Abstract
Background
Glomerular filtration rate (GFR) is considered the best measure of kidney function, but repeated assessment is not feasible in most research studies.
Study Design
Cross-sectional study of 1,433 participants from the Chronic Renal Insufficiency Cohort (CRIC) Study (i.e., the GFR subcohort) to derive an internal GFR estimating equation using a split sample approach.
Setting & Participants
Adults from 7 US metropolitan areas with mild to moderate chronic kidney disease; 48% had diabetes and 37% were black.
Index Test
CRIC GFR estimating equation
Reference Test or Outcome
Urinary 125I-iothalamate clearance testing (measured GFR)
Other Measurements
Laboratory measures including serum creatinine and cystatin C, and anthropometrics
Results
In the validation dataset, the model that included serum creatinine, serum cystatin C, age, gender, and race was the most parsimonious and similarly predictive of mGFR compared to a model additionally including bioelectrical impedance analysis phase angle, CRIC clinical center, and 24-hour urinary creatinine excretion. Specifically, the root mean square errors for the separate model were 0.207 vs. 0.202, respectively. The performance of the CRIC GFR estimating equation was most accurate among the subgroups of younger participants, men, non-blacks, non-Hispanics, those without diabetes, those with body mass index <30 kg/m2, those with higher 24-hour urine creatinine excretion, those with lower levels of high-sensitivity C-reactive protein, and those with higher mGFR.
Limitations
Urinary clearance of 125I-iothalamate is an imperfect measure of true GFR; cystatin C is not standardized to certified reference material; lack of external validation; small sample sizes limit analyses of subgroup-specific predictors.
Conclusions
The CRIC GFR estimating equation predicts measured GFR accurately in the CRIC cohort using serum creatinine and cystatin C, age, gender, and race. Its performance was best among younger and healthier participants.
Index words: glomerular filtration rate (GFR), kidney function, GFR estimation
An extensive body of information suggests that glomerular filtration rate (GFR) is the best overall index of kidney function.1-3 GFR is operationally measured as the clearance rate of a filtration marker from the plasma by the kidneys, but clearance tests are cumbersome and costly. In clinical practice and large-scale research projects, more convenient methods, such as determinations of serum creatinine and timed urinary creatinine clearance, have been used for diagnosis of reduced kidney function and for monitoring disease progression.4-10 However, factors other than GFR, including the production, tubular secretion, and extrarenal elimination of creatinine influence the serum concentration of this compound. Therefore, use of serum creatinine or creatinine clearance determinations alone may not always accurately estimate kidney function.1-3;7;8;10
GFR estimating equations that include demographic parameters and serum creatinine are available online, and are used extensively clinically and in research studies.6;11-13 Incorporation of measures of body size, creatinine generation, inflammation, and/or cystatin C into these estimating equations may address some of the limitations of serum creatinine-based equations. Improvements in GFR estimation were reported in several populations using equations that incorporate both serum creatinine and cystatin C.14-16
One of the main objectives of the ongoing National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-sponsored Chronic Renal Insufficiency Cohort (CRIC) Study is to improve the accuracy of assessing the level of kidney function and monitoring chronic kidney disease (CKD) progression within the cohort. As annual measurement of GFR was infeasible, this goal will be accomplished through GFR estimation. While existing GFR estimating expressions such as the Modification of Diet in Renal Disease (MDRD) Study equation and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations are used widely in clinical and research settings,11;13;16 their performance in the CRIC Study population may be limited due to differences in entry criteria between participants in CRIC and other studies, use of non-uniform GFR measurements, and limited sets of predictors. The objective of the current analysis was to utilize an extensive set of standardized data to derive an internal GFR estimating equation with optimal performance characteristics for future use in CRIC, describing the patterns of decline in kidney function in the entire cohort. Additionally, we compared the performance of the CRIC GFR estimating equation across study participant subgroups.
Methods
Participants
Study Population
The CRIC Study is a multi-center prospective cohort study of 3,939 racially and ethnically diverse adults aged 21 to 74 years old at baseline with mild to moderate CKD; 45% were female, 48% had diabetes, 42% were black, and 13% were Hispanic. Participants were recruited from clinical centers in Philadelphia, PA, Baltimore, MD, Cleveland, OH, Detroit, MI, Chicago, IL, New Orleans, LA, and Oakland, CA from June 2003 through September 2008. Inclusion was based on age-specific estimated GFR (eGFR) cutpoints calculated using the four-variable MDRD Study equation.11 The study design, methods, and baseline characteristics of the cohort have been described in detail elsewhere.17-19 Approximately one-third of the CRIC cohort (i.e., the GFR subcohort; N=1433) was selected, using stratified sampling by age, gender, race, diabetes status, MDRD Study equation eGFR, and CRIC clinical center, to have GFR measured at baseline, Year 2, and Year 4 study visits; data from these participants at baseline are used in the current analysis.
Data Collection
Numerous serum and urine measures are relevant to the current analysis beyond serum creatinine and serum cystatin C including: serum albumin (dye-binding assay), serum urea nitrogen, and plasma glucose (Hitachi Vitros 950 AT, www.roche.com); total plasma homocysteine (Abbott AxSYM, www.abbott.com); high-sensitivity C-reactive protein (hsCRP; Siemens BN™ II System, www.medical.siemens.com); plasma brain natriuretic protein (BNP), plasma fibrinogen, and urine neutrophil gelatinase-associated lipocalin (NGAL; Abbott Architect ci8200, www.abbott.com); insulin (Perkin-Elmer Gamma Counter, www.perkinelmer.com); interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), and interleukin 1 receptor-antagonist (IL-1ra) (Beckman Coulter FXp/Paradigm plate reader, www.beckmancoulter.com); 24-hour urine creatinine (Bio-Tek Plate Reader ELX 808, www.biotek.com); 24-hour urine total protein (Roche/Hitachi Modular P Chemistry Analyzer, www.roche.com); urine protein-creatinine ratio; 24-hour urine albumin (Siemens Immulite, www.medical.siemens.com); 24-hour urine urea nitrogen (Catachem, Inc. endpoint spectrophotometric assay, www.catacheminc.com); and creatinine clearance calculated using the Cockcroft-Gault formula.20 Most of these assays were batched and run after all baseline visits were completed. Insulin resistance and insulin sensitivity were estimated using homeostasis model assessment (HOMA) equations (HOMA-IR and SI-HOMA, respectively).21
Race and ethnicity were self-reported; diabetes mellitus was defined as a fasting plasma glucose ≥126 mg/dL or a non-fasting plasma glucose ≥ 200 mg/dL, or self-report of anti-diabetes medication use. Height was measured using a wall-mounted stadiometer, weight was measured using either balanced beam scale or regularly-calibrated electronic digital scale, and waist circumference was measured using a Gulick II or Gulick II Plus anthropometric tape measure just above the uppermost lateral border of the ilium.22 Body mass index (BMI) was calculated as weight in kg/square of height in meters. Bioelectrical impedance analysis (BIA) measures including resistance and reactance were taken using the Quantum II analyzer. BIA phase angle and impedance were calculated from resistance and reactance using standard equations.23 Fat-free mass was computed from gender-specific equations using weight, height, and resistance.24
Test Methods
Reference Standard
GFR was measured using a standardized protocol for urinary 125I-iothalamate clearance testing after a low-protein (<10 g) meal.25-27 The test was chosen due to its comparability to inulin clearance testing, and prior use in other NIDDK-sponsored major research studies.25;26 Exclusions for the 125I-iothalamate test were known iodine allergy, impaired urinary voiding, current breastfeeding or pregnancy, thallium stress test in the past 30 days, self-catheterization, renal replacement therapy, inadequate venous access, and radiation exposure to γ-emitting isotope other than technetium. Plasma and urine radioactive counts were analyzed at the Central GFR Laboratory for the CRIC Study (Cleveland Clinic Foundation, Cleveland, OH). Measured GFR (mGFR) was calculated with and without exclusion of the first clearance period due to concerns regarding systematic elevations during this period, and adjusted to 1.73 m2 of body surface area (BSA).26 Mean mGFR was 1.0 mL/min/1.73m2 lower overall with, compared to without, exclusion of the first clearance period. Due to increased precision of mGFR values with exclusion of the first clearance period, this measure was used as the reference standard in the analysis.
Index Tests
Serum creatinine assays were enzyme-based for all baseline visits and performed on the Hitachi Vitros 950 AT (www.roche.com; CV of 1.1%). Serum creatinine values were calibrated to standardized values at the Cleveland Clinic Research Laboratory (Cleveland, OH).28 Serum cystatin C was measured in stored baseline specimens using a particle-enhanced immunonephelometric assay on the Siemens BN™ II System (www.siemens.com) with a CV of 4.9%. An internal CRIC cystatin C standardization was implemented to correct for drift over time when using different calibrator lots and reagent lots.29
Statistical Methods
Descriptive Statistics
Baseline characteristics of the overall CRIC cohort, the GFR subcohort, and cohort members not included in the subcohort are described using means +/- standard deviations (SD) or medians (25th-75th percentiles) for continuous variables, and counts and percentages for categorical variables. Differences between the GFR subcohort and remaining participants were compared using the chi-square test for categorical variables and t-test for continuous variables. Median mGFR intra-test coefficients of variation (CV) were computed overall, and by CRIC clinical center and level of GFR. All factors with skewed distributions were log-transformed, including serum creatinine and cystatin C. Bivariate associations of characteristics with mGFR, before and after adjustment for age, gender, and race, were examined using linear regression models.
Model Development
CRIC GFR subcohort members at baseline were randomly divided into two groups; two-thirds (n=949) were used to derive a GFR estimating equation (development dataset), which was subsequently validated among the remaining one-third (n=484; validation dataset). Using the development dataset, an extensive set of candidate variables were explored as predictors of mGFR. These included fixed variables (gender, race, Hispanic ethnicity, and diabetes at study entry), variables available at all visits (set A: serum creatinine, serum cystatin C, serum albumin, serum urea nitrogen, age, height, weight, BMI, waist circumference, urine creatinine concentration, BIA resistance and reactance, fat-free mass, impedance, and BIA phase angle), and variables not available at all visits (set B: 24-hour urine protein, 24-h urine creatinine, 24-hour urine protein-creatinine ratio, 24-hour urine albumin, 24-hour urine urea nitrogen, plasma glucose, total plasma homocysteine, hsCRP, BNP, fibrinogen, insulin, creatinine clearance, HOMA-IR, SI-HOMA, IL-6, TNF-α, IL-1ra, and urine NGAL). Given the primary purpose of the equation is for CRIC internal use, CRIC clinical center was also considered as a predictor. Model selection was performed using linear regression models of natural log-transformed mGFR to satisfy the stable variance assumption of the model, and a forward selection strategy. Serum creatinine (log-transformed), age, gender and race were forced into the model. We first restricted the model building using set A predictors only. At each step, a single factor was added into the model that most improved the model fit measured by R2. Model-building stopped when no further variables had p-values < 0.01. Next, we explored set B factors as additional predictors using the same stepwise approach.
Once model-building was completed, the regression coefficients, root mean square error (RMSE) and R2 for each stepwise model were compared. Nonlinear relationships of the two key predictors of serum creatinine and cystatin C with mGFR were explored graphically and by adding higher-order terms into the models. Spline terms were explored, when appropriate. Knots for serum creatinine values at 0.7 and 0.9 mg/dL for women and men, respectively, from the CKD-EPI serum creatinine equation were not assessed given the lack of values below that range in the current population.13 Separate models in diabetic and non-diabetic groups were also fit and the coefficients for serum creatinine and cystatin C were compared. Formal tests of interaction between diabetes and both serum creatinine and cystatin C were implemented using cross-product terms.
Model Evaluation
All candidate models generated from the development were compared for performance in the validation dataset overall and within subgroups defined by age, gender, race, ethnicity, diabetes, BMI, 24-hour urine creatinine excretion, hsCRP and mGFR level. Measures of performance included the median difference (the difference between measured and estimated GFR) and percent median difference (median difference divided by measured GFR); the inter-quartile ranges for the median difference and percent median difference; P30 (percent of estimated GFR within 30% of measured GFR); and root mean square error (RMSE). Bootstrap re-sampling with 1000 repeated samples was employed to calculate 95% confidence intervals. Because CRIC participants were not randomly selected into the GFR subcohort, participants were re-weighted with the inverse of the sampling probability of being selected into the subcohort for the final model (not shown).
Informed Consent and Regulatory Approval
All aspects of the current study were approved by the Institutional Review Boards for each CRIC participating site and the Scientific and Data Coordinating Center. Informed consent was obtained from all participants prior to the baseline study visit. This study also conformed to the Health Insurance Portability and Accountability Act guidelines.
Results
Baseline characteristics for the entire CRIC cohort, the GFR subcohort, and those not in the subcohort are presented in Table 1. The mean mGFR levels with and without exclusion of the first clearance period were 48.0 ±19.9 (SD) and 49.0 ±21.0 mL/min/1.73m2, respectively. The median intra-test CV was 9.7% with exclusion of the first clearance period and 12.0% otherwise, and this measure varied by CRIC clinical center and level of kidney function. Among subcohort members, the mean age was 56.0 ±12.3 years; 43.8% were female and 48.1% had diabetes mellitus. Fifteen percent were Hispanic, and 37.3% reported black race. Median 24-hour urine protein excretion was 210.8 (25th-75th percentile, 76.5-1073.1) mg/d. The mean serum concentration of creatinine was 1.70 ±0.57 mg/dL and of cystatin C was 1.46 ±0.52 mg/L. Mean BMI was 31.3 ±6.9 kg/m2 and BSA was 2.04 ± 0.27 m2. Compared to the remainder of the CRIC Study cohort, subcohort members were younger, less likely to report black race, more likely to be Hispanic, and had lower levels of serum creatinine, cystatin C, plasma glucose, 24-hour urine albumin excretion, and urine NGAL. With the exception of weight, fat-free mass, and plasma glucose, all candidate predictors had a statistically significant relationship with mGFR after adjustment for age, gender, and race (Table 2).
Table 1. Demographic and clinical characteristics.
| Entire Cohort (n=3939) | Participants in GFR Subcohort | P-value | ||
|---|---|---|---|---|
| Yes (n=1433) | No (n=2506) | |||
| mGFR | ||||
| including first clearance period (mL/min/1.73 m2) | - | 49.0 ±21.0) | - | - |
| excluding first clearance period (mL/min/1.73 m2) | - | 48.0 ±19.9) | - | - |
| Age (y) | 58.2 ±11.0) | 56.0 ±12.3) | 59.4 ±10.0) | <0.001 |
| Female gender | 1778 (45.1) | 628 (43.8) | 1150 (45.9) | 0.2 |
| Black race | 1658 (42.1) | 534 (37.3) | 1124 (44.9) | <0.001 |
| Hispanic | 497 (12.6) | 215 (15.0) | 282 (11.3) | <0.001 |
| Diabetes present | 1907 (48.4) | 689 (48.1) | 1218 (48.6) | 0.8 |
| Height (cm) | 168.8 ±9.7) | 168.9 ±9.5) | 168.8 ±9.8) | 0.7 |
| Weight (kg) | 91.5 ±23.4) | 89.4 ±21.0) | 92.8 ±24.6) | <0.001 |
| BMI (kg/m2) | 32.1 ±7.8) | 31.3 ±6.9) | 32.5 ±8.3) | <0.001 |
| Waist circumference (cm) | 105.8 ±17.6) | 103.8 ±16.1) | 107.0 ±18.3) | <0.001 |
| BIA resistance (ohm) | 463.9 ±104.4) | 467.9 ±101.3) | 461.6 ±106.2) | 0.07 |
| BIA reactance (ohm) | 54.4 ±26.0) | 54.8 ±23.6) | 54.1 ±27.2) | 0.5 |
| BIA phase angle (degrees) | 6.9 ±4.2) | 6.8 ±3.7) | 6.9 ±4.5) | 0.5 |
| Fat-free mass† (kg) | 60.5 ±15.6) | 59.8 ±15.0) | 61.0 ±15.9) | 0.03 |
| Impedance† (ohm) | 468.2 ±102.7) | 472.2 ±99.9) | 465.9 ±104.2) | 0.07 |
| Serum creatinine‡ (mg/dL) | 1.74 ±0.58) | 1.70 ±0.57) | 1.77 ±0.58) | <0.001 |
| Serum cystatin C (mg/L) | 1.52 ±0.55) | 1.46 ±0.52) | 1.55 ±0.56) | <0.001 |
| Serum albumin (g/dL) | 3.9 ±0.5) | 3.9 ±0.5) | 4.0 ±0.5) | <0.001 |
| SUN (mg/dL) | 29.6 ±13.6) | 29.3 ±13.3) | 29.9 ±13.8) | 0.2 |
| 24-h urine protein (mg/d) | 184.3 [73.1-910.0] | 210.8 [76.5-1073.1] | 171.1 [71.4-839.5] | 0.01 |
| 24-h urine creatinine (mg/dL) | 70.8 ±36.9) | 68.0 ±35.0) | 72.4 ±37.9) | <0.001 |
| 24-h urine creatinine (g/d) | 1.3 ±0.6) | 1.4 ±0.6) | 1.3 ±0.6) | 0.002 |
| UPCR (mg/g)* | 152.5 [57.5-777.8] | 160.8 [59.1-941.4] | 145.9 [56.3-724.0] | 0.08 |
| 24-h urine albumin (mg/d) | 64.3 [10.3-558.7] | 84.1 [10.8-646.7] | 57.8 [9.9-508.6] | 0.004 |
| 24-h urine urea nitrogen (g/d) | 8.6 ±4.4) | 8.8 ±4.3) | 8.5 ±4.5) | 0.08 |
| Plasma glucose (mg/dL) | 115.4 ±51.7) | 112.7 ±48.8) | 117.0 ±53.2) | 0.01 |
| Total plasma homocysteine (mg/L) | 2.0 ±0.8) | 2.0 ±0.8) | 2.1 ±0.9) | <0.001 |
| hsCRP (mg/L) | 2.6 [1.1-6.5] | 2.2 [0.9-5.2] | 2.8 [1.1-7.1] | <0.001 |
| BNP (pg/mL) | 40.6 [17.0–96.0] | 36.7 [15.4-83.6] | 43.1 [18.0-101.9] | <0.001 |
| Fibrinogen (mg/dL) | 416.9 ±121.1) | 403.8 ±118.3) | 424.4 ±122.1) | <0.001 |
| Insulin (μU/mL) | 16.3 [10.9-25.4] | 15.6 [10.5-24.6] | 16.5 [11.2-25.9] | 0.01 |
| IL-6 (pg/mL) | 1.9 [1.2-3.2] | 1.7 [1.1-2.8] | 2.0 [1.2-3.4] | <0.001 |
| TNF-α (pg/mL) | 2.2 [1.5-3.2] | 2.2 [1.5-3.3] | 2.2 [1.5-3.2] | 0.6 |
| IL-1ra (pg/ml) | 715.7 [390-1551] | 715.0 [370-1508] | 717.1 [397-1569] | 0.1 |
| Urine NGAL (ng/ml) | 14.9 [5.0-35.4] | 13.9 [5.0-32.2] | 15.5 [5.0-38.0] | 0.004 |
| Creatinine clearance (mL/min) | 58.2 ±30.5) | 61.2 ±31.1) | 56.5 ±30.0) | <0.001 |
| HOMA-IR | 6.6 ±8.7) | 6.3 ±7.7) | 6.8 ±9.2) | 0.1 |
| SI-HOMA | 0.2 [0.1-0.4] | 0.3 [0.1-0.4] | 0.2 [0.1-0.4] | 0.02 |
| Body surface area (m2) | 2.06 ±0.29) | 2.04 ±0.27) | 2.07 ±0.30) | <0.001 |
Note: unless otherwise indicated, values for continuous variables given as mean ± SD or median [25th-75th percentile], while values for categorical variables given as number (percentage).
BIA – bioelectrical impedance analysis; BMI – body mass index; BNP – brain natriuretic protein; GFR – glomerular filtration rate; HOMA-IR – homeostasis model assessment – insulin resistance; hsCRP – high-sensitivity C-reactive protein; IL-1ra – interleukin 1 receptor antagonist; IL-6 – interleukin 6; mGFR – measured GFR; NGAL – neutrophil gelatinase-associated lipocalin; SI-HOMA – homeostasis model assessment – insulin sensitivity; Sun, serum urea nitrogen; TNF-α - tumor necrosis factor α; UPCR, urine protein-creatinine ratio.
Determined from 24-h urine sample
From BIA data
Traceable to isotope dilution mass spectrometry.
Note: Conversion factors for units: creatinine in mg/dL to μmol/L, ×88.4; creatinine clearance in mL/min to mL/s, ×0.01667; serum albumin in g/dL to g/L, ×10; SUN in mg/dL to mmol/L, ×0.357; plasma glucose in g/dL to mmol/L, ×0.05551; insulin in μU/mL to pmol/L, ×6.00.
Table 2. Associations of demographic and clinical characteristics with measured glomerular filtration rate (GFR) in the Chronic Renal Insufficiency Cohort (CRIC) Study GFR subcohort.
| Relationship with Measured GFR | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted for Age, Race and Gender | |||
| PE* (95% CI) | P-value | PE* (95% CI) | P-value | |
| Age (per 10 years) | -0.108 (-0.155, -0.060) | <0.001 | -0.108 (-0.155, -0.060) | <0.001 |
| Female (versus male) | -0.028 (-0.146, 0.091) | 0.7 | -0.023 (-0.141, 0.094) | 0.7 |
| Black (versus non-black) | -0.144 (-0.265, -0.023) | 0.02 | -0.143 (-0.263, -0.023) | 0.02 |
| Hispanic (versus non-Hispanic) | -0.332 (-0.527, -0.137) | 0.001 | -0.456 (-0.656, -0.257) | <0.001 |
| Diabetes (versus no diabetes) | 0.111 (0.053, 0.169) | <0.001 | -0.331 (-0.448, -0.214) | <0.001 |
| Height (cm) | -0.055 (-0.115, 0.004) | 0.07 | 0.168 (0.090, 0.246) | <0.001 |
| Weight (kg) | -0.332 (-0.527, -0.137) | <0.001 | -0.05 (-0.113, 0.013) | 0.1 |
| BMI (kg/m2) | -0.123 (-0.183, -0.064) | <0.001 | -0.107 (-0.167, -0.046) | 0.001 |
| Waist circumference (cm) | -0.138 (-0.196, -0.080) | <0.001 | -0.113 (-0.173, -0.054) | <0.001 |
| BIA resistance (ohm) | 0.058 (-0.003, 0.118) | 0.06 | 0.076 (0.007, 0.146) | 0.03 |
| Log (BIA reactance, ohm) | 0.218 (0.157, 0.279) | <0.001 | 0.207 (0.142, 0.271) | <0.001 |
| Log (BIA phase angle, degrees) | 0.205 (0.139, 0.271) | <0.001 | 0.206 (0.133, 0.28) | <0.001 |
| Fat-free mass† (kg) | -0.007 (-0.066, 0.052) | 0.8 | -0.021 (-0.099, 0.057) | 0.6 |
| Impedance† (ohm) | 0.058 (-0.001, 0.117) | 0.06 | 0.076 (0.008, 0.144) | 0.03 |
| Log (serum creatinine‡, mg/dL) | -0.736 (-0.776, -0.697) | <0.001 | -0.9 (-0.936, -0.865) | <0.001 |
| Log (serum cystatin C, mg/L) | -0.837 (-0.869, -0.806) | <0.001 | -0.838 (-0.869, -0.807) | <0.001 |
| Serum albumin (g/dL) | 0.288 (0.230, 0.347) | <0.001 | 0.305 (0.247, 0.363) | <0.001 |
| Serum urea nitrogen (mg/dL) | -0.623 (-0.670, -0.577) | <0.001 | -0.633 (-0.679, -0.587) | <0.001 |
| Log (24-hour urine protein, mg/d) | -0.323 (-0.380, -0.266) | <0.001 | -0.393 (-0.451, -0.335) | <0.001 |
| 24-hour urine creatinine (mg/dL) | 0.207 (0.149, 0.265) | <0.001 | 0.234 (0.174, 0.295) | <0.001 |
| Log (24-hour urine creatinine, g/d) | 0.214 (0.158, 0.271) | <0.001 | 0.246 (0.184, 0.309) | <0.001 |
| 24-hour urine protein:creatinine ratio (mg/g) | -0.298 (-0.359, -0.237) | <0.001 | -0.331 (-0.391, -0.270) | <0.001 |
| Log (24-hour urine albumin, mg/d) | -0.309 (-0.367, -0.251) | <0.001 | -0.371 (-0.430, -0.312) | <0.001 |
| Log (24-hour urine urea nitrogen, g/d) | 0.199 (0.141, 0.258) | <0.001 | 0.195 (0.135, 0.255) | <0.001 |
| Plasma glucose (mg/dL) | -0.056 (-0.116, 0.004) | 0.07 | -0.049 (-0.109, 0.010) | 0.1 |
| Log (total plasma homocysteine, mg/L) | -0.488 (-0.540, -0.436) | <0.001 | -0.499 (-0.552, -0.445) | <0.001 |
| Log (hsCRP, mg/L) | -0.099 (-0.159, -0.040) | 0.001 | -0.082 (-0.142, -0.022) | 0.008 |
| Log (BNP, pg/mL) | -0.312 (-0.369, -0.256) | <0.001 | -0.313 (-0.373, -0.254) | <0.001 |
| Log (fibrinogen, mg/dL) | -0.178 (-0.237, -0.119) | <0.001 | -0.16 (-0.219, -0.101) | <0.001 |
| Log (insulin, μU/mL) | -0.114 (-0.174, -0.055) | <0.001 | -0.104 (-0.164, -0.045) | 0.001 |
| Log (IL-6, pg/mL) | -0.261 (-0.318, -0.204) | <0.001 | -0.24 (-0.299, -0.182) | <0.001 |
| Log (TNF-α, pg/mL) | -0.327 (-0.384, -0.269) | <0.001 | -0.327 (-0.384, -0.270) | <0.001 |
| Log (IL-1ra, pg/mL) | -0.166 (-0.225, -0.107) | <0.001 | -0.174 (-0.233, -0.115) | <0.001 |
| Log (urine NGAL, ng/mL) | -0.361 (-0.418, -0.304) | <0.001 | -0.411 (-0.470, -0.352) | <0.001 |
| Log (creatinine clearance, mL/min) | 0.611 (0.565, 0.657) | <0.001 | 0.608 (0.562, 0.654) | <0.001 |
| Log (HOMA-IR) | -0.114 (-0.173, -0.055) | <0.001 | -0.103 (-0.162, -0.044) | 0.001 |
| Log (SI-HOMA) | 0.103 (0.047, 0.160) | <0.001 | 0.085 (0.027, 0.142) | 0.004 |
BIA – bioelectrical impedance analysis; BMI – body mass index; BNP – brain natriuretic protein; CI – confidence interval; GFR – glomerular filtration rate; HOMA-IR – homeostasis model assessment – insulin resistance; hsCRP – C-reactive protein; IL-1ra – interleukin-1 receptor antagonist; IL-6 – interleukin-6; NGAL – neutrophil gelatinase-associated lipocalin; PE – parameter estimate; SI-HOMA – homeostasis model assessment – insulin sensitivity; TNF- α - tumor necrosis factor-α.
Parameter estimate represents relative change in measured GFR per one standard deviation increase in predictors unless otherwise specified. Multiplying the parameter estimates by 100 provides approximate percent change;
From BIA data;
Traceable to isotope dilution mass spectrometry.
Model Development
The regression coefficients and model fit of five equations derived in the development dataset are provided in Table 3. The addition of cystatin C into the model decreased the magnitude of all other coefficients by one-half compared to the model with serum creatinine alone. Subsequent additions of BIA phase angle, CRIC clinical center, and 24-hour urine creatinine excretion had little impact on the coefficients of the first five variables (i.e., serum creatinine, cystatin C, age, gender, and race).
Table 3. Coefficients and performance of models to estimate GFR in the development dataset from the GFR subcohort.
| Model | Regression Coefficient (SE) | Model Performance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SCr | Age | Female Gender | Black Race | SCysC | BIA phase angle | 24-h UCr | RMSE (95% CI) | R2 (95% CI) | |
| 1. SCr, age, gender, race | -1.230 (0.026) | -0.059 (0.006) | -0.300 (0.017) | 0.096 (0.016) | 0.237 (0.223-0.249) | 0.696 (0.660-0.729) | |||
| 2. SCr, age, gender, race, SCysC | -0.598 (0.043) | -0.034 (0.006) | -0.184 (0.016) | 0.043 (0.015) | -0.636 (0.037) | 0.208 (0.194-0.220) | 0.767 (0.737-0.798) | ||
| 3. SCr, age, gender, race, SCysC, BIA phase angle | -0.604 (0.043) | -0.030 (0.006) | -0.172 (0.016) | 0.033 (0.015) | -0.618 (0.037) | 0.102 (0.029) | 0.206 (0.193-0.219) | 0.770 (0.739-0.801) | |
| 4. SCr, age, gender, race, SCysC, BIA phase angle, CRIC clinical center* | -0.578 (0.044) | -0.031 (0.006) | -0.167 (0.017) | 0.015 (0.015) | -0.623 (0.038) | 0.095 (0.030) | 0.204 (0.190-0.216) | 0.778 (0.747-0.808) | |
| 5. SCr, age, gender, race, SCysC, BIA phase angle, CRIC clinical center, 24-h UCr* | -0.603 (0.046) | -0.028 (0.006) | -0.135 (0.018) | 0.007 (0.016) | -0.583 (0.040) | 0.066 (0.030) | 0.199 (0.034) | 0.199 (0.187-0.212) | 0.787 (0.757-0.816) |
UCr – urine creatinine excretion; BIA – bioelectrical impedance analysis; CI – confidence interval; CRIC – Chronic Renal Insufficiency Cohort; SCysC – serum cystatin C; RMSE – root mean square error; SCr – serum creatinine; SE – standard error; GFR, glomerular filtration rate
Coefficients for CRIC clinical centers are not reported.
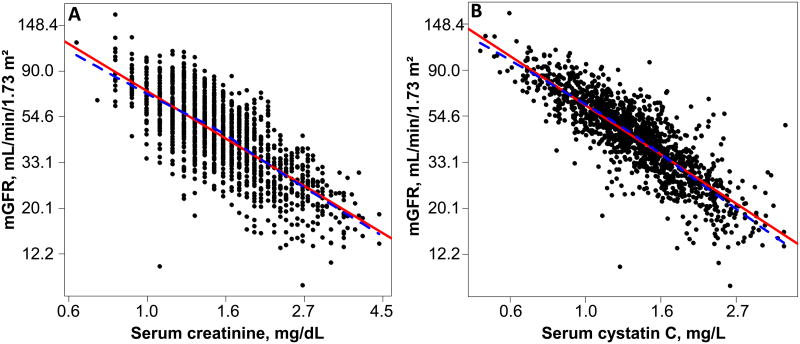
The relationships of log(serum creatinine) and log(cystatin C) with ln(mGFR) were explored graphically and both appeared linear (Figure 1). Quadratic terms for serum creatinine and cystatin C, when added to the models, were not statistically significant. Given the strongly linear relationship and lack of significant quadratic terms for these predictors, no further exploration of non-linear relationships using spline terms was performed. The interaction terms between diabetes and both serum creatinine and cystatin C were not statistically significant.
Figure 1.
Scat terplots of (A) mGFR with serum creatinine, and (B) mGFR with serum cystatin C within the Chronic Renal Insufficiency Cohort (CRIC) Study GFR subcohort. The solid line is the linear regression line and the dotted line represents the lowess fit. The scatterplots were created in the log scale, but axis labels have been converted back to the original scale.
Model Evaluation
In the validation dataset, the model incorporating both serum creatinine and cystatin C with age, gender, and race was unbiased, accurate, and had substantially better overall performance compared to the model with serum creatinine alone (Table 4). Performance of models was essentially unchanged after the addition of BIA phase angle, CRIC clinical center, and 24-hour urine creatinine excretion.
Table 4. Performance measures for models estimating GFR in the validation dataset from the GFR subcohort.
| Model | P30 (95% CI) | RMSE (95% CI) |
|---|---|---|
| 1. SCr, age, gender, race | 84 (80-87) | 0.232 (0.210-0.268) |
| 2. SCr, age, gender, race, SCysC | 89 (86-91) | 0.207 (0.183-0.242) |
| 3. SCr, age, gender, race, SCysC, BIA phase angle | 89 (86-92) | 0.206 (0.182-0.241) |
| 4. SCr, age, gender, race, SCysC, BIA phase angle, CRIC clinical center | 90 (87-92) | 0.205 (0.181-0.241) |
| 5. SCr, age, gender, race, SCysC, BIA phase angle, CRIC clinical center, 24hr UCr | 90 (87-92) | 0.202 (0.178-0.238) |
UCr – urine creatinine excretion; BIA – bioelectrical impedance analysis; CI – confidence interval; CRIC – Chronic Renal Insufficiency Cohort; SCysC – serum cystatin C; P30 – percentage of estimated GFR within 30% of measured GFR; RMSE – root mean square error; SCr – serum creatinine; GFR, glomerular filtration rate
Due to the similarity in the performance of Models 2 through 5 in the validation dataset, the most parsimonious model including serum creatinine, cystatin C, age, gender, and race was chosen as the final model. Table 5 displays its performance in the validation dataset by subgroup. The CRIC GFR estimating equation was most accurate among younger participants, men, non-blacks, and non-Hispanics. In addition, equation accuracy declined among those with diabetes, obese participants, those with the least 24-hour urine creatinine excretion, those with high hsCRP, and participants with eGFR levels below 45 mL/min/1.73m2.
Table 5. Performance of the CRIC Study GFR estimating equation in the validation dataset by subgroup.
| No. | Median Difference* | Median % Difference* | P30 (95% CI) | RMSE (95% CI) | |
|---|---|---|---|---|---|
| Age | |||||
| <45 y | 102 | 1 [-4, 7] | 2 [-9, 12] | 95 (89-99) | 0.170 (0.140-0.207) |
| 45-<60 y | 163 | 0 [-6, 7] | 0 [-14, 11] | 87 (81-92) | 0.212 (0.182-0.249) |
| ≥60 y | 219 | 1 [-4, 6] | 1 [-10, 11] | 87 (82-91) | 0.215 (0.175-0.279) |
| Gender | |||||
| Female | 217 | 0 [-5, 7] | 1 [-13, 11] | 86 (81-90) | 0.234 (0.192-0.300) |
| Male | 267 | 0 [-4, 6] | 1 [-10, 12] | 90 (87-94) | 0.181 (0.162-0.199) |
| Race | |||||
| Black | 196 | 1 [-4, 7] | 3 [-10, 14] | 84 (78-89) | 0.223 (0.198-0.248) |
| Non-black | 288 | 0 [-5, 5] | -1 [-12, 10] | 92 (88-95) | 0.194 (0.156-0.253) |
| Ethnicity | |||||
| Hispanic | 67 | -2 [-8, 2] | -5 [-21, 5] | 86 (76-94) | 0.216 (0.152-0.297) |
| Non-Hispanic | 417 | 1 [-5, 7] | 2 [-10, 12] | 89 (86-92) | 0.205 (0.180-0.242) |
| Diabetes | |||||
| Yes | 224 | 0 [-5, 6] | 1 [-15, 12] | 83 (77-88) | 0.240 (0.199-0.303) |
| No | 260 | 1 [-4, 7] | 1 [-10, 11] | 93 (90-96) | 0.172 (0.154-0.191) |
| BMI | |||||
| <25 kg/m2 | 89 | -2 [-6, 2] | -4 [-12, 5] | 93 (86-98) | 0.143 (0.116-0.178) |
| 25-<30 kg/m2 | 143 | 2 [-4, 7] | 4 [-9, 14] | 94 (89-97) | 0.174 (0.154-0.197) |
| ≥30 kg/m2 | 252 | 0 [-5, 7] | 1 [-13, 12] | 84 (79-88) | 0.239 (0.203-0.295) |
| 24-h urine creatinine | |||||
| <2.08 g/d | 174 | -1 [-6, 4] | -4 [-17, 8] | 85 (79-90) | 0.235 (0.179-0.314) |
| 2.08-<2.55 g/d | 160 | 0 [-4, 6] | -1 [-10, 11] | 88 (82-92) | 0.192 (0.167-0.220) |
| ≥2.55 g/d | 150 | 2 [-3, 8] | 4 [-6, 13] | 93 (88-96) | 0.188 (0.162-0.218) |
| hsCRP | |||||
| <1.22 mg/L | 162 | -1 [-5, 3] | -3 [-12, 7] | 92 (88-96) | 0.168 (0.145-0.190) |
| 1.22-<3.82 mg/L | 170 | 1 [-4, 7] | 2 [-12, 12] | 89 (84-94) | 0.221 (0.173-0.299) |
| ≥3.82 mg/L | 152 | 1 [-4, 7] | 2 [-9, 14] | 84 (76-89) | 0.225 (0.192-0.263) |
| eGFR | |||||
| <45 mL/min/1.73m2 | 233 | 0 [-4, 6] | 1 [-13, 14] | 82 (77-87) | 0.221 (0.200-0.242) |
| ≥45 mL/min/1.73m2 | 251 | 0 [-6, 7] | 1 [-11, 11] | 94 (91-97) | 0.190 (0.148-0.256) |
Abbreviations and definitions: BMI – body mass index; CI – confidence interval; CRIC – Chronic Renal Insufficiency Cohort; eGFR – estimated glomerular filtration rate; GFR, glomerular filtration rate; hsCRP – high-sensitivity C-reactive protein; P30 – percentage of eGFR within 30% of mGFR; RMSE – root mean square error; Median difference: median mGFR – eGFR; Median % difference: median ((mGFR – eGFR)/mGFR); mGFR, measured glomerular filtration rate.
25th and 75th percentile shown in parentheses.
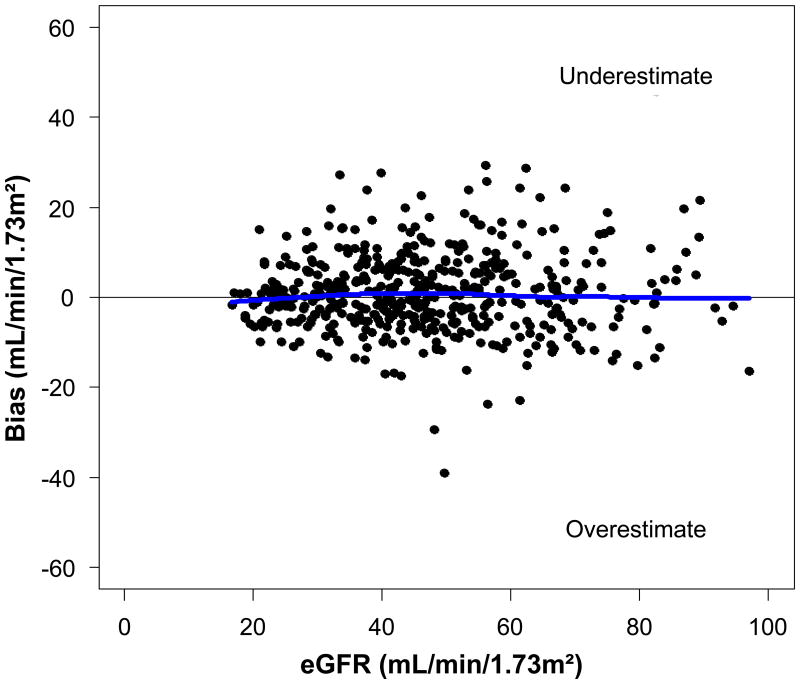
Performance of the final CRIC GFR estimating equation by level of estimated GFR in the validation dataset is shown in Figure 2. The CRIC GFR estimating equation was unbiased across the range of estimated GFR.
Figure 2.
Performance of the final Chronic Renal Insufficiency Cohort (CRIC) Study glomerular filtration rate (GFR) estimating equation refit in the validation dataset by level of estimated GFR.
Discussion
The CRIC Study is the largest prospective cohort study of CKD with detailed phenotypic assessment and measures of kidney function and cardiovascular disease. To achieve the aim of estimating GFR and tracking progression of CKD, a new CRIC GFR estimating equation was developed. After considering a broad set of candidate predictors including demographic and anthropometric measures, various biomarkers, and clinical characteristics, the final CRIC GFR estimating equation includes five variables; serum creatinine and cystatin C, and age, gender, and race. In the validation dataset, this equation demonstrated accuracy and precision similar to more complicated equations including BIA phase angle, CRIC clinical center, and 24-hour urine creatinine excretion, overall and within subgroups. Eighty-nine percent of estimated values were within 30% of measured GFR using the new equation. Compared across subgroups, the CRIC GFR estimating equation was most accurate among younger participants, men, non-blacks, non-Hispanics, those without diabetes, those with BMI < 30 kg/m2, and those with the highest 24-hour urine creatinine excretion levels and lowest hsCRP levels. Finally, GFR was most accurately predicted among participants with eGFR ≥45 mL/min/1.73m2.
Although GFR measurement using urinary 125I-iothalamate clearance tests correlates well with clearance of the gold standard, inulin,25;30-32 these tests have known substantial variability across clearance periods as evidenced by median CVs of 9.4% and 11.7% in the MDRD Study and the Diabetes Control and Complications Trial.26 Additionally, levels of mGFR tend to decline across clearance periods, especially at higher levels of GFR, further increasing the variability of the measure.26;33 In the CRIC Study, the goal to best characterize level of kidney function by both measured and estimated GFR motivated the exclusion of the first period of all clearance tests performed. Evidence of the success of this strategy was demonstrated by the increased precision of mGFR levels overall and a 1 mL/min/1.73m2 correction of bias. However, even with these improvements, performance of the CRIC GFR estimating equation was very similar with and without the exclusion of the first clearance period (data not shown).
The motivation to develop a GFR estimating equation specific to the CRIC Study came, in part, from our effort to identify novel factors which may significantly improve GFR estimation. However, among over 20 novel factors considered, including measures of body composition, and inflammation, none substantively improved GFR estimation once factors included in the CKD-EPI joint serum creatinine-cystatin C estimating equation (i.e., serum creatinine, cystatin C, age, gender, and race) were included.16 This was also true across all subgroups of interest included in our analyses, although small sample sizes in the validation dataset among subgroups limits our ability to compare equations (data not shown). The lack of additional informative predictors, along with the reduction in the coefficients for age, gender, and race once cystatin C was added to the model, supports recent findings that non-renal elimination of cystatin C may be minimal and that cystatin C levels may not be influenced by factors considered in the current analysis including level of inflammation.34-36 However, elucidation of non-GFR determinants of cystatin C levels awaits further study. Our findings provide reassurance that the performance of the most commonly used GFR estimating equations is not limited by the set of predictors available in typical research and clinical settings.
Variability in CRIC GFR estimating equation performance was observed across all subgroups examined, but none of these differences reached a level of statistical significance. Despite this, notable improvements to GFR prediction were present among demographic subgroups including participants <45 years of age, men, non-blacks, and non-Hispanics. Additionally, GFR was least accurately predicted among the least healthy including those with diabetes, obese individuals, those with low 24-hour urine creatinine excretion, those with high levels of inflammation, and among participants with more advanced CKD. Much of this variability in equation performance by age, gender, race, diabetes status, and BMI may largely be explained by known differences in these populations with non-renal elimination and generation of serum creatinine. The relatively small number of participants in any given subgroup precluded further exploration of factors that may influence GFR prediction in these subpopulations.
The current study has several strengths including the size of the subcohort that underwent urinary 125I-iothalamate clearance testing, allowing for large development and validation datasets. The goal of developing an internal equation allowed for the exploration of a varied set of candidate GFR predictors including body composition measures, and novel factors such as urine NGAL and insulin resistance. Another strength is the oversampling of non-Hispanic black/African-Americans and those with diabetes, and the inclusion of 215 Hispanics to generate a GFR estimating equation potentially more applicable in these subpopulations.
Our study had limitations as well. First, the CRIC Study equation was not developed to replace other published equations such as the CKD-EPI equation in the clinical setting.11;13;16 The CRIC Study enrolled selected individuals with mild to advanced CKD, and findings may not be applicable to certain types of kidney disease that did not occur frequently in the cohort, such as polycystic kidney disease and glomerulonephritis. Additionally, the CRIC GFR estimating equation has not been externally validated. Second, although main effect and interaction terms were explored for certain subpopulations such as those with diabetes, small effects cannot be ruled out due to the numbers of participants in these subgroups. Third, although exclusion of the first mGFR clearance period increased precision and corrected bias, estimates arising from the CRIC GFR estimating equation versus existing equations generated using unaltered urinary 125I-iothalamate clearance test data may not be entirely comparable. The impact of this is unlikely to be consequential owing to the principal goal of optimizing GFR estimation for use within the CRIC Study. Despite exclusion of the first clearance period, the precision of mGFR remained limited, potentially lowering accuracy of eGFR. Indeed, limitations to the precision of mGFR remain one of the largest barriers to improving GFR estimation.37 Fourth, the GFR subcohort was comprised of a slightly different population compared to the full CRIC cohort. To address this concern, coefficients in the final CRIC GFR estimating equation used internally were weighted back to the characteristics of the full cohort using several demographic and clinical characteristics. Finally, an initial 19% drift in cystatin C values among healthy population control samples was observed,29 necessitating that all cystatin C values be internally standardized to a specific calibrator and reagent lot. This limits the direct comparison of our data to other studies incorporating cystatin C data until CRIC's and other studies' values are standardized to certified reference material (ERM-DA471/IFCC).38;39
The CRIC GFR estimating equation provides accurate and unbiased GFR prediction within the CRIC study population, and will be used for estimating level of kidney function as well as trajectories of decline. While the CRIC estimating equation performs very well overall, accuracy declines among certain subgroups including older participants, women, blacks, those with diabetes, obese participants, those with higher levels of inflammation, and those with lower levels of kidney function.
Acknowledgments
Members of the CRIC Study Group are as follows; * denotes an Ancillary Investigator. University of Pennsylvania Scientific & Data Coordinating Center: Harold I. Feldman, MD, MSCE (PI); J. Richard Landis, PhD; Dina H. Appleby, MS; Shawn Ballard, MS; Denise Cifelli, MS; Robert M. Curley, MS; Jennifer Dickson; Marie Durborow; Stephen Durborow; Melanie Glenn, MPH; Asaf Hanish, MPH; Christopher Helker, MSPH; Elizabeth S. Helker, RN; Amanda Hyre Anderson, PhD, MPH; Marshall Joffe, MD, PhD, MPH; Scott Kasner, MD, MSCE, FAHA; Stephen E. Kimmel, MD, MSCE; Shiriki Kumanyika, PhD, MPH; Lisa Nessel, MSS, MLSP; Emile R. Mohler III, MD; Steven R. Messe, MD; Nancy Robinson, PhD; Leigh Rosen, MUEP; J. Sanford Schwartz, MD; Sandra Smith; Joan Stahl, MS; Kelvin Tao, PhD, MS; Valerie L. Teal, MS; Xin Wang, MS; Dawei Xie, PhD; Peter Yang, PhD; Xiaoming Zhang, MS. University of Pennsylvania Medical Center: Raymond R. Townsend, MD (PI); Manjunath Balaram; *Thomas P. Cappola, MD, ScM; Debbie Cohen, MD; Magdalena Cuevas; Mark J. Duckworth; *Daniel L. Dries, MD; Virginia Ford, MSN, CRNP; Colin M. Gorman; *Juan Grunwald, MD; Holly M. Hannah; Peter A. Kanetsky, PhD, MPH; Krishna Kellem; Lucy Kibe, MS; *Mary B. Leonard, MD, MSCE; *Maureen Maguire, PhD; Stephanie McDowell; John Murphy, MD; *Muredach Reilly, MB; *Sylvia E. Rosas, MD; Wanda M. Seamon; Angie Sheridan, MPH; Karen Teff, MD. The Johns Hopkins University: Lawrence J. Appel, MD, MPH (PI); Cheryl Anderson, PhD, MPH; Jeanne Charleston, RN; Nyya Etheredge; Bernard Jaar, MD, MPH; Kelly Mantegna; Carla Martin; Edgar “Pete” Miller, MD; Patience Ngoh; Julia Scialla, MD; Steve Sozio, MD, MHS; Sharon Turban, MD, MHS; Hemalatha Venkatesh. University of Maryland: Jeffrey Fink, MD, MS (Co-PI); Wanda Fink, RN, BSN; Afshin Parsa, MD, MPH; Beth Scism; Stephen Seliger, MD, MS; Matthew Weir, MD. University Hospitals of Cleveland Case Medical Center: Mahboob Rahman, MD (PI); Valori Corrigan RN; Renee Dancie, CMA; Genya Kisin MA; Radhika Kanthety; Louise Strauss, RN; Jackson T. Wright Jr, MD, PhD. MetroHealth Medical Center: Jeffrey Schelling, MD (Co-PI); Patricia Kao, MD (Co-PI); Ed Horowitz, MD (Co-PI); Jacqui Bjaloncik; Theresa Fallon; John R. Sedor, MD; Mary Ann Shella, RN, BSN; Jacqueline Theurer; J. Daryl Thornton, MD, MPH. Cleveland Clinical Foundation: Martin J. Schreiber, MD (Co-PI); Martha Coleman, RN; Richard Fatica, MD; Sandra Halliburton, PhD; Carol Horner, BSN, RN; Teresa Markle, BS; Mohammed A. Rafey, MD, MS; Annette Russo; Stephanie Slattery, RN; Rita Spirko, RN, MSN; Kay Stelmach, RN; Velma Stephens, LPN; Lara Danziger-Isakov, MD, MPH. University of Michigan at Ann Arbor: Akinlolu Ojo, MD, PhD (PI); Baskaran Sundaram, MD; Jeff Briesmiester; Denise Cornish-Zirker, BSN; Crystal Gadegbeku, MD; Nancy Hill; Kenneth Jamerson, MD; *Matthias Kretzler, MD; Bruce Robinson, MD; Rajiv Saran, MD; Bonnie Welliver, BSN, CNN; Jillian Wilson; Eric Young, MD, MS. St. John's Health System: Susan P. Steigerwalt, MD (Co-PI); Keith Bellovich, DO; Jennifer DeLuca; Sherry Gasko, BSRN; Gail Makos, RN, MSN; Chantal Parmelee; Shahan Smith; Kathleen Walls. Wayne State University: John M. Flack, MD, MPH (Co-PI); James Sondheimer, MD; Mary Maysura; Stephen Migdal, MD; M. Jena Mohanty, MD; Yanni Zhuang, BSN. University of Illinois at Chicago: James P. Lash, MD (PI); Jose Arruda, MD; Carolyn Brecklin, MD; Eunice Carmona, BA; Janet Cohan, MSN; Michael Fischer, MD, MSPH; Anne Frydrych, MS, RD; Amada Lopez; *Claudia Lora, MD; Monica Martinez; Adriana Matos; Alejandro Mercado; Brenda Moreno; Patricia Meslar, MSN; Ana Ricardo, MD, MPH; Thomas Stamos, MD; *Eve Van Cauter, PhD. Tulane University Health Science Center: Jiang He, MD, PhD (PI); Brent Alper, MD; Vecihi Batuman, MD; Lydia A. Bazzano, MD, PhD; Bernadette Borja; Adriana Burridge, MPH; Jing Chen, MD, MSc; Catherine Cooke; Patrice Delafontaine, MD; Karen B. DeSalvo, MD, MPH, MSc; Vivian A. Fonseca, MD; Lee Hamm, MD; Michelle R. Hurly, RN, BSN; Julie Legarde; Eva Lustigova, MPH; *Paul Muntner, PhD; Maria Patrocollo-Emerson, MPH; Lindsey Powers; Shea Shelton; Claire Starcke; Paul Whelton, MD, MSc. Kaiser Permanente of Northern California: Alan S. Go, MD (PI); Lynn M. Ackerson, PhD; Pete Dorin, MPA; Daniel Fernandez; Nancy G. Jensvold, MPH; Joan C. Lo, MD; Juan D. Ordonez, MD, MPH; Rachel Perloff; Thida Tan, MPH; Daphne Thompson; Gina M. Valladares; Annette Wiggins, RN; Diana B. Wong, RN, MPH; Jingrong Yang, MA. University of California, San Francisco: Chi-yuan Hsu, MD, MSc (Co-PI); Glenn M. Chertow, MD, MPH; *Nisha Bansal, MD; *Manju Kurella, MD, MPH; *Michael G. Shlipak, MD, MPH; *Kristine Yaffe, MD. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK): John W. Kusek, PhD; Andrew S. Narva, MD. Scientific Advisory Committee: Kathy Faber-Langendoen, MD; Bryce A. Kiberd, MD; Elisa T. Lee, PhD; Julia Lewis, MD; William McClellan, MD, MPH; Timothy Meyer, MD; David Nathan, MD; John B. Stokes, MD; Herman Taylor, MD; Peter W. Wilson, MD. University of New Mexico: *Vallabh Shah, PhD. George Washington University: *Dominic Raj MD, DM. University of Miami: *Myles Wolf, MD, MMSc. Consultant, Harvard School of Medicine: Paul M. Ridker, MD. Central Lab, University of Pennsylvania: Daniel J. Rader, MD; Anna DiFlorio; Ted Mifflin; Linda Morrell; Megan L. Wolfe. GFR Lab, Cleveland Clinic: Phillip Hall, MD; Henry Rolin; Sue Saunders. EBT Reading Center, UCLA: Mathew Budoff, MD; Chris Dailing. ECG Reading Center, Wake Forest: Elsayed Z. Soliman MD, MSc, MS; Zhu-Ming Zhang, MD. Echo Reading Center, University of Pennsylvania: Martin St. John Sutton, MBBS; Martin G. Keane, MD.
The authors would like to acknowledge and thank the CRIC Study participants, study coordinators, and investigators for their efforts. Additionally, the authors thank Robin Mogg, PhD for early contributions to this work and Qiang Pan for analytical support.
Support: In addition to funding under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (5U01DK060990, 5U01DK060984, 5U01DK06102, 5U01DK061021, 5U01DK061028, 5U01DK60980, 5U01DK060963, and 5U01DK060902), this work was supported in part by the following institutional Clinical Translational Science Awards (CTSA) and other National Institutes of Health grants: University of Pennsylvania UL1 RR-024134, K01DK092353, and K24DK002651, Johns Hopkins University UL1 RR-025005, University of Maryland General Clinical Research Center (GCRC) M01 RR-16500, Case Western Reserve University Clinical and Translational Science Collaborative (University Hospitals of Cleveland, Cleveland Clinic Foundation, and MetroHealth) UL1 RR-024989, University of Michigan GCRC M01 RR-000042 and CTSA UL1 RR-024986, University of Illinois at Chicago Clinical Research Center UL1 RR-029879 and M01 RR-013987-06, Tulane/LSU/Charity Hospital GCRC M01 RR-05096, and Kaiser NIH/NCRR UCSF-CTSI UL1 RR-024131.
Footnotes
This abstract was presented at the American Society of Nephrology Renal Week 2009 under the title, “Development of an equation to estimate glomerular filtration rate among participants of the Chronic Renal Insufficiency Cohort (CRIC) Study.”
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levey AS. Clinical evaluation of kidney function. In: Greenberg A, Cheung AK, Coffmann TM, Falk RJ, Jennette JC, editors. Primer on Kidney Disease. 2nd. San Diego, CA: Academic Press; 1998. pp. 20–26. [Google Scholar]
- 2.Kasiske BL, Keane WF. Laboratory assessment of renal disease: Clearance, urinalysis, and renal biopsy. In: Brenner BM, Rector FC, editors. The Kidney. 6th. Philadelphia, PA: W.B. Saunders; 2000. pp. 1129–1170. [Google Scholar]
- 3.Rahman M, Smith MC. Chronic renal insufficiency: a diagnostic and therapeutic approach. Arch Intern Med. 1998;158:1743–1752. doi: 10.1001/archinte.158.16.1743. [DOI] [PubMed] [Google Scholar]
- 4.Walser M. Assessing renal function from creatinine measurements in adults with chronic renal failure. Am J Kidney Dis. 1998;32:23–31. doi: 10.1053/ajkd.1998.v32.pm9669420. [DOI] [PubMed] [Google Scholar]
- 5.Toto RD, Kirk KA, Coresh J, et al. Evaluation of serum creatinine for estimating glomerular filtration rate in African Americans with hypertensive nephrosclerosis: results from the African-American Study of Kidney Disease and Hypertension (AASK) Pilot Study. J Am Soc Nephrol. 1997;8:279–287. doi: 10.1681/ASN.V82279. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 7.Walser M, Drew HH, LaFrance ND. Creatinine measurements often yielded false estimates of progression in chronic renal failure. Kidney Int. 1988;34:412–418. doi: 10.1038/ki.1988.196. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS. Measurement of renal function in chronic renal disease. Kidney Int. 1990;38:167–184. doi: 10.1038/ki.1990.182. [DOI] [PubMed] [Google Scholar]
- 9.Mitch WE, Walser M, Buffington GA, Lemann J., Jr A simple method of estimating progression of chronic renal failure. Lancet. 1976;2:1326–1328. doi: 10.1016/s0140-6736(76)91974-7. [DOI] [PubMed] [Google Scholar]
- 10.Coresh J, Toto RD, Kirk KA, et al. Creatinine clearance as a measure of GFR in screenees for the African-American Study of Kidney Disease and Hypertension pilot study. Am J Kidney Dis. 1998;32:32–42. doi: 10.1053/ajkd.1998.v32.pm9669421. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 12.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouvet Y, Bouissou F, Coulais Y, et al. GFR is better estimated by considering both serum cystatin C and creatinine levels. Pediatr Nephrol. 2006;21:1299–1306. doi: 10.1007/s00467-006-0145-z. [DOI] [PubMed] [Google Scholar]
- 15.Ma YC, Zuo L, Chen JH, et al. Improved GFR estimation by combined creatinine and cystatin C measurements. Kidney Int. 2007;72:1535–1542. doi: 10.1038/sj.ki.5002566. [DOI] [PubMed] [Google Scholar]
- 16.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 18.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis. 2011;58:214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Anthropometry Procedures Manual. Centers for Disease Control and Prevention. 2000 [Google Scholar]
- 23.Baumgartner RN, Chumlea WC, Roche AF. Bioelectric impedance phase angle and body composition. Am J Clin Nutr. 1988;48:16–23. doi: 10.1093/ajcn/48.1.16. [DOI] [PubMed] [Google Scholar]
- 24.Chumlea WC, Guo SS, Kuczmarski RJ, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–1609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
- 25.Perrone RD, Steinman TI, Beck GJ, et al. Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous comparison of 125I-iothalamate, 169Yb-DTPA, 99mTc-DTPA, and inulin. The Modification of Diet in Renal Disease Study. Am J Kidney Dis. 1990;16:224–235. doi: 10.1016/s0272-6386(12)81022-5. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Greene T, Schluchter MD, et al. Glomerular filtration rate measurements in clinical trials. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group. J Am Soc Nephrol. 1993;4:1159–1171. doi: 10.1681/asn.v451159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall PM, Rolin H. Iothalamate clearance and its use in large-scale clinical trials. Curr Opin Nephrol Hypertens. 1995;4:510–513. doi: 10.1097/00041552-199511000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Joffe M, Hsu CY, Feldman HI, Weir M, Landis JR, Hamm LL. Variability of creatinine measurements in clinical laboratories: results from the CRIC study. Am J Nephrol. 2010;31:426–434. doi: 10.1159/000296250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson AH, Xie D, Tao K, Landis JR, Rader D, Feldman HI. Internal standardization of cystatin C measurements in the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2010;21:176A. ASN abstract 287. [Google Scholar]
- 30.Israelit AH, Long DL, White MG, Hull AR. Measurement of glomerular filtration rate utilizing a single subcutaneous injection of 125I-iothalamate. Kidney Int. 1973;4:346–349. doi: 10.1038/ki.1973.127. [DOI] [PubMed] [Google Scholar]
- 31.Ott N, Wilson D. A simple technique for estimating glomerular filtration rate with subcutaneous injection of 125I-iothalamate. Mayo Clin Proc. 1975;50:664–668. [PubMed] [Google Scholar]
- 32.Adefuin PY, Gur A, Siegel NJ, Spencer RP, Hayslett JP. Single subcutaneous injection of iothalamate sodium I 125 to measure glomerular filtration rate. JAMA. 1976;235:1467–1469. [PubMed] [Google Scholar]
- 33.Anderson AH, Yang W, Landis JR, Xie D, Teal V, Feldman HI. Variation in the 125-I iothalamate glomerular filtration rate (GFR) procedure in the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2010;21:178A. ASN abstract 293. [Google Scholar]
- 34.Jonsson AS, Flodin M, Hansson LO, Larsson A. Estimated glomerular filtration rate (eGFRCystC) from serum cystatin C shows strong agreement with iohexol clearance in patients with low GFR. Scand J Clin Lab Invest. 2007;67:801–809. doi: 10.1080/00365510701397538. [DOI] [PubMed] [Google Scholar]
- 35.Grubb A, Bjork J, Nyman U, et al. Cystatin C, a marker for successful aging and glomerular filtration rate, is not influenced by inflammation. Scand J Clin Lab Invest. 2011;71:145–149. doi: 10.3109/00365513.2010.546879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grubb A. Non-invasive estimation of glomerular filtration rate (GFR). The Lund model: Simultaneous use of cystatin C- and creatinine-based GFR-prediction equations, clinical data and an internal quality check. Scand J Clin Lab Invest. 2010;70:65–70. doi: 10.3109/00365511003642535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwong YT, Stevens LA, Selvin E, et al. Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis. 2010;56:39–49. doi: 10.1053/j.ajkd.2010.02.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blirup-Jensen S, Grubb A, Lindstrom V, Schmidt C, Althaus H. Standardization of Cystatin C: development of primary and secondary reference preparations. Scand J Clin Lab Invest Suppl. 2008;241:67–70. doi: 10.1080/00365510802150067. [DOI] [PubMed] [Google Scholar]
- 39.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48:1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]