Abstract
Background
Task-oriented therapies such as treadmill exercise can improve gait velocity after stroke, but slow velocities and abnormal gait patterns often persist, suggesting a need for additional strategies to improve walking.
Objectives
To determine the effects of a 6-week visually guided, impedance controlled, ankle robotics intervention on paretic ankle motor control and gait function in chronic stroke.
Methods
This was a single-arm pilot study with a convenience sample of 8 stroke survivors with chronic hemiparetic gait, trained and tested in a laboratory. Subjects trained in dorsiflexion–plantarflexion by playing video games with the robot during three 1-hour training sessions weekly, totaling 560 repetitions per session. Assessments included paretic ankle ranges of motion, strength, motor control, and overground gait function.
Results
Improved paretic ankle motor control was seen as increased target success, along with faster and smoother movements. Walking velocity also increased significantly, whereas durations of paretic single support increased and double support decreased.
Conclusions
Robotic feedback training improved paretic ankle motor control with improvements in floor walking. Increased walking speeds were comparable with reports from other task-oriented, locomotor training approaches used in stroke, suggesting that a focus on ankle motor control may provide a valuable adjunct to locomotor therapies.
Keywords: stroke, rehabilitation, anklebot, ankle robot, hemiparetic gait
Introduction
Stroke is the leading cause of disability in the United States, with more than 795 000 new cases reported annually.1 For many survivors, hemiparetic gait is a persistent problem that limits mobility and imposes higher energy demands for performing basic daily activities.2,3 Gait and balance deficits contribute to more than 70% of stroke survivors sustaining a fall within 6 months,4 leading to higher risks for hip and wrist fractures in the first year.5–7 Contrary to traditional models that forecast a recovery plateau within months after a stroke,8 there is growing evidence that new intervention strategies can reduce impairment and improve motor function well beyond that time frame.
Among these new approaches, advances in robotics technology provide additional opportunities to enhance high-volume training with the capacity to regulate intensity and record performances over time. Most research using robotics with stroke has focused on improving arm function, with varied levels of success.9–18 For example, a recent multisite clinical trial demonstrated that interactive upper extremity robotic training produced long-lasting improvement in impairment, disability, and quality of life, which was superior to usual care with no training at comparable health care cost.18 The development of lower extremity robotics has followed with mixed results. Some studies have shown promise,19 but others suggest no advantage for improving hemiparetic gait over equivalent durations of traditional physical therapy.20–22 At this early stage, more research is needed to establish the efficacy and optimal approaches for using lower extremity robotics in poststroke therapies.
Here we report initial results from using an impedance-controlled ankle robot in a seated paradigm to train the paretic ankle of persons with chronic stroke. The anklebot (Interactive Motion Technologies, Watertown, MA) has been the focus of considerable preclinical characterization, safety, and feasibility testing prior to this pilot training study and is described in detail elsewhere.23 Briefly, the device allows normal range of motion in all 3 degrees of freedom of the foot relative to the shank. It can provide independent, active assistance in dorsi/plantar flexion and inversion/eversion, with passive internal/external rotation. It is highly backdriveable (ie, easily gets out of the way) and can be mounted or removed from patients within 2 minutes. It can deliver continuous torque of approximately 17 Nm in either actuated degree of freedom.
The focus on the hemiparetic ankle is due in part to the prevalence of foot drop in early stance that results from diminished control of the paretic dorsiflexors. Dorsiflexor control of the foot is also essential to clear the ground during the swing phase of gait. Lack of proper control during these 2 phases increases the likelihood of trips and falls. Furthermore, in unimpaired subjects the ankle is the largest source of mechanical power during terminal stance,24 as plantarflexors stabilize the forefoot rocker action.25 The plantarflexors contribute as much as 50% of positive mechanical work in a single stride to enable forward propulsion.26–29 In preswing, plantarflexors also act to advance the leg into swing phase while promoting knee flexion at toe-off.25 Additionally, the ankle helps maintain body weight support during gait.30–32 Patients often compensate by walking with increased hip circumduction, to propel the leg forward while preventing the toes from catching on the ground during swing phase and initial contact.33 Given the mechanical power deficits in hemiparetic gait,34 improving paretic ankle control during walking may help reduce such compensatory responses and increase walking speed. With these issues in mind, the anklebot can be programmed for different training approaches, including an interactive mode that assists as needed when users are unable to perform the prescribed movement. Evidence from upper extremity robotics studies suggests that interactive training produces the greatest gains in paretic arm compared with either passive, sensorimotor (hand-over-hand), or resistive modes.35
This study reports findings from a 6-week interactive seated anklebot training program using stroke survivors with chronic hemiparetic gait. Our purpose was to assess the potential benefits of paretic ankle training on impairment and whether reducing impairment would translate into functional improvement in overground walking speed. We hypothesized that subjects with mild-to-moderate hemiparesis would successfully complete regular training sessions of up to 60-minute duration and that the training would reduce impairments and improve motor control at the paretic ankle, potentially enhancing independent gait function through increased walking velocity and changes in spatiotemporal gait parameters.
Methods
Subjects
A convenience sample consisting of 8 consecutive volunteers with chronic stroke was recruited to participate in a 6-week, 3-times-weekly training program with the anklebot. Recruitment and informed consent procedures were approved by the University of Maryland, Baltimore Institutional Review Board and the Baltimore Veterans Affairs Research and Development Committee, and the Massachusetts Institute of Technology Committee on the Use of Humans as Experimental Subjects.
Inclusion criteria were as follows: (1) index stroke >6 months prior in women or men aged 18 to 85 years; (2) residual hemiparetic gait deficits, operationally defined as reduced stance phase in the paretic leg and ambulatory with or without any assistive device36,37; (3) completion of all conventional physical therapy; (4) adequate language and neurocognitive function to participate in testing and training, operationally defined by capability to follow 3-step commands; and (5) paretic dorsiflexor manual muscle test ≥2 of 4, gravity neutral. Exclusion criteria were as follows: (1) fixed or painful contractures at the paretic ankle that impede participation; (2) modified Ashworth spasticity score >2 at paretic ankle; (3) cerebellar ataxia; (4) sensory deficits defined by loss of pro-prioception at the great toe (≥4 mm at distal interphalangeal joint by manual test) and/or hemisensory neglect on neurologic exam; (5) neurological history of (a) dementia (Mini-Mental Status Examination ≤23), (b) nonstroke neurological disorder restricting exercise, or (c) untreated major depression; and (6) other medical conditions that interfere with participation in a 6-week program of lower extremity training.
Anklebot Setup
The anklebot’s proximal attachment was mounted anterior to an orthopedic knee brace that was lined with foam pads and had cushioned straps. The distal attachments were secured to a modified orthopedic shoe. Additional protection was provided by pads where subjects indicated sensitivity to pressure and a snug fit in the shoe was aided with the use of foam insoles and socks as required. For the seated tests and training, subjects sat in a “barber’s” chair, with the knee brace secured to a mounting plate for support and immobilization of the knee (Figure 1). A seatbelt was secured around the pelvis to further limit proximal hip and thigh motion. The paretic leg rested at approximately 45° on a cushioned support with the heel placed on a base to provide a pivot point, thus isolating the foot so it could move freely about the ankle. After the set-up subjects were introduced to the video “racer game” that constituted the primary activity in subsequent training.
Figure 1. The anklebot is depicted here during seated training with video feedback.
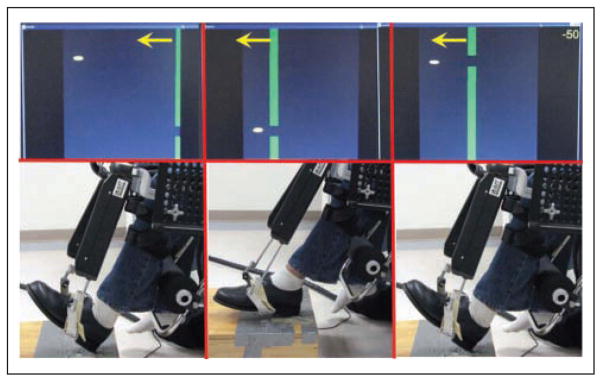
Arrows denote motion of vertical gates that serve as targets for the anklebot-controlled cursor. The knee brace is mounted to a fixed plate that supports the anklebot and restricts knee and hip motions, effectively isolating the ankle to move freely in either plantarflexion–dorsiflexion or inversion–eversion planes. The heel maintains contact with the base to provide a pivot for the foot.
Interactive Training
Training was visually evoked and guided (movement started at goal presentation and visual feedback was present during movement), with the goals displayed via a computer game played by moving the ankle in dorsiflexion or plantarflexion to guide a cursor through an approaching gate (see below). The interactive approach uses an impedance controller38 with similarities to the performance-based approach employed for the upper extremity.39 Here assistance is also provided only as needed, allowing subjects to reach targets unassisted if they are able. The controller tracks performance, and if the subject does not initiate the movement within 2 seconds or does not reach the target, the robot will provide assistive torques toward the target. The magnitude of assistance for a given trial is adjusted by changing the controller’s stiffness parameter, providing greater assistance if the cursor is farther away from the target.
A 6-week training program (3 times weekly for a total of 18 sessions) was conducted using the approach described above. Training sessions began with brief warm-ups by moving the paretic ankle in multiple directions, followed by plantarflexion–dorsiflexion training. The videogame required subjects to dorsiflex or plantarflex the paretic ankle to move a screen cursor “up or down” and pass through “gates” that approached from right to left at different vertical levels (Figure 1). Gate locations were predetermined for each subject, with highest and lowest locations scaled to 80% of dorsi– and plantar–active ranges of motion (AROMs), respectively. Two intermediate gate locations were set at approximately 40% of those ranges to give a total of four target locations that appeared in deterministic sinusoid-like sequences. Each session included 6 training blocks that followed an “easy-to-difficult” sequence where, as needed, the robot would deliver a stronger assist during the first 2 blocks (100 Nm/rad), reduced in the next 2 blocks (50 Nm/rad), and reduced further during the last 2 blocks (25 Nm/rad). One objective was to ensure an 80% success rate in one or more games to maintain subject enthusiasm, while providing a degree of challenge. These 6 blocks of 80 gates were preceded and followed by an abbreviated 40-gate racer game without any robotic assistance. Thus, each session amassed a total of 560 total targeted movements and was completed within 1 hour.
Assessments
Subjects were assessed at the beginning and completion of training with clinical and robot-based measures to evaluate ankle impairment and gait function. The robot-based assessment was also repeated at the 3-week midpoint. Clinical assessment included measurement of the paretic ankle AROMs, dorsiflexor strength, and self-selected overground walking. Dorsi- and plantar-AROMs were assessed with subjects’ legs and feet suspended to assess active ranges in a gravity environment, and goniometer measures taken from a neutral position defined at 90°.40 Dorsiflexor strength was measured with a handheld dynamometer (Chattilon, Largo, FL) placed on the dorsum of the foot adjacent to the metatarsal phalangeal joints. We focused initial strength measures on the dorsiflexors, consistent with the goal of retraining foot clearance and because dorsiflexors are often the more impaired ankle muscles after stroke. Average of AROMs and the peak force values from 3 trials were recorded.
Self-selected overground walking was performed on an 8-meter instrumented walkway (CIR Systems, Clifton, NJ) with at least 2 strides before the start and after the end for acceleration and deceleration. First and last steps were not included in the analyses to eliminate partial foot contacts at the extremes of the recording area. Spatiotemporal outcomes included mean speed (cm/s), stride lengths (cm), cadence (steps/min), and relative times in paretic single support and double support (%-cycle). All tests were repeated 3 times, allowing 1-minute rests between them, with the average across the 3 trials used for analysis. Anterior–posterior (A-P) propulsive impulses during paretic single support were derived from integrals of the A-P force profiles from a subset of 5 subjects who walked at self-selected speeds over a force plate (Bertec, Columbus, OH).
Robot-based metrics were calculated from positional data recorded by the robot41 during unassisted trials, each consisting of 40 targeted movements. These included average number of successful gate passages, peak and mean speed, and normalized jerk. Movement speed and acceleration were obtained from the first and second derivatives of position; the speed profiles were used to calculate mean and peak speed. Movement smoothness was characterized by mean jerk, that is, the average rate of change or first derivative of acceleration in a movement. Average jerk was divided by each subject’s peak speed to compute normalized jerk.
Statistical Analyses
Pre–post comparisons were tested with the nonparametric Wilcoxon sign rank test. Robot-based motor control variables were analyzed using the Kruskal–Wallis test with nonparametric multiple comparisons to detect significant differences across the 3 time points. The significance level was set at P < .05.
Results
Demographics of study participants are shown in Table 1. Subjects’ mean age was 62.4 ± 10.4 years and at the time of enrollment, they were 73 ± 37 months poststroke. Six subjects relied on some type of assistive device for ambulation. Based on self-report, wearing and using the anklebot in the seated context was tolerable for all participants. There was universal agreement that the robot could be worn comfortably for extended periods of exercise. All subjects completed the 18 sessions without any complications from fitting at the robot attachment points, nor were there any other adverse events.
Table 1.
Subject Characteristics
| ID# | Age (y)/Gender | Time Poststroke (mo) | Paretic Side | Assistive Device | Baseline Speed (cm/s) | Ashworth DF/PF |
|---|---|---|---|---|---|---|
| 1 | 53/F | 37 | R | AFO/SPC | 31.6 | 0/2 |
| 2 | 60/F | 89 | R | AFO | 71.9 | 1/1 |
| 3 | 75/M | 146 | R | SPC | 114.4 | 0/0 |
| 4 | 73/F | 84 | L | SPC | 28.7 | 1/1 |
| 5 | 66/M | 79 | L | AFO/4PC | 25.2 | 0/0 |
| 6 | 43/F | 60 | L | None | 68.1 | 0/0 |
| 7 | 64/F | 56 | R | None | 45.1 | 0/0 |
| 8 | 65/F | 29 | L | AFO/SPC | 26.7 | 2/1 |
Abbreviations: DF, dorsiflexion; PF, plantarflexion; M, male; F, female; R, right; L, left; AFO, ankle foot orthosis; SPC, single point cane; 4PC, quad cane.
Goniometry and Handheld Dynamometry
Mean paretic AROM in plantarflexion increased in 6 of the 8 subjects, from 125° ± 4° to 130° ± 4° (P < .05). Mean paretic AROM dorsiflexion increased from −7° ± 4° to −2° ± 3° but failed to achieve significance (P = .24). Isometric paretic dorsiflexion strength increased overall from 74.7 ± 15.9 N to 101.6 ± 11.0 N but failed to reach significance (P = .16).
Robot-Based Metrics
Improved paretic ankle motor control was seen in a number of metrics recorded during the unassisted racer games. As illustrated in Figure 2, there was substantial change toward smoother ankle movements. Analyses of trends across the baseline, 3-week, and 6-week time points are summarized in Table 2. All subjects increased the total number of successful gate passages at 3 weeks and made smaller additional gains by 6 weeks. Significant increases in the mean and peak velocities were observed at 6 weeks, with additional increases in peak values during the latter half of training. Subjects made smoother movements as well, characterized by decreases in normalized jerk over the 6 weeks but also significantly in the last 3 weeks of training.
Figure 2. Example of trajectory changes on 10 repetitions of a standard unassisted plantarflexion and dorsiflexion ankle targeting tasks before and after 6 weeks of performance-based ankle robot training.
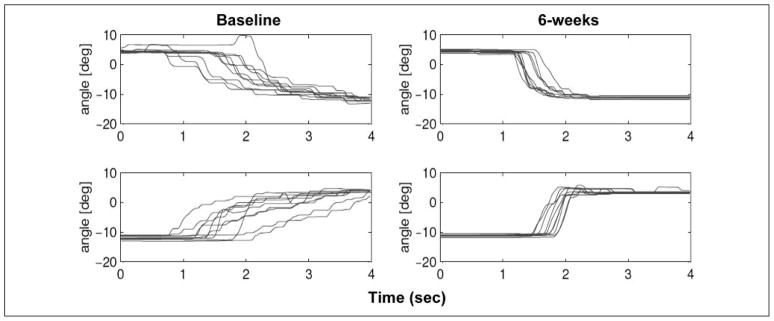
Note the improved consistency of responses, increased velocity (steeper slope), and smoother trajectories.
Table 2.
Robot-Based Variables at Baseline, 3 Weeks, and 6 Weeks of Ankle Robot Training (Mean ± SE) With Percentage Change at 6 Weeks
| Variable (Units) | Baseline | 3 Weeks | 6 Weeks | Change (%) |
|---|---|---|---|---|
| Successes (no. gates) | 21.6 ± 2.8 | 31.0 ± 2.4a | 34.3 ± 1.9b | 59 |
| Mean velocity (°/s) | 4.8 ± 0.5 | 6.9 ± 0.9 | 9.9 ± 1.7b | 106 |
| Peak velocity (°/s) | 41.4 ± 3.0 | 44.0 ± 2.9 | 48.2 ± 3.9c,d | 16 |
| Normalized jerk (s−2) | 303 ± 19 | 278 ± 21 | 209 ± 21b,d | −31 |
P < .05, baseline to 3 weeks.
P < .01, baseline to 6 weeks.
P < .05, baseline to 6 weeks.
P < .05, 3 to 6 weeks.
Gait Speed and Spatiotemporal Results
Results from the overground walking assessments are presented in Table 3. Subjects significantly increased the primary functional outcome of self-selected walking speed over the 6-week period of training. Analyses of spatiotemporal gait parameters showed that speed increased through a combination of longer stride lengths and faster cadence. Also of note were significantly longer percentage cycle time in paretic single support and reduced time in double support. The paretic A-P impulses were calculated from trials reflecting similar velocity increases to those reported for the spatiotemporal analysis. These impulses increased 18% in 4 of 5 participants after ankle robot training (pre, 32.4 ± 39.8 Ns/kg; post, 40.2 ± 32.6 Ns/kg).
Table 3.
Selected Spatiotemporal Gait Parameters Before and After 6 Weeks’ Ankle Robot Training (Mean ± SE)
| Variable (Units) | Baseline | 6 Weeks | Change (%) | P |
|---|---|---|---|---|
| Walking speed (cm/s) | 51.4 ± 11.1 | 61.7 ± 10.9 | 20 | .032 |
| Stride length (cm) | 78.2 ± 10.5 | 86.3 ± 9.3 | 10 | .048 |
| Cadence (steps/min) | 75.3 ± 7.5 | 83.4 ± 8.1 | 11 | .045 |
| Paretic: Single support (%) | 21.1 ± 2.4 | 24.2 ± 2.4 | 15 | .033 |
| Double support (%) | 46.6 ± 4.6 | 40.3 ± 4.0 | −14 | .010 |
Discussion
This study provides evidence that suggests the potential for seated visuomotor ankle robot training to improve chronic hemiparetic gait velocity with concomitant gains in multiple indices of paretic ankle motor control, including speed, accuracy, and smoothness. Time profile analysis reveals that control of targeting accuracy increased during the first 3 weeks, whereas maximum improvements in mean and peak velocities and normalized jerk were made in the last 3 weeks. The 20% increase in overground walking velocity suggests that seated robotics training to reduce ankle impairments may translate into improved functional mobility.
Paretic Ankle Motor Control
There were marked gains in paretic ankle motor control indexed by increased targeting accuracy, speed, and smoothness of unassisted movements in the dorsiflexion–plantarflexion range during unassisted movements. This is similar to training results from upper extremity robotics therapy, where the mean jerk values were reduced significantly for chronic stroke but not for subacute stroke.41 These improved motor control metrics suggest neural plasticity and motor learning in the chronic hemiparetic condition. Analyses of trends in the selected motor control variables showed that gains in target accuracy tended to occur before significant increases in movement speed and smoothness. In unimpaired subjects, Perez et al42 showed that short-term motor skill ankle training increased cortical excitability to the tibialis anterior, whereas equal amounts of unskilled and passive ankle training did not. The increased excitability was associated with reduced errors on an ankle motor performance task, indicating improved motor control of ankle musculature. If a similar mechanism is evoked by the anklebot training in our subjects this may contribute to improved walking speeds.
Gait Performance
The main functional result was the significant increase in overground walking speed with isolated robotic training of the paretic ankle in a seated, non–weight-bearing position. Analysis of functional categories based on walking speeds43 showed that of the 8 subjects, 2 transitioned from being home ambulators (<0.4 m/s) to being limited community ambulators, whereas 2 other subjects went from being limited community ambulators (≥0.4 and ≤0.8 m/s) into being community ambulators (≥0.8 m/s). These results challenge prevailing views that task specificity in motor skill training is the optimal or requisite model for designing neurorehabilitation interventions.44 It is possible that separately addressing fundamental impairments such as ankle motor control may provide an important piece in the therapist’s treatment arsenal, and these findings are similar to some of the results obtained previously for the upper extremity.45,46
To probe this possibility, we analyzed propulsive impulses during paretic single support in a subset of 5 participants. Paretic propulsion increased in 4 of the participants after ankle robot training. Although these data are limited, they suggest that the seated anklebot training may have enhanced paretic contributions to propulsion and increased gait velocity. Future studies need to include these and other measures (eg, electro-myography) to better discern the role of the paretic ankle in gait changes.
The effects of seated ankle robot training on gait function compare favorably with those from a number of task-oriented locomotor interventions. A recent comparison between robotic partial body weight–supported treadmill training (BWSTT; eg, Lokomat) and therapist-assisted BWSTT therapy showed similar gains to those reported here (0.07 and 0.13 m/s, respectively), although those were achieved with 12 sessions versus the 18 used here.20 Notably in that study, subjects receiving robotics-assisted training did not improve their paretic single support duration whereas subjects receiving therapist-assisted training improved from 20% to 22% of the gait cycle, similar to results reported in this study (21% to 24%). A similar range for the Lokomat training was observed in another 4-week/12-session study.21 For subacute stroke, a 9-week pilot crossover design employing Lokomat and conventional therapy showed an overall improvement in 10-meter walk speeds from 0.13 to 0.27 m/s, a range comparable to the current findings.22 To note, the same group failed to replicate and observe any differences between robotics and conventional training in a subsequent larger randomized clinical trial.47 Peurala et al48 employed the Gait Trainer I with chronic stroke patients for 3 weeks (fifteen 20-minute sessions) and observed speed gains in self-selected 10-meter walks from 0.25 to 0.33 m/s, slightly less than the gains found with the seated anklebot approach. It is worth noting that more substantial velocity gains (0.31 m/s) were reported by Pohl et al49 from combining robotic gait training with conventional therapy in a group of very impaired subjects in the early subacute phase after stroke. Some of the improvement was likely because of natural recovery; however, this group performed significantly better than a cohort receiving only conventional therapy. Our earlier work showed that 6 months of treadmill exercise improved 10-meter walking speed in subjects with chronic stroke by 17%,36 compared with the 20% increase after only 6 weeks with the ankle robot. Taken together, distinct locomotor training approaches have produced about the same degree of overground speed improvement demonstrated in the current pilot study. Yet not all of those studies have shown significant improvements in spatiotemporal gait metrics such as paretic single support.
Using the Robot to Structure Practice and Feedback for Motor Learning
Participants’ reactions to training were very positive, with the videogame format reported as engaging, which could optimize compliance and outcomes.50 Incremental reduction of robotic support across training was well tolerated, introducing increasing challenge without causing undue frustration. This may be further refined, drawing on the performance-based approach in upper extremity robotics41 and the challenge-point framework for structure of practice,51 which proposes that motor skill learning is best mediated by an optimal mix of task difficulty versus skill (deficit) level to enhance learning and neuroplasticity responses. This challenge-point framework approach, used successfully in motor learning studies in Parkinson’s disease,52 could be used to customize the levels of challenge in ankle robotics protocols. This also is consistent with the view that modulating the level of challenge in training may be crucial for eliciting neuroplasticity responses.42
The nature of feedback is also highlighted in a recent report that visual virtual reality and verbal augmentation of isolated ankle robotics training improves overground walking velocity in chronic hemiparetic stroke, whereas robotics training alone does not.53 It emphasizes the importance of an enriched learning environment. Thus, further studies are needed to investigate feedback paradigms to enhance the functional benefits of robotic training, such as use of contextual interference and delayed or irregular feedback,54 and varying practice schedules to emphasize different task features such as speed and torque requirements, predictability of target locations, and multidirectional movements.
Study Limitations
This pilot study was designed to assess the safety and feasibility for using an impedance-controlled modular ankle robot during seated visuomotor training. The sample size was small, limiting conclusions about generalizability to the wider realm of stroke deficits. The lack of multiple baseline tests and comparisons to a control group require that the results be interpreted with caution. Another potential source of bias stems from self-selection of these participants compared to the general population, as they had previously participated in other research studies. Absence of retention measures prevents assessing the durability of performance gains, and establishing translation into community activity would be enhanced with portable home-based step monitoring. We also limited training to the dorsi–plantar ranges, leaving out the potential benefits of training inversion–eversion movements to improve control of the mediolateral stabilizer muscles. Incorporating these additional degrees of freedom into the training may reduce impairments further and enhance functional outcomes, especially in balance. Future studies are planned to investigate potential for seated anklebot training in the earlier stages of stroke recovery as an adjunct to standard physical therapy, treadmill and overground training, and to test different motor learning paradigms.
Conclusion
This study has demonstrated promise for the use of a modular impedance controlled anklebot in the treatment of post-stroke hemiparesis. Seated anklebot training had a significant impact in reducing ankle impairment and a concomitant improvement in gait function. The positive experiences of users in the chronic phase of stroke suggest that the device may be useful in earlier phases of stroke rehabilitation, either alone or in combination with other therapies, especially when weight-bearing activities may be limited. Beyond the realm of intervention development, the anklebot has additional potential as a means to probe the central nervous system control of the lower extremity and to study the effects of varied feedback and motivation on motor learning.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was funded by the Department of Veterans Affairs Rehabilitation Research and Development Service (VA RR&D) “Center of Excellence on Task-Oriented Exercise and Robotics in Neurological Diseases,” B3688R; VA RR&D Advanced Career Development Award, B3390K; NIH-NIA Claude D. Pepper OAIC Pilot Study Award, P30 AG028747; and the Department of Veterans Affairs and Veterans Affairs Medical Center Baltimore Geriatric Research, Education and Clinical Center (GRECC).
Footnotes
Reprints and permission: http://www.sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The author(s) declared a potential conflict of interest (e.g. a financial relationship with the commercial organizations or products discussed in this article) as follows: Dr. H. I. Krebs is a co-inventor in the MIT patents for the robotic devices. He holds equity positions in Interactive Motion Technologies, Inc., the company that manufactures this type of technology under license to MIT.
References
- 1.American Heart Association. Heart Disease and Stroke Statistics– 2009 Update. http://www.americanheart.org/statistics.
- 2.Macko RF, Smith GV, Dobrovolny CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. 2001;82:879–884. doi: 10.1053/apmr.2001.23853. [DOI] [PubMed] [Google Scholar]
- 3.Silver KH, Macko RF, Forrester LW, Goldberg AP, Smith GV. Effects of aerobic treadmill training on gait velocity, cadence, and gait symmetry in chronic hemiparetic stroke: a preliminary report. Neurorehabil Neural Repair. 2000;14:65–71. doi: 10.1177/154596830001400108. [DOI] [PubMed] [Google Scholar]
- 4.Forster A, Young J. Incidence and consequences of falls due to stroke: a systematic inquiry. BMJ. 1995;311:83–86. doi: 10.1136/bmj.311.6997.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramnemark A, Nyberg L, Borssén B, Olsson T, Gustafson Y. Fractures after stroke. Osteoporos Int. 1998;8:92–95. doi: 10.1007/s001980050053. [DOI] [PubMed] [Google Scholar]
- 6.Dennis MS, Lo KM, McDowall M, West T. Fractures after stroke: frequency, types, and associations. Stroke. 2002;33:728–734. doi: 10.1161/hs0302.103621. [DOI] [PubMed] [Google Scholar]
- 7.Kanis J, Oden A, Johnell O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke. 2001;32:702–706. doi: 10.1161/01.str.32.3.702. [DOI] [PubMed] [Google Scholar]
- 8.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27–32. doi: 10.1016/s0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 9.Aisen ML, Krebs HI, Hogan N, McDowell F, Volpe BT. The effect of robot-assisted therapy and rehabilitative training on motor recovery following a stroke. Arch Neurol. 1997;54:443–446. doi: 10.1001/archneur.1997.00550160075019. [DOI] [PubMed] [Google Scholar]
- 10.Volpe BT, Krebs HI, Hogan N. Robot-aided sensorimotor training in stroke rehabilitation. Adv Neurol. 2003;92:429–433. [PubMed] [Google Scholar]
- 11.Ferraro M, Palazzolo JJ, Krol J, Krebs HI, Hogan N, Volpe BT. Robot-aided sensorimotor arm training improves outcome in patients with chronic stroke. Neurology. 2003;61:1604–1607. doi: 10.1212/01.wnl.0000095963.00970.68. [DOI] [PubMed] [Google Scholar]
- 12.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair. 2008;22:111–121. doi: 10.1177/1545968307305457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Emken JL, Cramer SC, Reinkensmeyer DJ. Learning to perform a novel movement pattern using haptic guidance: slow learning, rapid forgetting, and attractor paths. In. Proceedings of the 2005 IEEE 9th International Conference on Rehabilitation Robotics; 2005. pp. 37–40. [Google Scholar]
- 14.Patton JL, Mussa-Ivaldi FA, Rymer WZ. Altering movement patterns in healthy and brain-injured subject via custom designed robotic forces. Proceedings of the Annual International Conference: IEEE Engineering Medicine Biology. 2001;2:1356–1359. [Google Scholar]
- 15.Housman S, Scott K, Reinkensmeyer D. A randomized controlled trial of gravity-supported, computer-enhanced arm exercise for individuals with severe hemiparesis. Neurorehabil Neural Repair. 2009;23:505–514. doi: 10.1177/1545968308331148. [DOI] [PubMed] [Google Scholar]
- 16.Lum PS, Burgar CG, Van der Loos M, Shor PC, Majmundar M, Yap R. MIME robotic device for upper-limb neurorehabilitation in subacute stroke subjects: a follow-up study. J Rehabil Res Dev. 2006;43:631–642. doi: 10.1682/jrrd.2005.02.0044. [DOI] [PubMed] [Google Scholar]
- 17.Hesse S, Schulte-Tigges G, Konrad M, Bardeleben A, Werner C. Robot-assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic subjects. Arch Phys Med Rehabil. 2003;84:915–920. doi: 10.1016/s0003-9993(02)04954-7. [DOI] [PubMed] [Google Scholar]
- 18.Lo A, Guarino PD, Richards LG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362:1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehrholz J, Werner C, Kugler J, Pohl M. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2007;(4):CD006185. doi: 10.1002/14651858.CD006185.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke. 2008;39:1786–1792. doi: 10.1161/STROKEAHA.107.504779. [DOI] [PubMed] [Google Scholar]
- 21.Westlake KP, Patten C. Pilot study of Lokomat versus manual-assisted treadmill training for locomotor recovery post-stroke. J Neuroeng Rehabil. 2009;6:18. doi: 10.1186/1743-0003-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayr A, Kofler M, Quirbach E, Matzak H, Fröhlich K, Saltuari L. Prospective, blinded, randomized crossover study of gait rehabilitation in stroke patients using the Lokomat gait orthosis. J Neurorehabil Neural Repair. 2007;21:307–314. doi: 10.1177/1545968307300697. [DOI] [PubMed] [Google Scholar]
- 23.Roy A, Krebs HI, Williams DJ, et al. Robot-aided neurorehabilitation: a novel robot for ankle rehabilitation. IEEE Trans Robotics. 2009;25:569–582. [Google Scholar]
- 24.Robertson DGE, Winter DA. Mechanical energy generation, absorption, and transfer amongst segments during walking. J Biomech. 1980;13:845–854. doi: 10.1016/0021-9290(80)90172-4. [DOI] [PubMed] [Google Scholar]
- 25.Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992. [Google Scholar]
- 26.Sawicki GS, Ferris DP. A pneumatically powered knee-ankle-foot orthosis (KAFO) with myoelectric activation and inhibition. J Neuroeng Rehabil. 2009;6:23. doi: 10.1186/1743-0003-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eng JJ, Winter DA. Kinetic analysis of the lower limbs during walking: what information can be gained from a three-dimensional model? J Biomech. 1995;28:753–758. doi: 10.1016/0021-9290(94)00124-m. [DOI] [PubMed] [Google Scholar]
- 28.Teixeira-Salmela LF, Nadeau S, Milot MH, Gravel D, Requiao LF. Effects of cadence on energy generation and absorption at lower extremity joints during gait. Clin Biomech. 2008;23:769–778. doi: 10.1016/j.clinbiomech.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Umberger BR, Martin PE. Mechanical power and efficiency of level walking with different stride rates. J Exp Biol. 2007;210:3255–3265. doi: 10.1242/jeb.000950. [DOI] [PubMed] [Google Scholar]
- 30.Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34:1387–1398. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- 31.Meinders M, Gitter A, Czerniecki JM. The role of ankle plantar flexor muscle work during walking. Scand J Rehabil Med. 1998;30:39–46. doi: 10.1080/003655098444309. [DOI] [PubMed] [Google Scholar]
- 32.Gottschall JS, Kram R. Energy cost and muscular activity required for propulsion during walking. J Appl Physiol. 2003;94:1766–1772. doi: 10.1152/japplphysiol.00670.2002. [DOI] [PubMed] [Google Scholar]
- 33.Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: Characteristics. Gait Posture. 1996;4:136–148. [Google Scholar]
- 34.Olney SJ, Griffin MP, Monga TN, McBride ID. Work and power in gait of stroke patients. Arch Phys Med Rehabil. 1991;72:309–314. [PubMed] [Google Scholar]
- 35.Hogan N, Krebs HI, Rohrer B, et al. Motions or muscles? Some behavioral factors underlying robotic assistance of motor recovery. J Rehabil Res Dev. 2006;43:605–618. doi: 10.1682/jrrd.2005.06.0103. [DOI] [PubMed] [Google Scholar]
- 36.Macko RF, Ivey F, Forrester LW, et al. Treadmill training improves fitness and ambulatory function in chronic stroke patients. Stroke. 2005;36:2206–2211. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 37.Luft AR, Macko RF, Forrester LW, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke. 2008;39:3341–3350. doi: 10.1161/STROKEAHA.108.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norkin C, White D. Measurement of Joint Motion: A Guide to Goniometry. Philadelphia, PA: F. A. Davis; 1985. [Google Scholar]
- 39.Rohrer B, Fasoli S, Krebs HI, et al. Movement smoothness changes during stroke recovery. J Neurosci. 2002;22:8297–8304. doi: 10.1523/JNEUROSCI.22-18-08297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hogan N. Adaptive control of mechanical impedance by coactivation of antagonist muscles. IEEE Trans Automat Control. 1984;AC-29:681–690. [Google Scholar]
- 41.Krebs HI, Palazzolo JJ, Dipietro L, et al. Rehabilitation robotics: performance-based progressive robot-assisted therapy. Auton Robots. 2003;15:7–20. [Google Scholar]
- 42.Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- 43.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 44.NINDS Stroke Progress Review Group. http//www.ninds.nih.gov/find_people/groups/stroke_prg/09_2006_stroke_prg.report.htm.
- 45.Krebs HI, Mernoff S, Fasoli SE, Hughes R, Stein J, Hogan N. Transport of the arm and manipulation of objects in chronic stroke: a pilot study. NeuroRehabilitation. 2008;23:81–87. [PMC free article] [PubMed] [Google Scholar]
- 46.Conroy S, Bever C, Krebs HI. Rehabilitation robotics: overview and controversy on spatial movements. Evaluation des Méthodes de Rééducation at the 22nd Entretiens Annuels des Fondation Garches; Paris, France: Frison-Roche; 2006. [Google Scholar]
- 47.Saltuari L. Deep brain stimulation. Paper presented at: 5th World Congress for Neurorehabilitation (WCNR); September 24–27, 2008; Brasilia, Brazil. [Google Scholar]
- 48.Peurala SH, Tarkka IM, Pitkänen K, Sivenius J. The effectiveness of body weight-supported gait training and floor walking in patients with chronic stroke. Arch Phys Med Rehabil. 2005;86:1557–1564. doi: 10.1016/j.apmr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Pohl M, Werner C, Holzgraefe M, et al. Repetitive locomotor training and physiotherapy improve walking and basic activities of daily living after stroke: a single-blind, randomized multicenter trails (DEutsche GAngtrainerStudie, DEGAS) Clin Rehabil. 2007;21:17–27. doi: 10.1177/0269215506071281. [DOI] [PubMed] [Google Scholar]
- 50.Schmid M. Reinforcing motor re-training and rehabilitation through games: a machine-learning perspective. Front Neuroeng. 2009;2:3. doi: 10.3389/neuro.16.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guadagnoli MA, Lee TD. Challenge point: a framework for conceptualizing the effects of various practice conditions in motor learning. J Mot Behav. 2004;36:212–224. doi: 10.3200/JMBR.36.2.212-224. [DOI] [PubMed] [Google Scholar]
- 52.Onlaor S, Winstein CJ. Determining the optimal challenge point for motor skill learning in adults with moderately severe Parkinson’s disease. Neurorehabil Neural Repair. 2008;22:385–395. doi: 10.1177/1545968307313508. [DOI] [PubMed] [Google Scholar]
- 53.Mirelman A, Bonato P, Deutsch JE. Effects of training with a robot-virtual reality system compared with a robot alone on the gait of individuals after stroke. Stroke. 2009;40:169–174. doi: 10.1161/STROKEAHA.108.516328. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt RA, Lee T. Motor Control and Learning: A Behavioral Emphasis. Champaign, IL: Human Kinetics; 2005. [Google Scholar]


