Abstract
Drug abuse and HIV infection are interlinked. From the onset of the HIV/AIDS epidemic, the impact of illicit drug use on HIV disease progression has been a focus of many investigations. Both laboratory-based and epidemiological studies strongly indicate that drug abuse may exacerbate HIV disease progression and increase mortality and morbidity in these patients Increase susceptibility to opportunistic infection has been implicated as one of the major cause for this detriment. Furthermore, opioids are known to elicit prevalence of neurodegenerative disorders in HIV-infected patients. Numerous authors have delineated various molecular as well as cellular mechanisms associated with neurological complications in these patients. This review gives an overview of these findings. Understanding the mechanisms will allow for the development of targeted therapies aimed at reducing the progression of neurocognitive decline in the drug abusing HIV infected individuals.
Keywords: Opioid, HAND, microglia, astrocyte, neuron, TLR, opportunistic infection
Introduction
Worldwide, the seroprevalence of human immunodeficiency virus (HIV) among intravenous drug users (IDUs) is alarmingly high. Out of approximately 16 million IDUs worldwide, an estimated 3 million are HIV infected [1, 2]. From the beginning of HIV epidemic, IDU has directly and indirectly accounted for more than one-third (36%) of AIDS cases in the United States (http://www.cdc.gov/hiv/resources/Factsheets/idu.htm). This disturbing trend appears to be still continuing. Injection heroin (an opioid), one of the major drug of abuse among IDUs, has been reported to be directly involved in the morbidity and mortality associated with HIV infection and AIDS. Even though the pervasiveness of heroin abuse in USA over the last decade has been relatively stable, the misuse of prescription opioids has markedly increased [3]. In the past year, about 400,000 Americans used heroin, while 12 million misused prescription opioids (http://www.oas.samhsa.gov/NSDUH/2k7NSDUH/2k7results.cfm). Patients with comorbid HIV infection and opioid dependence are less likely to have access to HIV clinical care, less likely to receive or follow antiretroviral therapy and more likely to have rapid disease progression [4–8]. Immunosuppressive properties of chronic opioid have been postulated to promote accelerates the incidence of infection with secondary opportunistic pathogens in HIV infected individuals [9].
Opioid Drug Abuse, Immunosuppression and susceptibility to Infection
Several laboratories as well as epidemiological studies strongly indicate that drug abuse may exacerbate risk factor for HIV acquisition and disease progression [10–13]. Increased susceptibility to opportunistic infection has been implicated as one of the major causes for this detriment. The evidence implicating high prevalence of viral hepatitis A, B, and C, bacterial pneumonias, tuberculosis, abscesses, and other soft tissue infections in heroin abusers continues to rise [14, 15]. Parallel morphine exposure in animals show increased lethality to the fungus, Candida albicans, and sensitizations to intraperitoneally administered Klebsiella pneumoniae [16]. S. pneumoniae is one of the most common diagnoses among opiate abusers and is responsible for more than 25% of all cases of pneumonia [17]. It is estimated to have an overall mortality rate of 23% among hospitalized patients. Pneumococcal clearance requires the cooperation of reactions from both innate and adaptive immunity. We have previously shown that chronic morphine treatment followed by intranasal inoculation with S. pneumoniae markedly delayed neutrophil recruitment, increased bacterial burden in the lung, spleen, and blood with a subsequent increase in mortality. In addition we also show significant dissemination of bacteria into the central nervous system (CNS) [18]. Based on these studies, we postulate that the synergistic immunosuppressive effect of opioid and HIV infection, increase risk of secondary infection and exacerbate neurocognitive impairment in infected individuals.
Infection and neuropathogenesis
It is well known that systemic infections cause flare-ups of disease in individuals with asthma and rheumatoid arthritis, and that relapses in multiple sclerosis can often be associated with upper respiratory-tract infections [19]. In chronic neurodegenerative diseases such as Alzheimer’s disease, systemic infections and inflammation can cause acute exacerbations of symptoms and drive the progression of neurodegeneration [19]. In a retrospective general-practitioner database study, the presence of two or more infections over a 4-year follow-up period increased the odds of developing Alzheimer’s disease by around twofold [20]. Evidence that systemic inflammation in general is a risk factor for the future development of Alzheimer’s disease has been found in a number of studies. Inflammatory proteins in the plasma, notably C-reactive protein and IL-6, were found to be increased 5 years before the clinical onset of dementia in several studies. Animal models suggest that when activated microglia are further activated by a subsequent systemic infection a significant increase in proinflammatory cytokines are observed within the CNS, which in turn potentiates neurodegeneration [21]. Chronic or recurrent infections contribute to sustained high levels of viremia and thereby accelerate HIV disease progression, immune deterioration and neuropathogenesis [22].
Opioid drug abuse and NeuroAIDS
HIV-1 can cause several neurological disorders, collectively known as HIV-associated neurocognitive disorders (HAND). HAND manifests as a subcortical dementia characterized by psychomotor slowing, changes in mood and anxiety levels and deficits in memory, abstraction, information processing, verbal fluency, decision-making, and attention [23–25]. HIV exerts its neurotoxic effects through several secreted proteins including gp120, Tat, Nef and Vpr [26, 27]. A strong relationship exists between opiate usage and HIV-1 neuropathogenesis, as the former correlates with the severity of the CNS disease in heroin-abusing cohorts [28]. However, precise molecular mechanisms which characterize the complex interaction among opiates and HIV neurotoxicity remain a question and area of research that need to be better developed. Accumulating evidence from in vitro and in vivo experimental systems indicates that opiates promote HIV-1 propagation in immune cells while suppressing immune functions [17, 18, 29–31]. Opioid can exacerbate dysregulation of neural cells in HIV positive individuals and accelerate neuropathogenesis by regulating pathogen entry in the immune cells, influencing the trafficking of the infected immune cells and viral transmission in CNS. A recent study by Olin et al., (2011) demonstrates that morphine profoundly exacerbates lymphocyte infiltration into the CNS following a neuroinflammatory stimulus. Opiates enhance the cytotoxicity of HIV-1 viral proteins via mechanisms that involve intracellular calcium modulation and release of pro-inflammatory cytokines, thereby inducing synergistic neurotoxicity affecting the cells of the CNS, making them an important cellular target for HIV-opiate interactions [32]. Chronic opioid drug exposure often leads to altered patterns of gene activation and increased oxidative stress that eventually lead to synaptodendritic miscommunications and neuronal injury [33–35]. Opiates interact with HIV-1 at multiple levels in the pathogenesis of AIDS by disrupting blood brain barrier (BBB), dysregulate glial homeostasis, increasing inflammation, and decreasing the threshold for pro-apoptotic events in neurons. The mechanism by which opioid modulate the different compartment of CNS are the focus of the next section.
Blood–brain barrier (BBB) integrity and HIV
The BBB is present at the interface between the brain and the periphery. It strictly controls the exchanges between both compartments, by actively transporting nutrients to the brain and protecting the CNS from various xenobiotics and pathogens. It’s extremely low permeability is due to endothelial tight junctions and the activity of multiple efflux transport systems. In addition, migration of circulating cells, notably activated leukocytes, across the BBB is a finely regulated dynamic process, responsible for the low-level but significant immunological survey of the brain in physiological conditions. Any dysregulation of the BBB could facilitate virus or other opportunistic pathogens entry to the CNS and eventually lead to aggravation of CNS associated complications. The properties of the BBB can be altered by modifying the expression of certain major molecular components of the tight junction proteins which includes transmembranous and structural proteins, occludin, junctional adhesion molecule (JAM) and claudins, and the submembranous peripheral zona occludin (ZO) proteins. HIV gp120 proteins and HIV-1 gp160 peptides both alter the functional and molecular properties of the BBB [36]. HIV gp120 increase brain endothelial cell permeability by disrupting and downregulating the tight junction proteins (ZO-1, ZO-2 and occludin) expression which could results in increase trafficking of HIV infected cells into the CNS [37]. In addition, HIV-1 Tat protein also reported to compromises BBB integrity by decreasing claudin-1, claudin-5, and ZO-2 expressions [38].
Opioid effect on BBB in the context of HIV
Morphine alone or in combination with Tat, results in alterations in the expression of certain TJ proteins (ZO-1 and occludin), thereby resulting in the breakdown of BBB integrity [32]. Disruptions of BBB integrity by morphine can be further reflected by enhanced transendothelial migration and a significant decrease in TEER across the BBB. Further, a combined treatment of Tat and morphine resulted in a synergistic increase in intracellular Ca2+ levels and activation of myosin light chain kinase in primary brain microvascular endothelial cells (BMVEC) resulting in enhanced transendothelial migration across the BBB [32]. In addition, Lynch and Banks, (2008) reported both chronic as well as morphine withdrawal modulates proinflammatory cytokines (IL-1alpha, IL-2, and TNF-alpha) transport across the BBB [39]. Moreover, morphine by itself can cross the BBB in sufficient amounts to affect brain function.
Microglia and their role in HAND
Infection and activation of microglia is now recognized as an important factor in the development of HAND. Microglia are the only brain cell type that can be productively infected by HIV, and—because of their ability to harbor viral particles intracellularly - are also potential reservoirs of the virus [40, 41]. Virus crosses BBB through infected monocytes (“Trojan Horse” method) that later differentiate into macrophages [42]. It is now recognized that the number of activated microglia and macrophages in the CNS better correlates with HAND than viral load or the number of HIV-1-infected cells [43, 44] and microglial cell activation is a better correlate of neuronal damage than productive HIV-1 infection in the CNS [45]. These observations underscore the importance of activated microglia/macrophages as potential biomarkers of HIV-1 neuropathogenesis. Microglial nodules, which are focal aggregates of multinuclear giant cells from the fusion of microglial cells and macrophages [46] is a histological marker of HIV brain infection. In brains from HAD patients, apoptotic neurons do not co-localize with infected microglia, supporting the hypothesis that neurotoxic mediators released from microglia play a major role in HIV-1 neuropathogenesis [47]. In an SIV model of neuroAIDS when studies were carried out to investigate potential CSF markers to predict disease stage, consistently MCP-1 and IL-6 correlated with HAND [48, 49]. Indeed, it is now recognized that HIV-1- infected microglia and other brain macrophages actively secrete both neurotoxins such as TNF-α, IL-6, MCP-1, glutamate, quinolinic acid, platelet activating factor, eicosanoids, and nitric oxide as well as neurotoxic viral proteins such as Tat, gp120, and gp41 [50]. Ultimately, a complex interaction between activated microglia/macrophages, astrocytes, and neurons triggers the onset of neuronal dysfunction or apoptosis and progression of CNS damage.
Opioid effect on microglia in the context of HIV
Morphine augments the cytotoxic effect of HIV-1 Tat1–72 observed in mouse BV-2 microglial cells and primary mouse microglia. A dramatic increase in the expression of the microglial activation marker CD11b, mRNA expression of nitric oxide synthase (nitric oxide synthase (iNOS), CD40 ligand, Interferon-gamma-inducible protein 10 (IP-10) and proinflammatory cytokines (TNF-α, IL-1β, IL-6) was observed in BV-2 microglial cell line [28]. Various independent studies have shown that morphine and other μ-receptor agonists exacerbates the expression of major HIV-1 coreceptors i.e. β-chemokine receptor 5 (CCR5) and α-chemokine receptor 4 (CXCR4) on the peripheral as well as on microglia cells [28, 51– 57]. These chemokine receptors cooperate sequentially with CD4 and facilitate virus entry into target cells. Presently, there is no clear mechanism by which morphine induces these chemokine receptors. This could be due to the release of cytokines (TNF-α or IL-2) which are known to stimulate chemokine receptor expression or, alternatively, morphine may inhibit the synthesis of certain chemokines (e.g., RANTES, macrophage inflammatory protein MIP-1a (CCL3), or MIP-1b (CCL4) that cause internalization of receptors [58]. In a recent study Zou et al., (2011) document that morphine can potentiate neurodegenerative effects of HIV-1 Tat through actions at μ-opioid receptor-expressing glia [59]. Opioid receptors form an oligomeric protein complex with CCR5 on the cell membrane of immune cells. The activation of opioid receptors by morphine simultaneously induces the expression of CCR5 and promotes viral entry to the cell. In addition, to viral entry, morphine can modulate the trafficking/recruitment of HIV infected immune cells to CNS. There are many published data which reports that systemic morphine markedly potentiate the production of CCL5, CCL2, and IL-6 in astrocytes of mice treated with intrastriatal HIV-1 Tat protein [60–62]. Increase secretion of chemokine ligands in CNS is postulated to draw HIV infected leukocyte trafficking into the brain resulting in neuropathogenesis.. Molecular mechanisms involved in opiate induced HIV replication is shown to be associated with concurrent inhibition of anti-HIV microRNA (miRNA-28, 125b, 150, and 382) expression [63]. Heroin-dependent subjects had significantly lower levels of anti-HIV miRNAs in peripheral blood mononuclear cells than the healthy subjects. In vitro and in vivo findings indicate that opioid use impairs intracellular innate anti-HIV mechanisms in monocytes which than function as cofactors in the immunopathogenesis of HIV disease.
Astrocytes and HIV
Astrocytes are essential in regulating neuronal function and support, and participate in neurogenesis, synaptic transmission, brain repair, and in the formation and preservation of the BBB. When brain homeostasis is disrupted, astrocytes become activated, producing a variety of factors, including nitric oxide, neuropeptides and cytokines. Astrocytes fulfill many functions in the CNS, disturbances of which are likely to have serious consequences. Numerous studies show that HIV infects astrocytes, resulting in persistent infection [64–66]. Ultrastructural observation through transmission electron microscopic reveals the presence of viral particles in HIV encephalitis (HIVE) brain samples within the astrocyte cytoplasm. The presence of intermediate filaments, as well as multinucleated giant cells were also identified [67–69]. HIVp17 and p24 were also demonstrated in astrocytes using immunocytochemical methods in HIVE cases [70]. A number of studies employing the in situ PCR technique have shown that a proportion of astrocyte nuclei contain HIV-1 DNA both in adult and pediatric biopsy samples of AIDS [71–75]. Interestingly most of the infected cells were in the gray than in white matter and that the total number of infected cells significantly correlates with the severity of neurocognitive symptoms [72, 73]. The mechanism of HIV entry into astrocytes in the intact brain remains unknown. Entry of HIV into astrocytes may occur by a receptor-mediated endocytic pathway [76], rather than by fusion of HIV with cell surface membrane. In addition, report shows some astrocytes express the chemokine receptor CCR5, major HIV-1 coreceptor [77]. HIV-1 infection is known to upregulate expression of astrocytic adhesion molecules, namely ICAM-1 and VCAM-1 [78], which support adherence of lymphocytes and monocytes. Considering the significance of these cells for the function of the CNS and their potential to fulfill both protective and detrimental roles in HIV-induced neuropathogenesis, astrocytes play a significant role in neuroAIDS. The unique location of astrocytes at the BBB suggests that they could protect the CNS from the effects of extensive virus production, especially during the pre-AIDS phase, by storing incoming HIV in a more or less dormant state.
Opioid effect on astrocytes in the context of HIV
Astrocytes can express mu-opioid receptors, and are likely targets for abused opiates, which preferentially activate mu-opioid receptors and are the key intermediaries in opiate-HIV interactions [79]. Disruptions in astroglial function and inflammatory signaling may contribute to an accelerated neuropathogenesis in HIV-infected individuals who abuse opiates. Opiates act by exacerbating the astroglial response to HIV-1, which involves the release of cytokines (e.g., TNF-α, IL-1β and IL-6) and chemokines (e.g., CCL2/MCP-1, CCL5/RANTES, and CCL3/MIP-1 α), which are potent chemokines thought to be involved in the progression of neuroAIDS and propagating HIV by virtue of its ability to recruit and activate macrophages/microglia [79,80]. On the contrary a significant study on primary normal human astrocytes showed morphine induced significant downregulation of the gene expression of beta chemokines, MCP-1, and MIP-1 beta, while reciprocally upregulating the expression of their specific receptors, CCR2b, CCR3, and CCR5 as detected by real-time quantitative PCR [54]. Morphine-induced effects on astrocytes were reversed by the opioid mu receptor antagonist, naloxone. Further, results indicate that morphine-induced effects are mediated via the modulation of MAPK and CREB signaling pathways. Data further validate the notion that opiates act as co-factors in the neuropathogenesis of HIV infection. A synergistic increase in intracellular Ca2+ was observed by astrocytes treated with opiates and HIV-1 Tat. According to profiling studies, screening 152 transcription factors indicated that the NF-kappaB subunit, c-Rel, was a likely candidate for Tat or Tat plus opiate-induced increases in cytokine and chemokine production by astrocytes [80]. Together with the report that opioids breakdown the BBB and increases the number of infiltrating HIV-susceptible cells, opioid exposure may increase the astrocytic reservoir for HIV and augment HIV spread and increase of virus load in the brain. Infected astrocytes may also contribute to HIV-associated neuropathogenesis by producing cellular and/or viral neurotoxic factors.
Toll like receptors and HIV
Toll-like receptors (TLRs) are transmembrane receptors that activate cells of the innate immune systems upon recognition of variety of bacterial, mycobacterial, spirochetal, and viral pathogen. TLRs are a crucial link between the innate immune system and the CNS [81, 82]. Pathogens, injury and stressors stimulate TLRs of glia, recruit adaptor proteins, and initiate the pro-inflammatory signal-transduction pathways that ultimately trigger cytokine transcription [81, 82]. The limited immune surveillance of the CNS makes it important that resident cells be able to rapidly recognize and respond to infection. Innate immunity in the CNS depends primarily on the functions of glial cells which are important for the early control of pathogen replication and direct the recruitment and activation of cells of the adaptive immune system required for pathogen clearance [83]. While ability to mount an effective innate immune response in the CNS is critical to eliminate pathogens and often vital to host survival, however, it is also evident that chronic or dysregulated inflammation in the CNS can cause tissue damage and neurodegeneration. Under resting conditions in vivo, TLRs 1–9 has been detected in the CNS by quantitative real time PCR [84]. The levels of TLRs in the CNS can be upregulated by viral and bacterial infection, treatment with TLR stimuli, or CNS autoimmunity [85, 86], providing a mechanism for amplification of inflammatory responses to pathogens infecting the CNS. Microglia as well as astrocyte produces a variety of chemokines in response to stimulation with TLRs or pathogens, including CCL2, CCL3, CCL4, CCL5 and CXCL10, which are important for recruiting leukocytes into the inflamed CNS [87–90].
HIV-infected patients are frequently co-infected with multiple organisms that can induce HIV replication synergistically or additively. Additive immunosuppressive effect of opioids and HIV provide room for greater secondary infections. Profound infection could lead to costimulation of various TLR ligands, such as TLR4 and TLR2 or TLR9 induce NF-kB activation and inflammatory cytokine production synergistically leading to a synergistic or additive increase in HIV transcription and HIV replication. TLRs represent an attractive therapeutic target for numerous CNS disorders and infectious diseases.
Opioid and TLR cascade
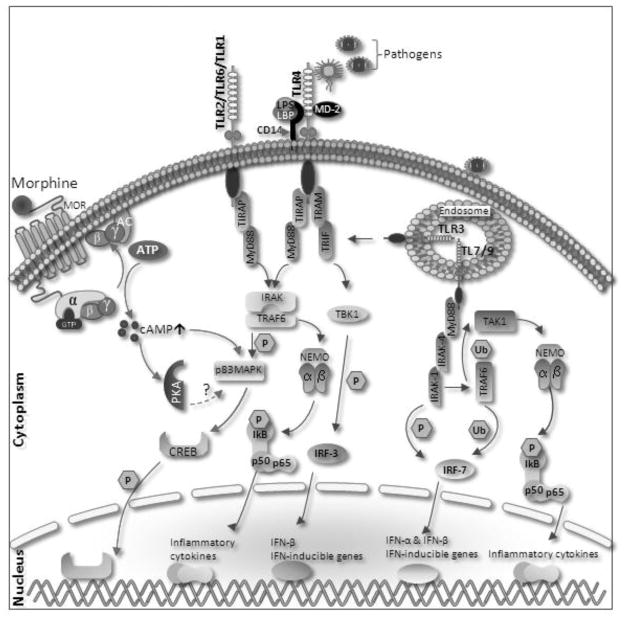
HIV-infected patients displayed enhanced expression of TLR2 but not TLR4 on monocytes [91, 92]. TLR2 expression on the surface of monocytes was significantly increased upon stimulation of HIV type 1 envelope protein gp120, and that TLR2 stimulation in HIV-infected patients induced increased viral replication and TNF-α response. Recently, El-Hage et al reported modulation of TLR2, 4 and 9 expression on astroglia on exposure to morphine alone as well as in addition of Tat, gp120 [93]. A significant increase in TLR2 with a reciprocal decrease in TLR9 expression in astroglia was observed in response to Tat or gp120 in the presence and absence of morphine treatment. In addition, morphine alone cause significantly decrease the TLR9 expression. Activation of TLR9 signaling triggers activation of proapoptotic signals, and causes cell apoptosis in various systems [94]. In addition, TLR9 signaling mainly activates inflammatory responses, including proinflammatory cytokines. Morphine can induce TLR9 expression through a classical mu-opioid receptor (MOR) dependent mechanism and induced apoptosis in microglia [95]. Hence morphine by itself or in the presence of HIV and HIV-related opportunistic infections can differentially modulate the TLR signaling resulting into diverse neurological alterations. Figure 1 we illustrate the mechanism by which opioid activation of MOR modulate TLR signaling.
Figure 1.
Proposed mechanism for opioid modulation for TLR. Morphine leads to superactivation of adenylyl cyclase and increase intracellular cAMP levels. cAMP activates p38MAPK in turn leading to CREB (cAMP response element-binding)phosphorylation and nuclear translocation, leading transcription of its target genes. Surface receptor (TLR4, TLR2) and endosomal receptors (TLR7 and TLR9) mediate through MyD88 dependent pathway. Upon activation of TLR 2 and 4 by their cognate ligands, MyD88 recruits IRAK4, thereby allowing the association of IRAK1. IRAK4 then induces the phosphorylation of IRAK1. TRAF6 (Tumour Necrosis Factor-Receptor-Associated Factor-6) is then recruited to the receptor complex, by associating with phosphorylated IRAK1. Phosphorylated IRAK1 and TRAF6 then dissociate from the receptor to form a complex with TBK1 (TGF-Beta-Activated Kinase-1)- a ubiquitin ligases (Ub). This leads to the ubiquitylation of TRAF6, which induces the activation of TAK1. TAK1, in turn, phosphorylates p38MAPK (mitogen activated protein kinase). p38 phosphorylates CREB and induce the expression of its target genes. TAK1 also phosphorylates NEMO (nuclear factor-κB (NF-κB) essential modulator) which having IKK complex (Inhibitor of Kappa Light Polypeptide Gene Enhancer in B-Cells Kinase). The IKK complex then phosphorylates I-KappaB, which leads to its ubiquitylation and subsequent degradation. This allows NF-KappaB to translocate to the nucleus and induce the expression of its target genes. TRIF and TRAM link TLR4 to pathways that lead to TBK1 (TANK Binding Kinase-1) and IRF3 activation (i.e., the MyD88-independent pathway).
Effect on Neurons
HIV-1 does not infect neurons directly, instead, soluble neurotoxic factors (Tat, gp120, pro-inflammatory cytokines, chemokines, excitotoxins and proteases) secreted from either virus and/or infected glia eventually induce programmed cell death in neurons through p38 mitogen-activated protein kinase signaling cascade.
Opioid effect on neural cells
Various authors have reported synergistic neuronal death by morphine and HIV-1 Tat in vitro [96,97]. In addition, morphine transiently exacerbated X4-tropic gp120 (IIIB)-induced neuronal death. In combination with dual-tropic gp120 (MN), morphine caused sustained increases in the rate of neuronal death compared to gp120 (MN) alone that were prevented by naloxone [98]. Various in vitro studies indicate a wide-ranging expression of TLR mRNA in neural cell [99, 100], although the expression of neuronal TLR mRNA is more controversial. So, any direct effect of secondary infection on neural TLRs, in the presence of morphine may be potential mechanism for the various molecular and cellular mechanisms associated with neurodegeneration in HIV/AIDS patients.
Summary
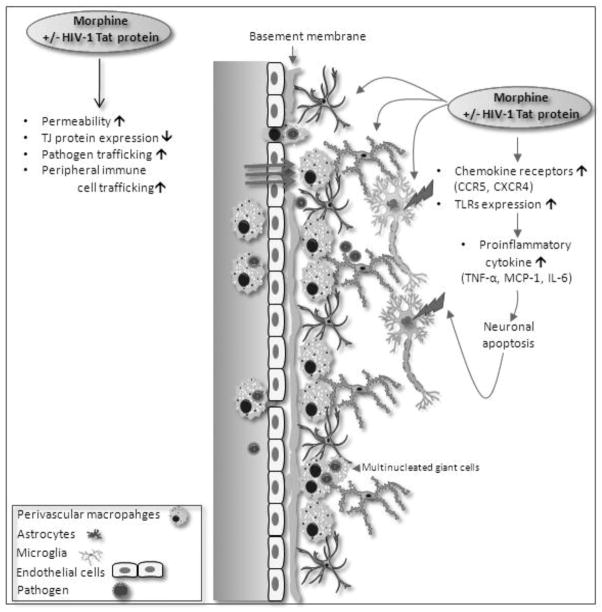
Complete eradication of HIV-1 from infected patients’ remains problematic, although the use of HAART has improved the prognosis of patients who are HIV-positive, complete eradication of HIV-1 from infected patients remains problematic. The persistence of viral reservoirs and continuous rounds of de novo virus infection of host cells with rapid turnover of both free virus and virus-producing cells are major obstacles that must be surmounted for complete eradication. Wealth of literature implicates induction of immune activation, by opportunistic pathogens, affect the infectability, transmissibility, and pathogenesis of HIV by influencing several aspects of HIV biology. Monocytes/macrophage are major reservoirs for HIV and infection of monocytes/macrophages by many pathogens can induce immune activation of monocytes and T lymphocytes, thus increasing the infectivity of these cells to HIV. Infections promote disease progression by creating a cytokine environment that favours faster replication of HIV. Furthermore, infectious agents can modulate the expression of coreceptors on the surfaces of permissive cellular targets, thus altering the cellular tropism. Such immune pressures can lead to emergence of viral variants that not only use multiple coreceptors, but also are more virulent. Opioid drug abuse significantly modulates these mechanisms thus contributing to HIV disease progression and neuropathogenesis (figure 2).
Figure 2.
A schematic diagram outlining mechanisms by which opioid drug modulate HIV neuropathogenesis. Opioid abuse (morphine) in the presence or absence of HIV-1 TAT on central nervous system results into accelerated neuropathogenesis. CCR5 (C-C Chemokine receptor type 5), Tight junction (TJ) protein, CXCR4 (C-X-C chemokine receptor type 4), TLRs (Toll-like receptors), TNFα (Tum.or necrosis factor-alpha), MCP1 (Monocyte chemotactic protein-1), IL-6 (Interleukin-6).
Acknowledgments
Funded by: RO1 DA 12104, RO1 DA 022935, RO1 DA031202, K05DA033881, P50 DA 011806, 1R01DA034582
Reference List
- 1.Mathers BM, Degenhardt L, Ali H, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375:1014–1028. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- 2.Vlahov D, Robertson AM, Strathdee SA. Prevention of HIV infection among injection drug users in resource-limited settings. Clin Infect Dis. 2010;50 (Suppl 3):S114–21. doi: 10.1086/651482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83 (Suppl 1):S4–7. doi: 10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Celentano DD, Vlahov D, Cohn S, Shadle VM, Obasanjo O, Moore RD. Self-reported antiretroviral therapy in injection drug users. JAMA. 1998;280:544–546. doi: 10.1001/jama.280.6.544. [DOI] [PubMed] [Google Scholar]
- 5.Strathdee SA, Palepu A, Cornelisse PG, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- 6.Moore RD, Keruly JC, Chaisson RE. Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. J Acquir Immune Defic Syndr. 2004;35:46–51. doi: 10.1097/00126334-200401010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Poundstone KE, Chaisson RE, Moore RD. Differences in HIV disease progression by injection drug use and by sex in the era of highly active antiretroviral therapy. AIDS. 2001;15:1115–1123. doi: 10.1097/00002030-200106150-00006. [DOI] [PubMed] [Google Scholar]
- 8.Malta M, Strathdee SA, Magnanini MM, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction. 2008;103:1242–1257. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- 9.Bruce RD, Altice FL. Clinical care of the HIV-infected drug user. Infect Dis Clin North Am. 2007;21:149–79. ix. doi: 10.1016/j.idc.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray M, Hogg R, Lima V, et al. The effect of injecting drug use history on disease progression and death among HIV-positive individuals initiating combination antiretroviral therapy: collaborative cohort analysis. HIV Med. 2011 doi: 10.1111/j.1468-1293.2011.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Tan N, Douglas SD, Zhang T, Wang YJ, Ho WZ. Morphine inhibits CD8+ T cell-mediated, noncytolytic, anti-HIV activity in latently infected immune cells. J Leukoc Biol. 2005;78:772–776. doi: 10.1189/jlb.0305167. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Douglas SD, Peng JS, et al. Naltrexone inhibits alcohol-mediated enhancement of HIV infection of T lymphocytes. J Leukoc Biol. 2006;79:1166–1172. doi: 10.1189/jlb.1105642. [DOI] [PubMed] [Google Scholar]
- 13.Purohit V, Rapaka R, Shurtleff D. Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Mol Neurobiol. 2011;44:102–110. doi: 10.1007/s12035-011-8195-z. [DOI] [PubMed] [Google Scholar]
- 14.Quaglio G, Lugoboni F, Talamini G, Lechi A, Mezzelani P. Prevalence of tuberculosis infection and comparison of multiple-puncture liquid tuberculin test and Mantoux test among drug users. Scand J Infect Dis. 2002;34:574–576. doi: 10.1080/00365540110080791. [DOI] [PubMed] [Google Scholar]
- 15.Nath A, Hauser KF, Wojna V, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31 (Suppl 2):S62–9. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- 16.Reichman LB, Felton CP, Edsall JR. Drug dependence, a possible new risk factor for tuberculosis disease. Arch Intern Med. 1979;139:337–339. [PubMed] [Google Scholar]
- 17.Wang J, Barke RA, Charboneau R, Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol. 2005;174:426–434. doi: 10.4049/jimmunol.174.1.426. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Barke RA, Charboneau R, Schwendener R, Roy S. Morphine induces defects in early response of alveolar macrophages to Streptococcus pneumoniae by modulating TLR9-NF-kappa B signaling. J Immunol. 2008;180:3594–3600. doi: 10.4049/jimmunol.180.5.3594. [DOI] [PubMed] [Google Scholar]
- 19.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 20.Dunn N, Mullee M, Perry VH, Holmes C. Association between dementia and infectious disease: evidence from a case-control study. Alzheimer Dis Assoc Disord. 2005;19:91–94. doi: 10.1097/01.wad.0000165511.52746.1f. [DOI] [PubMed] [Google Scholar]
- 21.Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Power C, Johnson RT. Neuroimmune and neurovirological aspects of human immunodeficiency virus infection. Adv Virus Res. 2001;56:389–433. doi: 10.1016/s0065-3527(01)56034-0. [DOI] [PubMed] [Google Scholar]
- 23.Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J Neuroimmune Pharmacol. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krebs FC, Ross H, McAllister J, Wigdahl B. HIV-1-associated central nervous system dysfunction. Adv Pharmacol. 2000;49:315–385. doi: 10.1016/s1054-3589(00)49031-9. [DOI] [PubMed] [Google Scholar]
- 25.Kolson DL, Lavi E, Gonzalez-Scarano F. The effects of human immunodeficiency virus in the central nervous system. Adv Virus Res. 1998;50:1–47. doi: 10.1016/s0065-3527(08)60804-0. [DOI] [PubMed] [Google Scholar]
- 26.Ozdener H. Molecular mechanisms of HIV-1 associated neurodegeneration. J Biosci. 2005;30:391–405. doi: 10.1007/BF02703676. [DOI] [PubMed] [Google Scholar]
- 27.Jones G, Power C. Regulation of neural cell survival by HIV-1 infection. Neurobiol Dis. 2006;21:1–17. doi: 10.1016/j.nbd.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Bokhari SM, Yao H, Bethel-Brown C, et al. Morphine enhances Tat-induced activation in murine microglia. J Neurovirol. 2009;15:219–228. doi: 10.1080/13550280902913628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy S, Ninkovic J, Banerjee S, et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol. 2011;6:442–465. doi: 10.1007/s11481-011-9292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brack A, Rittner HL, Stein C. Immunosuppressive effects of opioids-clinical relevance. J Neuroimmune Pharmacol. 2011;6:490–502. doi: 10.1007/s11481-011-9290-7. [DOI] [PubMed] [Google Scholar]
- 31.Madera-Salcedo IK, Cruz SL, Gonzalez-Espinosa C. Morphine decreases early peritoneal innate immunity responses in Swiss-Webster and C57BL6/J mice through the inhibition of mast cell TNF-alpha release. J Neuroimmunol. 2011;232:101–107. doi: 10.1016/j.jneuroim.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Mahajan SD, Aalinkeel R, Sykes DE, et al. Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability. J Clin Immunol. 2008;28:528–541. doi: 10.1007/s10875-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 33.Turchan-Cholewo J, Dimayuga FO, Gupta S, et al. Morphine and HIV-Tat increase microglial-free radical production and oxidative stress: possible role in cytokine regulation. J Neurochem. 2009;108:202–215. doi: 10.1111/j.1471-4159.2008.05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turchan-Cholewo J, Dimayuga FO, Ding Q, et al. Cell-specific actions of HIV-Tat and morphine on opioid receptor expression in glia. J Neurosci Res. 2008;86:2100–2110. doi: 10.1002/jnr.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 36.Kanmogne GD, Kennedy RC, Grammas P. HIV-1 gp120 proteins and gp160 peptides are toxic to brain endothelial cells and neurons: possible pathway for HIV entry into the brain and HIV-associated dementia. J Neuropathol Exp Neurol. 2002;61:992–1000. doi: 10.1093/jnen/61.11.992. [DOI] [PubMed] [Google Scholar]
- 37.Kanmogne GD, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol. 2005;64:498–505. doi: 10.1093/jnen/64.6.498. [DOI] [PubMed] [Google Scholar]
- 38.Andras IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res. 2003;74:255–265. doi: 10.1002/jnr.10762. [DOI] [PubMed] [Google Scholar]
- 39.Lynch JL, Banks WA. Opiate modulation of IL-1alpha, IL-2, and TNF-alpha transport across the blood-brain barrier. Brain Behav Immun. 2008;22:1096–1102. doi: 10.1016/j.bbi.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Kaul M, Lipton SA. Mechanisms of neuroimmunity and neurodegeneration associated with HIV-1 infection and AIDS. J Neuroimmune Pharmacol. 2006;1:138–151. doi: 10.1007/s11481-006-9011-9. [DOI] [PubMed] [Google Scholar]
- 41.Liu NQ, Lossinsky AS, Popik W, et al. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J Virol. 2002;76:6689–6700. doi: 10.1128/JVI.76.13.6689-6700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albright AV, Soldan SS, Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol. 2003;9:222–227. doi: 10.1080/13550280390194073. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 44.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 45.Adle-Biassette H, Chretien F, Wingertsmann L, et al. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 1999;25:123–133. doi: 10.1046/j.1365-2990.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- 46.Corr P. Imaging of neuro-AIDS. J Psychosom Res. 2006;61:295–299. doi: 10.1016/j.jpsychores.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186 (Suppl 2):S193–8. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 48.Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 50.Rock RB, Peterson PK. Microglia as a pharmacological target in infectious and inflammatory diseases of the brain. J Neuroimmune Pharmacol. 2006;1:117–126. doi: 10.1007/s11481-006-9012-8. [DOI] [PubMed] [Google Scholar]
- 51.Burbassi S, Aloyo VJ, Simansky KJ, Meucci O. GTPgammaS incorporation in the rat brain: a study on mu-opioid receptors and CXCR4. J Neuroimmune Pharmacol. 2008;3:26–34. doi: 10.1007/s11481-007-9083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Happel C, Steele AD, Finley MJ, Kutzler MA, Rogers TJ. DAMGO-induced expression of chemokines and chemokine receptors: the role of TGF-beta1. J Leukoc Biol. 2008;83:956–963. doi: 10.1189/jlb.1007685. [DOI] [PubMed] [Google Scholar]
- 53.Mahajan SD, Aalinkeel R, Reynolds JL, et al. Morphine exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in U373 astrocytoma cells. Curr HIV Res. 2005;3:277–288. doi: 10.2174/1570162054368048. [DOI] [PubMed] [Google Scholar]
- 54.Mahajan SD, Schwartz SA, Aalinkeel R, Chawda RP, Sykes DE, Nair MP. Morphine modulates chemokine gene regulation in normal human astrocytes. Clin Immunol. 2005;115:323–332. doi: 10.1016/j.clim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309:99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki S, Carlos MP, Chuang LF, Torres JV, Doi RH, Chuang RY. Methadone induces CCR5 and promotes AIDS virus infection. FEBS Lett. 2002;519:173–177. doi: 10.1016/s0014-5793(02)02746-1. [DOI] [PubMed] [Google Scholar]
- 57.Miyagi T, Chuang LF, Doi RH, Carlos MP, Torres JV, Chuang RY. Morphine induces gene expression of CCR5 in human CEMx174 lymphocytes. J Biol Chem. 2000;275:31305–31310. doi: 10.1074/jbc.M001269200. [DOI] [PubMed] [Google Scholar]
- 58.Hu S, Chao CC, Hegg CC, Thayer S, Peterson PK. Morphine inhibits human microglial cell production of, and migration towards, RANTES. J Psychopharmacol. 2000;14:238–243. doi: 10.1177/026988110001400307. [DOI] [PubMed] [Google Scholar]
- 59.Zou S, Fitting S, Hahn YK, et al. Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at mu-opioid receptor-expressing glia. Brain. 2011 doi: 10.1093/brain/awr281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Hage N, Wu G, Ambati J, Bruce-Keller AJ, Knapp PE, Hauser KF. CCR2 mediates increases in glial activation caused by exposure to HIV-1 Tat and opiates. J Neuroimmunol. 2006;178:9–16. doi: 10.1016/j.jneuroim.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El-Hage N, Wu G, Wang J, et al. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53:132–146. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Avdoshina V, Biggio F, Palchik G, Campbell LA, Mocchetti I. Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia. 2010;58:1630–1639. doi: 10.1002/glia.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Ye L, Zhou Y, Liu MQ, Zhou DJ, Ho WZ. Inhibition of anti-HIV microRNA expression: a mechanism for opioid-mediated enhancement of HIV infection of monocytes. Am J Pathol. 2011;178:41–47. doi: 10.1016/j.ajpath.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atwood WJ, Tornatore CS, Meyers K, Major EO. HIV-1 mRNA transcripts from persistently infected human fetal astrocytes. Ann N Y Acad Sci. 1993;693:324–325. doi: 10.1111/j.1749-6632.1993.tb26298.x. [DOI] [PubMed] [Google Scholar]
- 65.Yao K, Akyani N, Donati D, et al. Detection of HHV-6B in post-mortem central nervous system tissue of a post-bone marrow transplant recipient: a multi-virus array analysis. J Clin Virol. 2006;37 (Suppl 1):S57–62. doi: 10.1016/S1386-6532(06)70013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78:7319–7328. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Epstein LG, Sharer LR, Cho ES, Myenhofer M, Navia B, Price RW. HTLV-III/LAV-like retrovirus particles in the brains of patients with AIDS encephalopathy. AIDS Res. 1984;1:447–454. doi: 10.1089/aid.1.1983.1.447. [DOI] [PubMed] [Google Scholar]
- 68.Mirra SS, del Rio C. The fine structure of acquired immunodeficiency syndrome encephalopathy. Arch Pathol Lab Med. 1989;113:858–865. [PubMed] [Google Scholar]
- 69.Gyorkey F, Melnick JL, Gyorkey P. Human immunodeficiency virus in brain biopsies of patients with AIDS and progressive encephalopathy. J Infect Dis. 1987;155:870–876. doi: 10.1093/infdis/155.5.870. [DOI] [PubMed] [Google Scholar]
- 70.Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bagasra O, Lavi E, Bobroski L, et al. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 72.Nuovo GJ, Alfieri ML. AIDS dementia is associated with massive, activated HIV-1 infection and concomitant expression of several cytokines. Mol Med. 1996;2:358–366. [PMC free article] [PubMed] [Google Scholar]
- 73.Nuovo GJ, Gallery F, MacConnell P, Braun A. In situ detection of polymerase chain reaction-amplified HIV-1 nucleic acids and tumor necrosis factor-alpha RNA in the central nervous system. Am J Pathol. 1994;144:659–666. [PMC free article] [PubMed] [Google Scholar]
- 74.Sharer LR, Saito Y, Epstein LG, Blumberg BM. Detection of HIV-1 DNA in pediatric AIDS brain tissue by two-step ISPCR. Adv Neuroimmunol. 1994;4:283–285. doi: 10.1016/s0960-5428(06)80268-8. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- 76.Hao HN, Lyman WD. HIV infection of fetal human astrocytes: the potential role of a receptor-mediated endocytic pathway. Brain Res. 1999;823:24–32. doi: 10.1016/s0006-8993(98)01371-7. [DOI] [PubMed] [Google Scholar]
- 77.Rottman JB, Ganley KP, Williams K, Wu L, Mackay CR, Ringler DJ. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am J Pathol. 1997;151:1341–1351. [PMC free article] [PubMed] [Google Scholar]
- 78.Seilhean D, Dzia-Lepfoundzou A, Sazdovitch V, et al. Astrocytic adhesion molecules are increased in HIV-1-associated cognitive/motor complex. Neuropathol Appl Neurobiol. 1997;23:83–92. [PubMed] [Google Scholar]
- 79.El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.El-Hage N, Bruce-Keller AJ, Yakovleva T, et al. Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca(2+)](i), NF-kappaB trafficking and transcription. PLoS One. 2008;3:e4093. doi: 10.1371/journal.pone.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carpentier PA, Duncan DS, Miller SD. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav Immun. 2008;22:140–147. doi: 10.1016/j.bbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lehnardt S, Lehmann S, Kaul D, et al. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 83.Bailey SL, Carpentier PA, McMahon EJ, Begolka WS, Miller SD. Innate and adaptive immune responses of the central nervous system. Crit Rev Immunol. 2006;26:149–188. doi: 10.1615/critrevimmunol.v26.i2.40. [DOI] [PubMed] [Google Scholar]
- 84.McKimmie CS, Fazakerley JK. In response to pathogens, glial cells dynamically and differentially regulate Toll-like receptor gene expression. J Neuroimmunol. 2005;169:116–125. doi: 10.1016/j.jneuroim.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 85.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 86.Laflamme N, Rivest S. Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. FASEB J. 2001;15:155–163. doi: 10.1096/fj.00-0339com. [DOI] [PubMed] [Google Scholar]
- 87.Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Babcock AA, Wirenfeldt M, Holm T, et al. Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. J Neurosci. 2006;26:12826–12837. doi: 10.1523/JNEUROSCI.4937-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Quandt J, Dorovini-Zis K. The beta chemokines CCL4 and CCL5 enhance adhesion of specific CD4+ T cell subsets to human brain endothelial cells. J Neuropathol Exp Neurol. 2004;63:350–362. doi: 10.1093/jnen/63.4.350. [DOI] [PubMed] [Google Scholar]
- 90.Trifilo MJ, Lane TE. The CC chemokine ligand 3 regulates CD11c+CD11b+CD8alpha- dendritic cell maturation and activation following viral infection of the central nervous system: implications for a role in T cell activation. Virology. 2004;327:8–15. doi: 10.1016/j.virol.2004.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heggelund L, Muller F, Lien E, et al. Increased expression of toll-like receptor 2 on monocytes in HIV infection: possible roles in inflammation and viral replication. Clin Infect Dis. 2004;39:264–269. doi: 10.1086/421780. [DOI] [PubMed] [Google Scholar]
- 92.Heggelund L, Damas JK, Yndestad A, et al. Stimulation of toll-like receptor 2 in mononuclear cells from HIV-infected patients induces chemokine responses: possible pathogenic consequences. Clin Exp Immunol. 2004;138:116–121. doi: 10.1111/j.1365-2249.2004.02595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.El-Hage N, Podhaizer EM, Sturgill J, Hauser KF. Toll-like Receptor Expression and Activation in Astroglia: Differential Regulation by HIV-1 Tat, gp120, and Morphine. Immunol Invest. 2011 doi: 10.3109/08820139.2011.561904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen L, Shi W, Li H, et al. Critical role of toll-like receptor 9 in morphine and Mycobacterium tuberculosis-Induced apoptosis in mice. PLoS One. 2010;5:e9205. doi: 10.1371/journal.pone.0009205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.He L, Li H, Chen L, et al. Toll-like receptor 9 is required for opioid-induced microglia apoptosis. PLoS One. 2011;6:e18190. doi: 10.1371/journal.pone.0018190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fitting S, Xu R, Bull C, et al. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 2010;177:1397–1410. doi: 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Malik S, Khalique H, Buch S, Seth P. A Growth Factor Attenuates HIV-1 Tat and Morphine Induced Damage to Human Neurons: Implication in HIV/AIDS-Drug Abuse Cases. PLoS One. 2011;6:e18116. doi: 10.1371/journal.pone.0018116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Podhaizer EM, Zou S, Fitting S, et al. Morphine and gp120 Toxic Interactions in Striatal Neurons are Dependent on HIV-1 Strain. J Neuroimmune Pharmacol. 2011 doi: 10.1007/s11481-011-9326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mishra BB, Mishra PK, Teale JM. Expression and distribution of Toll-like receptors in the brain during murine neurocysticercosis. J Neuroimmunol. 2006;181:46–56. doi: 10.1016/j.jneuroim.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang SC, Arumugam TV, Xu X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]