145 shops composed of 85 ADDOs and 60 DLDBs were visited. We used two female RAs to obtain more information < in that a wider range of roles could be fulfilled, such as a mother with a baby, and to ensure consistency. Since we had nine case situations which needed to be assessed, our RAs separately visited the same shop on different days and each addressed four to five different case scenarios.
The RAs visited drug outlets, where they posed as a patient or guardian of a sick child. After presenting a case scenario or disease condition to an ADDO or DLDB, they requested buying antibiotics. Among the cases presented were those requiring antibiotics and those that could be managed by other drugs, such as oral rehydration salts, analgesics, antihistamines, expectorant cough syrups, and others. The RAs also pretended they wanted to open an ADDO business. The following are the nine case scenarios used by the simulated clients for data collection:
In the first scenario, the RA indicated that she had been coughing for the past 3 days and told the dispenser that she had once before experienced the same cough, which was cured by using an antibiotic. She then enquired about the types of antibiotics that were available in the shop. The RA wrote down all the names that were mentioned by the shop’s dispensing personnel and left the shop, promising to return.
The RA posed as a woman with a 4-year-old male child troubled with a headache and diarrhea and requested to buy ciprofloxacin, as she suspected that it could be typhoid.
The RA presented a self-written prescription of amoxicillin capsules, 500 mg every 8 hours for 5 days, and then indicated that she needed to buy only 10 capsules, which would be enough for her pneumonia.
The RA informed the drug dispenser that, that morning, her 6-year-old daughter had been injured on her left hip by a piece of metal. She then stated that, in such cases, she normally uses ampicillin capsules or applies procaine penicillin forte (PPF) powder to the injury, and therefore wanted to buy PPF powder.
The RA told the drug dispenser that she had a 3-week-old baby who was presenting with fever and diarrhea. She explained that this might be cholera, since some people in her neighborhood had been having the same problems for the past few weeks. She asked to buy tetracycline capsules for her baby, explaining that she would dissolve the powder in water.
The RA requested to buy chloramphenicol syrup for her 3-week-old neonate who had been vomiting and had frequent diarrhea. She told the drug dispenser that she suspected the baby had typhoid.
The RA mentioned that she had had a persistent cough for the past 2 days and suspected that she had contracted pneumonia. She explained that her husband, a nurse, normally injected her with PPF for 2 days followed by ampicillin capsules. She therefore requested to buy two vials of PPF injection powder and two ampoules of water for injection.
The RA presented herself as a patient with a urinary tract infection, complaining of yellowish urethral discharge with a bad smell. She told the drug dispenser that it was possibly gonorrhea. She requested to know the price of benzyl phenoxy penicillin injection. She enquired about the possibility of receiving an intramuscular injection of benzyl phenoxy penicillin in the shop. In the event the drug dispenser agreed to her request, she told the dispenser that she would return with the money to buy the medicine.
The RA entered the ADDO shop and initiated a conversation to identify whether the staff employees had been trained by the ADDO program and for how long. The RA explained that, being a business woman, she would not like to spend more than 3 months in training. Depending on the responses she received from the dispensing personnel during the conversation, the RA was able to identify whether the dispensing personnel and/or owner were trained.
Documentation of dispensing practice by the simulated client
The RAs had well-designed forms containing questions that were intended to elicit information with regard to the posed case scenario. Immediately after leaving the shop, the RA filled out a checklist with the responses.
Ethical considerations
The study was granted ethical clearance from Muhimbili University of Health and Allied Sciences Research and Publications Committee. The TFDA, being the trainer and mentor for the ADDO program, was not consulted for permission to conduct the study. Because of the nature of this study, drug dispensers were not informed in advance nor asked for consent to participate. All information obtained was kept strictly confidential, and none of the dispenser’s responses or practices was reported to the shop owners. No names of the dispensers were recorded in the checklist forms, and data were entered into the computer using only study code numbers.
Data analysis
We compared the proportion of ADDO and DLDB shops that were found to be violating the regulations for dispensing antibiotics. We used STATA software (v9; Stata Corp LP, College Station, TX, USA) to carry out a two-sample chi-square test for comparison of proportions (proportion test) between ADDO and DLBD shops.
Results
We visited 104 ADDOs in the three districts; 19 ADDOs were found to be inactive, of which eleven (58%) had converted to food stores and other small businesses. We also managed to assess 60 DLDB shops in Kibaha district that were not yet part of the ADDO program. Both ADDO and DLDB shops were selected based on their easy accessibility and availability of their services during our visits. In all studied districts, only 60% of the trained ADDO dispensers were still retained; the rest had left their jobs and had been replaced by untrained dispensers.
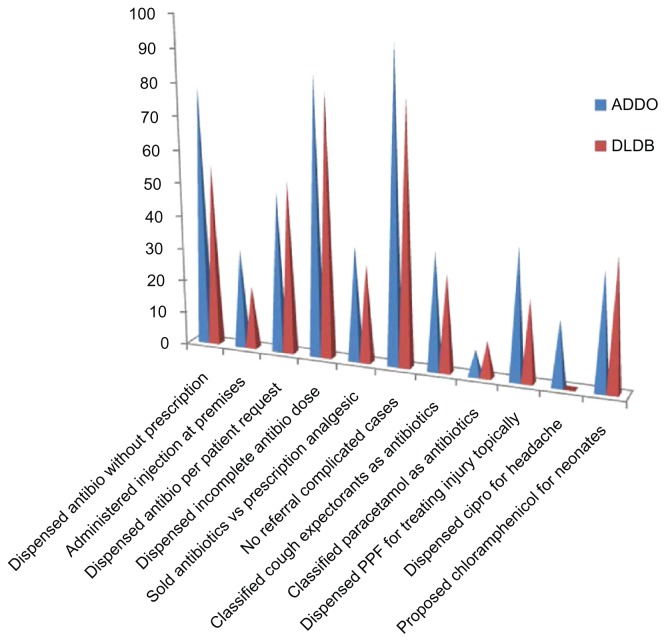
Tables 1 and 2 summarize the findings obtained from the ADDO and DLDB shops. Performance between the two types was similar in many cases; however, in some circumstances, eg, antibiotic sales without prescription and no referral made for complicated cases, the ADDOs performed worse than the DLDB shops. A larger proportion of ADDOs dispensed antibiotics without prescriptions (P = 0.004), and ADDOs had a higher incidence of not referring patients to health facilities (P = 0.008). For the most part, in both shops, antibiotics were verbally prescribed and, frequently, incomplete doses of antibiotics were dispensed. In addition, PPF powder was dispensed as a topical application to treat injuries in both shops. A statistically significant larger number of ADDOs dispensed ciprofloxacin to patients for a headache (Table 2). In both types of shops, the dispensers failed to differentiate the use of cough syrups containing expectorants and antihistamines from those containing antibiotics. Figure 1 summarizes the dispensing practices of antibiotics in the two types of shops, and Figures 2–5 show some of the purchased products from the shops.
Table 1.
Comparison of proportions between ADDOs and part II medical shops (DLDBs) in terms of antibiotic-dispensing practice
| Dispensing malpractice | DLDB (n = 60) % of shops | ADDO (n = 85) % of shops | P-value |
|---|---|---|---|
| Sold antibiotics (oral dosage and injectibles) without prescriptions | 55 | 79 | 0.004082 |
| Willing to administer benzyl phenoxy penicillin intramuscular injection at the premises | 19 | 30 | 0.140554 |
| Dispensed antibiotics to clients who asked for a drug without explanation of reasons | 53 | 49 | 0.766776 |
| Dispensed ten capsules of 250 mg amoxicillin for adults to clients who had a prescription for 5 days | 80 | 85 | 0.6061 |
| Offered antibiotics to clients instead of the analgesics shown in the prescription | 30 | 35 | 0.625518 |
| Did not refer a patient to a dispensary or hospital for a complicated case scenario | 80 | 95 | 0.008643 |
Abbreviations: ADDO, accredited drug dispensing outlet; DLDB, duka la dawa baridi.
Table 2.
Comparison of knowledge between ADDO and part II (DLDB) dispensers
| Personnel knowledge | DLDB (n = 60) % of shops | ADDO (n = 85) % of shops | P-value |
|---|---|---|---|
| Mentioned cough expectorant syrups as an antibiotic | 30 | 36 | 0.526697 |
| Mentioned paracetamol as an antibiotic | 11 | 8 | 0.686526 |
| Dispensed PPF as a topical for treating an injury | 25 | 40 | 0.088663 |
| Dispensed ciprofloxacin for headache | 0 | 20 | 0.000615 |
| Proposed chloramphenicol powder from capsules for neonates | 40 | 36 | 0.796689 |
| Proposed tetracycline powder from capsules for neonates | 34 | 37 | 0.831259 |
Abbreviations: ADDO, accredited drug dispensing outlet; DLDB, duka la dawa baridi; PPF, procaine penicillin forte.
Figure 1.
Summary of observed dispensing practices in ADDOs and part II medical shops (DLDBs) in the visited districts.
Abbreviations: ADDOs, accredited drug dispensing outlets; antibio, antibiotics; cipro, ciprofloxacin; DLDBs, duka la dawa baridi.
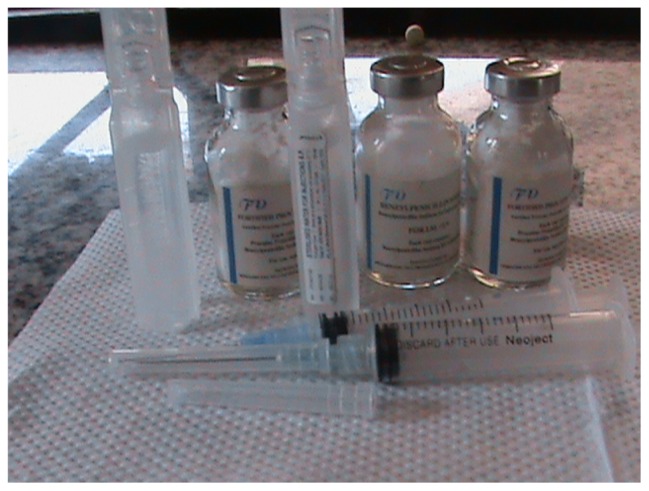
Figure 2.
An illustration of benzyl phenoxy penicillin with syringes bought from one of the ADDO shops without a prescription.
Note: The seller was willing to inject our simulated client at the ADDO premises.
Abbreviation: ADDO, accredited drug dispensing outlet.
Figure 5.
Four amoxicillin capsules as incomplete dose for an adult patient bought from a DLDB.
Abbreviation: DLDB, duka la dawa baridi.
Discussion
Unregulated prescribing and dispensing of prescription-only medicines is a common practice in developing countries, and this makes antibiotics vulnerable to developing resistance.1,9–13,25 The current study revealed the existence of irrational dispensing of antibiotics in both ADDO and DLDB shops (Figure 1), indicating that there has been little change in terms of dispensing practice by transforming DLDB shops into ADDOs. The simulated client (mystery shopper) method used in this study is a useful means of assessing health care provider behavior and practice in a first-hand way while minimizing observation bias.23,27
Poor knowledge on the basic pharmacology of antibiotics was substantiated by dispensing practices and misinformation provided by the dispensers. For instance, dispensers instructed the simulated client to apply PPF powder topically to treat fresh injuries. Some dispensers failed to distinguish cough expectorants from antibiotics and chlorpheniramine from cloxacillin; they also classified paracetamol as an antibiotic, and some were willing to dispense chloramphenicol and tetracycline to treat infections in neonates and headaches in adults. Tetracycline is contraindicated in neonates since it impairs teeth and bone formation, while chloramphenicol suppresses bone marrow, causing aplastic anemia, and may also cause gray baby syndrome.
We found no statistical significant difference (P = 0.141) between the two types of shops with respect to willingness to administer injections at the premises or selling injectable antibiotics without prescriptions. Despite an incidence of only 30%, this is a notable level since our sample size was small. Administration of injections in drug outlets has serious health risks as these premises do not have specific rooms for giving injections or a well-organized system for biological garbage disposal. Injection administration requires availability of trained personnel and a place to rest, if needed, following administration of an injection.
A notable malpractice detected in this study was an attempt to substitute other medicines for a given condition; this was particularly common when the prescribed medicine was not available in the visited shop. In this scenario, the dispenser tried to convince the patient to buy other products, claiming that they had the same indications. Because these personnel were untrained in medicinal use, this often led to providing misinformation on the correct medicine, as in the case where the ADDO dispenser substituted cloxacillin with chlorpheniramine claiming that they had the same spectra of indications. Misinformation about medications has also been reported in one study conducted in rural Ghana.17 A cadre of ADDO personnel were exposed to a mini-training session of only 26 days, so it was not surprising to find one proposing chlorpheniramine syrup as a substitute of cloxacillin syrup.
Inadequately trained personnel who are predominantly available in the ADDO and DLDB shops are major sources of misinformation and hence lead to irrational use of medicine.
The Tanzanian Food, Drugs, and Cosmetics Act of 2003 empowers TFDA with regulatory responsibility of all pharmaceuticals and food products in the country. However, in practice the implementation of drug retailer regulation has remained broadly unchanged. DLDB shops are allowed to stock basic medical supplies and OTC medicines but are not permitted to sell any prescription-only drugs, including all nontopical antibiotics and injectables of any kind. Despite this restriction and the seller’s knowledge of the prohibition, DLDB shops were found stocking and selling antibiotics.
Ideally, failure to comply with regulations could lead to the drug store being fined or closed down. To our knowledge, there is no single reported case in which the TFDA has impounded an ADDO infringing the drug regulations nor a penalty imposed due to regulatory violations. The TFDA is the lead agency for the ADDO program and is responsible for training, deploying, and conducting supportive supervision. The TFDA is a drug-regulatory body with powers to take legal action against those ignoring the drug regulations, but the TFDA may fail to take legal action against the ADDO due to conflict of interest. Consideration should be given to moving the responsibility for training within the ADDO program to training institutions. This would enable the TFDA to operate more effectively as a regulatory body and to more easily impose sanctions or penalties against ADDOs found infringing the drug regulations. Potential causes for frequent infringements of health-related regulations are poor knowledge of regulations, lack of inspections, lack of sanctions, successful concealment of regulatory violations, and the tacit permission of inspectors.22
The ADDO program was an attempt to reduce the problem of irrational dispensing of medicines by DLDB shops. Although some regulatory responsibilities have been transferred from district to ward-level officials, who would presumably be more aware of the shops’ day-to-day operations, the situation in the ADDOs appears to be worse than before the transformation from DLDBs.
In this study, we showed that there is a low retention rate of trained personnel at ADDOs, resulting in some services being provided by untrained personnel. If the retention of trained ADDO staff is not addressed, then the sustainability of an effective ADDO program is unlikely. The Government is now trying to expand the ADDO initiative with the aim to eventually eliminate all nonaccredited DLDBs, replacing them with ADDOs. It is essential that expansion of the program is tied to strengthening the mechanisms for monitoring and offering training and supportive supervision to enable these shops to provide high quality services. A recently published study indicated that part I pharmacies in Tanzania were mainly located in urban areas, with more than 50% in the commercial capital Dar es Salaam, leaving the rural areas underserved.28 Equipping the ADDOs with better trained dispensers would enhance the provision of quality service in such areas as well.
Conclusion
Despite training and supervision being provided to ADDOs, these shops ignore pharmaceutical regulations. In this study, both ADDOs and DLDBs dispensed antibiotics irrationally and all shops disregarded dispensing regulations of antibiotics, making the change from DLBD to ADDO counterproductive. The Pharmacy Council of Tanzania in Dar es Salaam has prepared a training curriculum for dispensers, in which those who completed secondary school education, including previously trained ADDO personnel, can undergo modular training of at least 1 year (as opposed to 26-day training as offered by the TFDA) to qualify as a dispenser (Pharmacy Council personnel, personal communication, March 2012). This will ensure an adequate training period for this type of cadre who, after completion of a 1-year course, can dispense drugs not only in ADDOs but also in higher ranking drug outlets.
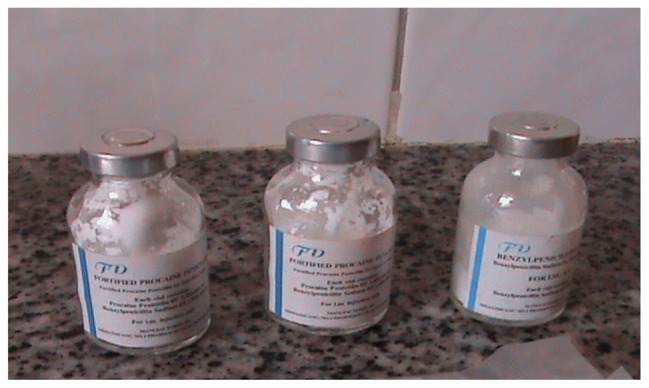
Figure 3.
Two vials of fortified procaine penicillin and one of benzyl phenoxy penicillin bought from a part II medical shop (DLDB) without a prescription.
Abbreviation: DLDB, duka la dawa baridi.
Figure 4.
Ciprofloxacin tablets bought from an ADDO upon request, without a prescription.
Abbreviation: ADDO, accredited drug dispensing outlet.
Acknowledgments
This study was financially supported by Sida through the Directorate of Research and Publication at Muhimbili University of Health and Allied Sciences through its small grants funding mechanism. We thank our research assistants for data collection. We also thank the dispensing personnel for cooperating with our research assistant even though this was a blind simulation method. Mr Ngaimisi provided data analysis support and is hereby acknowledged.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Goud SR, Nagesh L, Fernandes S. Are we eliminating cures with antibiotic abuse? A study among dentists. Niger J Clin Pract. 2012;15:151–155. doi: 10.4103/1119-3077.97291. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez CM, Schaeffer AJ. Treatment of urinary tract infection; what’s new and what works. World J Urol. 1999;6:372–382. doi: 10.1007/s003450050163. [DOI] [PubMed] [Google Scholar]
- 3.Hillier SL, Magee JT, Howard AJ, Palmer SR. How strong is the evidence that antibiotic use is risk factor for antibiotic resistant community – acquired urinary tract infection? J Antimicrob Chemother. 2002;50:241–247. doi: 10.1093/jac/dkf121. [DOI] [PubMed] [Google Scholar]
- 4.Rattanaumpawan P, Thamlikitkul V, Chokepaibulkit K, Lohsiriwat D, Aswapokee N. Vancomycin overuse in Siriraj Hospital. J Med Assoc Thai. 2006;89( Suppl 5):S125–S132. [PubMed] [Google Scholar]
- 5.Rattan A, Kumar A. Antibiotics – use and misuse. J Acad Hospital Admin. 1995;7:19–22. [PubMed] [Google Scholar]
- 6.Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 7.Epstein BJ, Gums JG, Drlica K. The changing face of antibiotic prescribing: the mutant selection window. Ann Pharmacother. 2004;38:1675–1682. doi: 10.1345/aph.1E041. [DOI] [PubMed] [Google Scholar]
- 8.Potz NA, Hope R, Warner M, Johnson AP, Livermore DM London and South East ESBL Project Group. Prevalence and mechanisms of cephalosporin resistance in Enterobacteriaciae in London and South East England. J Antimicrob Chemother. 2006;58:320–326. doi: 10.1093/jac/dkl217. [DOI] [PubMed] [Google Scholar]
- 9.Towner KJ, Pearson NJ, Mhalu FS, O’Grady F. Resistance to antimicrobial agents of Vibrio cholerae E1 Tor strains isolated during the fourth cholera epidemic in the United Republic of Tanzania. Bull World Health Organ. 1980;5:747–751. [PMC free article] [PubMed] [Google Scholar]
- 10.Mudur G. Drug resistant cholera in India attributed to antibiotic misuse. BMJ. 2000;321:1368–1369. [Google Scholar]
- 11.Trap B, Hansen EH. Cotrimoxazole prescribing by dispensing and non-dispensing doctors: do they differ in rationality? Trop Med Int Health. 2002;7:878–885. doi: 10.1046/j.1365-3156.2002.00923.x. [DOI] [PubMed] [Google Scholar]
- 12.Awad AI, Ball DE, Eltayeb IB. Improving rational drug use in Africa: the example of Sudan. East Mediterr Health J. 2007;13:1202–1211. doi: 10.26719/2007.13.5.1202. [DOI] [PubMed] [Google Scholar]
- 13.Olayemi SO, Akinyede AA, Oreagba AI. Prescription pattern at primary health care centres in Lagos State. Niger Postgrad Med J. 2006;13:220–224. [PubMed] [Google Scholar]
- 14.Kumar R, Indira K, Rizvi A, Rizvi T, Jeyaseelan L. Antibiotic prescribing practices in primary and secondary health care facilities in Uttar Pradesh, India. J Clin Pharm Ther. 2008;33:625–634. doi: 10.1111/j.1365-2710.2008.00960.x. [DOI] [PubMed] [Google Scholar]
- 15.Finch MJ, Morris JG, Jr, Kaviti J, Kagwanja W, Levine MM. Epidemiology of antimicrobial resistant cholera in Kenya and East Africa. Am J Trop Med Hyg. 1988;39:484–490. doi: 10.4269/ajtmh.1988.39.484. [DOI] [PubMed] [Google Scholar]
- 16.Okeke IN, Laxminarayan R, Bhutta ZA, et al. Antimicrobial resistance in developing countries. Part 1: Recent trends and current status. Lancet Infect Dis. 2005;5:481–493. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 17.Wolf-Gould C, Taylor N, Horwitz S, Barry M. Misinformation about medications in rural Ghana. Soc Sci Med. 1991;33:83–89. doi: 10.1016/0277-9536(91)90459-p. [DOI] [PubMed] [Google Scholar]
- 18.Buttler CC, Rollinck S, Pill R, Maggs-Rapport F, Stott N. Understanding the culture of prescribing: qualitative study of general practitioner’s and patients’ perception of antibiotics for sore throats. BMJ. 1998;317:637–642. doi: 10.1136/bmj.317.7159.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagashe GA, Minzi O, Matowe L. An assessment of dispensing practices in private pharmacies in Dar-es-Salaam, Tanzania. Int J Pharm Pract. 2011;19:30–35. doi: 10.1111/j.2042-7174.2010.00075.x. [DOI] [PubMed] [Google Scholar]
- 20.Kumaranayake L, Lake S, Mujinja P, Hongoro C, Mpembeni R. How do countries regulate the health sector? Evidence from Tanzania and Zimbabwe. Health Policy Plan. 2000;15:357–367. doi: 10.1093/heapol/15.4.357. [DOI] [PubMed] [Google Scholar]
- 21.Goodman C, Kachur SP, Abdulla S, Bloland P, Mills A. Drug shop regulation and malaria treatment in Tanzania – why do shops break the rules, and does it matter? Health Policy Plan. 2007;22:393–403. doi: 10.1093/heapol/czm033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fassin D. Illicit sale of pharmaceuticals in Africa: sellers and clients in the suburbs of Dakar. Trop Geogr Med. 1988;40:166–170. [PubMed] [Google Scholar]
- 23.Chalker J, Chuc NT, Falkenberg T, Do NT, Tomson G. STD management by private pharmacies in Hanoi: practice and knowledge of drug sellers. Sex Transm Infect. 2000;76:299–302. doi: 10.1136/sti.76.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stenson B, Syhakhang L, Ericksson B, Tomson G. Real world pharmacy: assessing the quality of private pharmacy practice in the Lao People’s Democratic Republic. Soc Sci Med. 2001;52:393–404. doi: 10.1016/s0277-9536(00)00142-8. [DOI] [PubMed] [Google Scholar]
- 25.Goel P, Ross-Degnan D, Berman P, Soumerai S. Retail pharmacies in developing countries: a behavior and intervention framework. Soc Sci Med. 1996;42:1155–1161. doi: 10.1016/0277-9536(95)00388-6. [DOI] [PubMed] [Google Scholar]
- 26.Sigonda-Ndomondo M. Accredited drug dispensing outlets: improving access to quality drugs and services in rural and peri-urban areas with few or no pharmacies. Paper presented at: Second International Conference on Improving Use of Medicines; July 2004; Chiang Mai, Thailand. [Google Scholar]
- 27.Aung T, Montagu D, Schlein K, Khine TM, McFarland W. Validation of a new method for testing provider clinical quality in rural settings in low- and middle-income countries: the observed simulated patient. PLoS One. 2012;7:e30196. doi: 10.1371/journal.pone.0030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ministry of Health and Social Welfare. Assessment of the Pharmaceutical Human Resources in Tanzania and The Strategic Framework. Dar es Salaam: Ministry of Health and Social Welfare; 2010. Available from: http://apps.who.int/medicinedocs/documents/s17397e/s17397e.pdf. Accessed. [Google Scholar]