Abstract
Anticancer agents act, at least in part, by inducing reactive oxygen and nitrogen species (RONS). We examined the redox effect on SW480 and HT-29 colon cancer cells of four anticancer compounds, arsenic trioxide, phospho-aspirin, phospho-sulindac and nitric oxide-donating aspirin (NO-ASA). All compounds inhibited the growth of both cell lines (IC50s 10–90 μM) and induced RONS detected by a general RONS molecular probe. NO-ASA, which induced at least four individual RONS (NO, H2O2, superoxide anion and peroxynitirte) induced apoptotic and necrotic cell death that was RONS-mediated (cell death paralleled RONS levels and was abrogated by N-acetyl cysteine, but not by diphenylene iodonium, which displayed prooxidant activity and enhanced cell death). NF-κB and MAPKs were modulated by RONS. Thioredoxin-1 (Trx-1), an oxidoreductase involved in redox-regulation, was heavily oxidized in response to RONS and mediated the growth inhibitory effect of the anticancer agents; knocking-down trx-1 expression by siRNA abrogated cell death induced by them. These compounds also inhibited the activity of thioredoxin reductase that reduces oxidized Trx-1, whereas the thioredoxin reductase inhibitor aurothiomalate synergized with NO-ASA in the induction of cell death. Our findings indicate that the Trx system mediates to a large extent redox-induced cell death in response to anticancer agents. This mechanism of action may be shared by more anticancer agents and deserves further assessment as a candidate mechanism for the pharmacological control of cancer.
Keywords: Thioredoxin, thioredoxin reductase, reactive oxygen species, cell death, anticancer drugs
INTRODUCTION
Thioredoxin (Trx) is an a oxidoreductase involved in redox-regulation and cell signaling (1–4). Trx is a member of the Trx system that also includes Trx reductase (TrxR) and NADPH. The ubiquitously expressed Trx system and glutathione (GSH) are the two main antioxidant systems that reduce thiol (-SH) groups. Three distinct forms of Trx have been identified: The 12 kDa cytosolic Trx-1; Trx-2, the mitochondrial isoform; and SpTrx which is highly expressed in spermatozoa. Trx-1 acts as an intracellular reductase using two vicinal cysteine residues (Cys32 and Cys35) at its conserved active site (–Cys–Gly–Pro–Cys–). The redox-active center of reduced Trx-1 interacts with the oxidized protein that it is about to reduce, which has a disulfide bond (S-S) (1). Trx-1 reduces the target protein by converting its disulfide bond to two SH groups, while, in the process, Trx-1 itself becomes oxidized, with its two –SH groups forming a disulfide. TrxR and NADPH reduce Trx-1 back to its original state. Trx-1 has three additional cysteines, Cys62, Cys69, and Cys73. Further oxidation of Trx-1 first leads to the formation of a disulfide bond between Cys62 and Cys69, and next to a disulfide bond between Cys73 of two different Trx-1 molecules, which ultimately leads to the formation of a dimer.
Numerous proteins are redox-regulated by the Trx system (5). Examples of such regulation include the reduction by Trx-1 of a cysteinylresidue of the p50 subunit of NF-κB, which is required for its DNA binding, and the activation through similar reduction reactions of many transcription factors. Trxs, implicated in a number of diseases (2, 3) seem to play a vital role in cancer biology and in cancer response to chemotherapeutic agents. Trx, overexpressed in pancreas, colon, lung and other cancers, suppresses apoptosis by activating the Akt pathway or through the apoptosis signal-regulating kinase-1 (ASK1) (6).
In recent years, it has become clear that anticancer agents act, at least in part, by inducing reactive oxygen and nitrogen species (RONS). At low concentrations RONS appear to protect the cell, while at higher concentrations they can damage many biological molecules, such as DNA, proteins, and lipids, and may initiate cell death (7). Among the anticancer compounds that generate RONS is nitric oxide-donating aspirin (NO-ASA), a promising chemopreventive agent (8). NO-ASA generates a state of oxidative stress through which it affects redox-sensitive signaling pathways, leading ultimately to the elimination of the neoplastic cell via apoptosis or necrosis (9).
Given the apparent importance of RONS in the mechanism of action of these compounds, we studied this phenomenon in human colon cancer cell lines. While we have focused on NO-ASA, we also have included in our study phospho-aspirin, a structurally similar anticancer derivative of aspirin, which is devoid of the NO-releasing moiety (10), phospho-sulindac, a derivative of sulindac, that is also chemopreventive against colon cancer (our unpublished observations), and arsenic trioxide (As2O3), an agent that is highly effective in acute promyelocytic leukemia (11). Here, we present results demonstrating that these anticancer agents induce oxidative stress leading to apoptosis and necrosis and that the Trx system plays a key role in the induction of cell death by them.
MATERIALS AND METHODS
Reagents and culture media
McCoy’s 5a medium (modified), Minimum Essential Medium (Eagle), RPMI 1640 and antibiotics were from Fisher-Mediatech. Fetal calf serum (FCS) was from Hyclone. NO-ASA [2-(acetyloxy) benzoic acid 4-(nitrooxymethyl)-phenyl ester], phospho-aspirin [2-(acetyloxy) benzoic acid 4-(diethylphospho)-phenyl ester] and phospho-sulindac were synthesized by us (12). Dihydroethidium (DHE) and 2′, 7′-dichlorofluorescine diacetate (DCFDA) were from Calbiochem. Dihydrorhodamine (DHR), MitoSOX™ Red, annexin V and propidium iodide (PI) were from Invitrogen. Anti-MAPK antibodies were from Cell Signaling, anti-ASK1 was from Santa Cruz Biotechnology, and antibodies to Trx-1 and TrxR were from Abcam. Arsenic trioxide (ATO), aurothiomalate (ATM) and all other reagents were from Sigma Chemical, St. Louis, MO.
Cell culture and cell viability assays
The HT-29 and SW480 human colon adenocarcinoma cell lines were from American Type Culture Collection (ATCC, Manassas, VA). Cells, grown as recommended by ATCC, were seeded at 5×104 cells/cm2 and allowed to attach for 24 h, followed by various treatments as indicated. Cell viability was measured by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay, following the protocol of the manufacturer (Roche Diagnostics).
Cell death assays
PI incorporation assay
Cells were seeded in 12-well plates and treated as indicated. After each treatment, cells were detached using a cell scraper, fixed in 70% ethanol for 1 h, stained with PI (50 μg/ml) and RNase A (4 U/ml) for 30 min and subjected to flow cytometry analysis.
LDH assay
After cells were treated with the test drug for 24 h, the LDH concentration was determined in the culture medium using the Cytotoxicity Detection KitPLUS (Roche Diagnostics).
Annexin V and PI staining
After cells were treated with the test drugs in 12-well plates, they were trypsinized and stained with annexin V-FITC (100X dilution) and PI (0.5 μg/ml) for 15 min. Annexin V-FITC and PI fluorescence intensities were analyzed by FACScaliber (BD Bioscience). Annexin V (+)/PI (−) cells are apoptotic cells, annexin V (+)/PI (+) cells have undergone secondary necrosis, and annexin V (−)/PI (+) cells are necrotic cells.
Determination of Reactive Oxygen and Nitrogen Species (RONS)
Determination of RONS levels by confocal microscopy
To determine mitochondrial superoxide anion production, cells were seeded in 35 mm glass bottom culture dishes (MatTek, Ashland, MA). After each treatment, cells were stained with 5 μM MitoSOX™ Red for 10 min or with 5 μM DHR for 15 min. Live cells were kept in a 5% CO2 chamber and examined under a Zeiss LSM510 meta confocal microscope.
Determination of RONS levels by SpectraMax M5
Cells seeded in 96 well plates were loaded with either 5 μM DCFDA for 30 min or 5 μM DAF-2 for 30 min or 5 μM Amplex Red for 15 min in plain RPMI medium, and then treated with the test drug as indicated. The emission for each dye was read by SpectraMax M5 (Molecular Devices). In experiments using N-acetyl-cysteine (NAC), cells were pretreated with 20 mM NAC for 4 h in complete medium, followed by DCFDA for 30 min in medium without phenol red and serum and then were treated with the test drug as indicated.
Determination of RONS by FACScaliber
After treatment with the test drug in 12-well plates, cells were trypsinized and stained with 10 μM DCFDA for 30 min at 37°C and the fluorescence intensity was analyzed by FACScaliber (BD Bioscience).
Western blotting
After each treatment, cells were lysed on ice with 1% Triton X-100 lysis buffer with 2.5 mM 4-nitrophenylphosphate, 1% SDS and 0.25% sodium deoxycholate for 30 min. 30 μg of the cell lysate were loaded onto SDS-electrophoresis gel and transferred onto a nitrocellulose membrane. The membrane was then immunoblotted with anti-phospho-p38, anti-phospho-ERK or anti-phospho-JNK antibodies followed by secondary antibodies conjugated with HRP from Santa Cruz Biotechnology. ECL was used to visualize the bands on X-ray film.
Electrophoretic Mobility Shift Assay (EMSA)
Following treatment with the test drug cells were gently scraped into the medium and EMSAs were performed on nuclear extracts as previously described. (13) To assay NF-κB binding, we used a commercially available Gel Shift Assay System (Promega Corporation, Madison, WI).
NF-κB (human p50) transcription factor assay by ELISA
Following treatment with the test drug, cells were gently scraped into the medium and nuclear extracts were isolated as previously described (13). The NF-κB activity was analyzed using an ELISA kit from Panomics.
Assay for ASK1-Trx-1 complex formation
Immunoprecipitation of ASK1-Trx-1 complexes was performed using Protein A/G PLUS–Agarose beads as per the instructions of the manufacturer (Santa Cruz Biotechnology). Briefly, cells were lysed in RIPA buffer and half of each cell lysate was incubated with 1 mM DTT for 30 min. The lysates were then precleared by adding 40 μl Protein A/G PLUS-Agarose beads and incubating for 30 min at 4°C. Cell lysates were then incubated with 6 μg anti-Trx-1 and 40 μl Protein A/G PLUS-Agarose beads for 4 h at 4°C. The immunoprecipitates were boiled in 1X electrophoresis sample buffer and samples were subjected to SDS-PAGE analysis
Thioredoxin redox status assay
106 cells were lysed in 6 M guanidinium chloride, 50 mM Tris/HCl, pH 8.3, 3 mM EDTA, 0.5% Triton-X-100 containing 50 mM iodoacetic acid (14). After 30 min at 37°C, the excess iodoacetic acid was removed using Microspin G-25 columns (GE Healthcare Life Sciences). Oxidized and reduced Trx-1 were separated by native PAGE. The gel was electroblotted onto a nitrocellulose membrane and probed with a Trx-1 antibody, followed by HRP-conjugated secondary antibody. Bands corresponding to Trx-1 were visualized by ECL.
Trx gene silencing by transfecting specific siRNA
0.8×105 SW480 cells seeded in 12-well plates were transfected with 100 nM Trx siRNA or nonspecific control siRNA (Dharmacon) for 72 h using lipofectamine 2000 (Invitrogen).
Thioredoxin reductase activity assay
Following treatment with the test drug(s), cells were lysed and TrxR activity was determined in the protein lysate using a commercially available kit as per the instructions of the manufacturer (Cayman Chemical). In this assay TrxR uses NADPH to reduce 5, 5′-dithiobis-(2-nitrobenzoic acid) (DTNB) to 5-thio-2-nitrobenzoic acid (TNB).
RESULTS
Anticancer agents induce multiple reactive oxygen and nitrogen species in colon cancer cell lines
As expected, all four of the anticancer compounds studied (structures in Suppl. Fig. 1) inhibited the growth of SW480 and HT-29 human colon cancer cells. Under our experimental protocol, the IC50s of growth inhibition after 24 h of treatment were as follows: a) NO-ASA: HT-29 cells = 25 ± 2.3 μM (mean±SEM, for this and all subsequent values), SW480 cells=38 ± 4.0 μM; b) phospho-aspirin: HT-29 cells = 39.3 ± 2.9 μM, SW480 cells = 90.3 ± 2.8 μM; c) phospho-sulindac: HT-29 cells = 65 ± 2.3 μM, SW480 cells = 98 ± 4.0 μM, and d) arsenic trioxide: SW480 cells = 10 ± 1.3 μM.
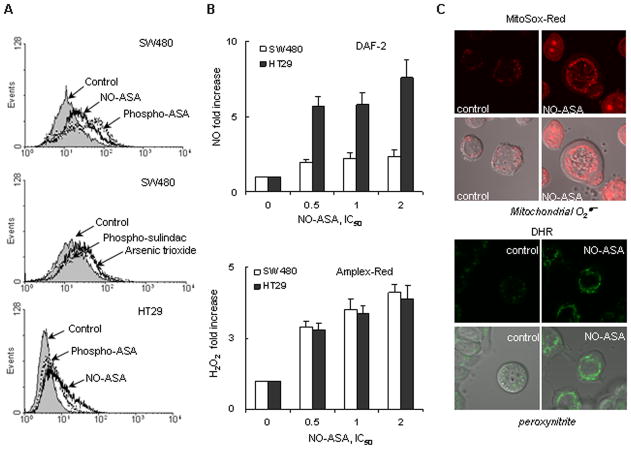
As shown in Fig. 1, all four compounds induced RONS, as detected by the general molecular probe DCFDA, which responds to at least 10 individual reactive species (15, 16). Of note, at identical multiples of their respective IC50s sulindac produced more RONS than phospho-sulindac; ASA 5 mM failed to induce any RONS, whereas NO-ASA and phospho-aspirin did (Supplemental Fig 2). We then investigated the effect of NO-ASA on the levels of four individual RONS, NO, H2O2, ONOO− and . After 3 h of treatment, NO-ASA increased in both cell lines the levels of NO and H2O2 in a concentration-dependent manner. NO levels were increased 2–7 fold over control and H2O2 levels 3.5–4.5 fold over control. Imaging by confocal microscopy showed that NO-ASA promoted and ONOO− formation in mitochondria, as early as 30 min after they were exposed to NO-ASA.
Figure 1. Anticancer agents induce multiple RONS in colon cancer cell lines.
A: HT-29 and SW480 cells were treated with various compounds for 3h as indicated, followed by DCFDA staining for 30 min. The fluorescent intensity of each sample was read by flow cytometry. B: Upper panel, HT-29 and SW480 cells were preincubated with the NO probe, DAF-2, for 30 min, followed by treatment with the test agent for 3 h, when intracellular NO levels were determined by flow cytometry as in Methods. Results are shown as fold increase compared to control cells. Lower panel, HT-29 and SW480 cells were preincubated for 15 min with the H2O2 probe Amplex Red, followed by treatment with each of the test drugs for 3 h, when intracellular H2O2 levels were determined based on their fluorescence as in Methods. Results are expressed as fold increase compared to untreated controls. C: HT-29 cells were treated with 50 μM NO-ASA (2xIC50) for 30 min, followed by 5 μM MitoSox ( probe) for 10 min (upper panel) or 5μM DHR (peroxynitrite probe) for 15min (lower panel). The production of (upper panel) and peroxynitrite (lower panel) was observed using a Zeiss LSM510-meta confocal microscope. In each panel, the upper row represents fluorescent images and the lower row represents them merged with the corresponding Differential Interference Contrast (DIC) image. Values are mean±SEM.
NO-ASA induces RONS-dependent cell death
The two cell lines, SW480 and HT-29, responded to NO-ASA in a similar manner. As shown in Fig. 2A, NO-ASA induced colon cancer cell death both by apoptosis and necrosis. The induction of apoptosis by NO-ASA was greatest at the 1xIC50 concentration, when 20% of SW480 cells and 16% of HT-29 cells were apoptotic, diminishing progressively as the concentrations of NO-ASA increased further. The induction of necrosis showed a steady increase in response to increasing concentrations of NO-ASA; at 4xIC50, the highest NO-ASA concentration studied, 74% and 53% of SW480 and HT-29 cells, respectively, were necrotic.
Figure 2. NO-ASA induces oxidative stress-dependent cell death.
A: SW480 and HT-29 cells were treated with various concentrations of NO-ASA for 24 h and cells were collected for apoptotic or necrotic cell death analysis. PI incorporation determined by flow cytometry was used to detect apoptotic cell death (sub-G1 population in DNA histograms) shown on the left of Fig. A. LDH assay from cultured supernatants was used to determine necrosis, shown on the right of Fig. A. Please note the difference in scale between the two graphs. B: SW480 cells were pretreated with either NAC for 4 h or DPI for 2 h, followed by treatment with NO-ASA for 18 h as indicated. Annexin V and PI staining was used to detect necrosis and apoptosis, shown on the left of panel B. The isobologram, on the right of panel B, is based on the results of cell death by annexin V and PI staining and was used to analyze potential synergy on cell death between NO-ASA and DPI. The additivity line connects the IC50 value of each compound used alone. A and B represent two different dose pairs of each compound (their respective concentrations are shown for each point). Both A and B appear well below the additivity line signifying synergy. C: RONS production was detected by staining with DCFDA (general RONS probe). On the left, both HT-29 and SW480 cells were pretreated with NAC, followed by NO-ASA treatment for 3 h; the DCFDA fluorescence intensity was measured by SpectraMax. The induction of RONS in treated samples is shown as fold increase compare to control cells. On the right, SW480 cells were pretreated with DPI for 2 h, followed by NO-ASA treatment for 3 h; the DCFDA fluorescent intensity was measured by flow cytometry. Values are mean±SEM.
RONS are known to induce cell death in various cancer cell lines (7, 17). On the other hand, RONS can be produced during cell death (18). To distinguish whether the cell death induced by NO-ASA was RONS-dependent or whether RONS production was the result of cell death, we employed two antioxidants, N-acetyl cysteine (NAC) and diphenylene iodonium (DPI) (19–22). However, the results obtained with these two compounds were different. As seen in Fig. 2B, after treatment with NO-ASA for 16 h, 20 mM NAC blocked cell death significantly. For example, at 2xIC50 NO-ASA cell death (annexin V positive cells) was reduced by 50% by NAC (30.4% in control, 14.2% in NAC). In contrast, 25 μM DPI potentiated NO-ASA-induced cell death, increasing the annexin V positive cells from 30.4% to nearly 100% at NO-ASA 2xIC50. Examined by isobolograms (23), the combined effects of DPI and NO-ASA on cell death represent a clear-cut case of pharmacological synergy.
We also examined RONS levels in response to NAC and DPI. As shown in Fig. 2C, 20 mM NAC prevented the induction of RONS by NO-ASA, keeping their levels at baseline. In the DPI study, NO-ASA at 1xIC50 increased RONS levels 83% over control. DPI, in contrast to NAC, increased both basal RONS levels (17% over control) and NO-ASA-induced RONS levels (204% increase over cells treated solely with NO-ASA and nearly 3-fold over control).
In these cells, the level of RONS paralleled the degree of cell death either by apoptosis or necrosis As Figs. 2A and 2C demonstrate, such a correlation existed also for the extent of cell death following treatment with NAC or DPI (Fig. 2B and 2C). The type of cell death (apoptosis vs. necrosis) appears to be dependent on the levels of RONS. For example, lower NO-ASA concentrations (e.g., 1xIC50) induced lower RONS levels, which activated the apoptotic signaling pathway. Higher NO-ASA concentrations (e.g., ≥2xIC50) led to higher RONS levels, in which case cell death was due predominantly to necrosis. (Fig. 1A, 1B, 2A). In DPI-pretreated SW480 cells, the massive RONS production by NO-ASA (3-fold over control) caused necrosis in nearly all cells.
Anticancer drug-induced RONS modulate NF-κB and MAPK signaling in colon cancers cells
NF-κB and MAPK are major determinants of cell renewal and cell death. In general, NF-κB, whose activation and binding to DNA can be modified by RONS (24), modulates cell kinetics, but in each case the specific response depends upon the biological context (24). MAPKs are serine/threonine-specific protein kinases that respond to extracellular stimuli and regulate various cellular activities, such as gene expression, mitosis, differentiation, cell survival and cell death (25). The three major MAPKs, p38, ERK and JNK, are at least partial mediators of RONS-induced cellular response.
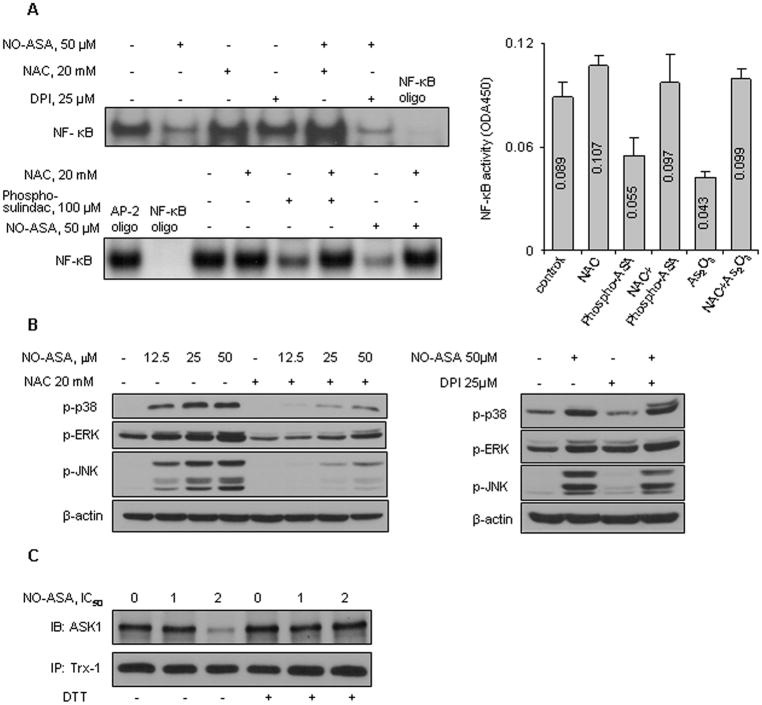
In our system, we studied the effect of the altered RONS levels on NF-κB and MAPK activation. NO-ASA inhibited NF-κB activation after 3 h of treatment (Fig. 3A). Pretreatment with 20 mM NAC for 4 h restored NF-κB activity to control levels. Pretreatment with 25 μM DPI, however, could not restore NF-κB activity; in fact, it inhibited NF-κB activity slightly more than NO-ASA alone. Similar results were obtained with the other three compounds that we studied. In all cases the activity of NF-κB (assayed either by an ELISA method or by EMSA, Fig. 3A) was inhibited by each one of them and this inhibition was abrogated by pretreatment with 20 mM NAC for 16 h.
Figure 3. NO-ASA activates RONS-dependent signaling in colon cancer cells.
A: Upper left: SW480 cells were preincubated with 20 mM NAC for 4 h or 10 μM DPI for 2 h, followed by NO-ASA treatment for 3 h. NF-κB activity was determined by EMSA in nuclear extracts. Upper right: NF-κB activity (mean ± SD) in SW480 cells treated with phospho-aspirin or As2O3, each at 2xIC50 for 4 h; pretreatment with NAC 20 mM was for 16 h. Numbers in columns indicate the ODA450 values. Lower left: HT-29 cells were treated with phospho-sulindac or NO-ASA for 4 h and pretreated with NAC 20 mM for 16 h as indicated. Nuclear proteins were subjected to EMSA. AP-2 oligonucleotide was used as a nonspecific competitor. B: MAPK phosphorylation was determined by immunoblotting of total cell lysates. Cells were pretreated with either NAC (left) or DPI (right). Loading control: β-actin. C: HT-29 cells were treated with NO-ASA for 1 h. Cell protein lysates were treated with or without DTT 1 mM for 30 min and then subjected to immunoprecipitation (IP) using an anti-Trx-1 antibody and the precipitates were immunoblotted (IB) for ASK1.
As indicated by their increased phosphorylation, NO-ASA activated p38, ERK and JNK as early as 1 h after initiation of treatment in a concentration-dependent manner (Fig. 3B). The activation of these MAPKs could be blocked by pretreatment with 20 mM NAC. DPI had no effect on the activation of MAPKs induced by NO-ASA.
NO-ASA dissociates the ASK1-Trx-1 complex
ASK1, also known as mitogen-activated protein kinase kinase kinase 5 (abbreviated as MAP3K5), is part of the MAPK cascade (26). ASK1 is inactive when bound to reduced Trx. When Trx is oxidized by RONS, Trx and ASK1 dissociate, and ASK1 autophosphorylates, becoming an active MAPKKK. Phosphorylation of ASK1 protein can lead to apoptosis or other cellular responses, depending on cell type. We explored the status of the ASK1-Trx-1 complex in HT-29 cells in response to NO-ASA.
As shown in Fig. 3C, treatment of HT-29 cells for 1 h with NO-ASA at 2xIC50 decreased dramatically the amount of ASK1 associated with Trx-1. This decrease resulted from the dissociation of the ASK1-Trx-1 complex due to NO-ASA-induced oxidation of Trx-1 (Fig. 4). This conclusion is supported by the finding that incubation of the cell lysate with the reducing agent DTT restored the integrity of the ASK1-Trx-1 complex.
Figure 4. NO-ASA oxidizes Trx-1, which mediates oxidative stress-induced cell death.
A: The level of Trx-1 was detected by Western blot in whole cell lysates from SW480 cells treated with NO-ASA for 1 h with or without pretreatment with NAC or DPI as indicated. B: After the same treatment as in A, cell lysates were processed as in Materials and Methods to identify Trx-1 redox forms indicated as red = reduced form and ox = oxidized form; the uppermost ox band is the one most oxidized. Cell lysates from HT-29 cells produced similar results. C: SW480 cells were transfected with Trx-1 siRNA or non-specific siRNA for 72 h and total cell lysates were analyzed by Western blotting (upper small panel). After transfection with siRNA, SW480 cells were treated with NO-ASA (left lower panel), phospho-sulindac, arsenic trioxide, or phospho-aspirin, as indicated, and cell death was evaluated by annexin V staining. All drugs increased annexin V (+) cells, a response severely suppressed in the absence of Trx-1. Values are mean±SEM.
Oxidized thioredoxin participates in oxidative stress-induced cell death
Trx-1 is one of the most important molecules linking RONS to NF-κB and MAPK (5). Trx-1 modulates the activity of NF-κB by interacting directly with it and of MAPK through the ASK1-Trx signaling complex. In our system, NO-ASA dramatically influenced RONS-dependent NF-κB and MAPK activities, as well as cell death. Consequently, we determined whether NO-ASA together with NAC or DPI could influence the cellular levels and the redox status of Trx-1.
As shown in Fig. 4A, NO-ASA did not change the total protein level of Trx-1, and pretreatment with NAC or DPI had no effect on it. However, we found significant changes in the oxidation status of Trx-1 in response to treatment with NO-ASA. As described in Methods, to detect the oxidized and reduced forms of Trx-1, protection of the oxidation status of its thiols during protein extraction was essential. When fully reduced, the five cysteines of Trx-1, generate its reduced form, whereas when oxidized they generate its oxidized forms (1). Following 1 h of treatment of HT-29 cells with NO-ASA, the redox status of Trx-1 changed in a concentration-dependently manner (Fig. 4B). At low NO-ASA concentrations (1/8, 1/4, 1/2, and 1xIC50), we observed both reduced and oxidized forms of Trx-1, whereas at high concentrations (2 and 4xIC50), the reduced band was undetectable and only two oxidized forms were present. SW480 cells gave similar results (data not shown).
In both HT-29 and SW480 cells, the changes in the redox state of Trx-1 in response to NO-ASA corresponded closely to the changes in RONS levels. Furthermore, 20 mM NAC, which completely suppressed RONS, reduced the oxidized form of Trx-1 that was generated by NO-ASA. However, 25 μM DPI, which greatly enhanced RONS levels in the presence of NO-ASA (Fig. 2C), also enhanced further the oxidation of Trx-1. Combined with NO-ASA, DPI generated two oxidized forms in comparison to one oxidized and one reduced form in cells treated with NO-ASA alone (Fig 4B). These findings suggest that RONS generated by NO-ASA (and DPI when it was used) modulated the redox status of Trx-1.
We then silenced the expression of Trx-1 in SW480 cells using siRNA and determined whether the absence of Trx-1 interfered with the cell death effect of NO-ASA. As shown in Fig. 4C, 72 h after transfection the protein level of Trx-1 decreased dramatically, with only a faint band being detected in the Western blot. Compared to cells treated with control siRNA, NO-ASA induced much less death in cells transfected with Trx-1 siRNA. Tested at three different concentrations of NO-ASA, the absence of Trx-1 reduced the extent of apoptosis-related cell death by 65–70%. (annexin V (A) positive cells represent either apoptosis [A(+)/PI(−)] or secondary necrosis [A(+)/PI(+)]). Trx-1 was also required for the ability of the other three compounds to induce cell death. When SW480 cells with knocked down expression of Trx-1 were treated in a similar manner with each of the other three compounds, apoptosis-related cell death was reduced significantly in each case (85% by phospho-sulindac, 50% by arsenic trioxide and 86% by phospho-aspirin). The latter finding indicates a generalized property of Trx-1 rather than one restricted to the effect of NO-ASA.
Anticancer agents inhibit thioredoxin reductase
Of the four compounds that we studied, only arsenic trioxide has been previously evaluated for its effect on Trx-1 (27). This pro-apoptotic agent is known to oxidize Trx-1 by inducing RONS production and by inhibiting the activity of TrxR, the enzyme which normally reduces oxidized Trx-1. We studied, therefore, the effect of all four compounds on both the expression and activity of TrxR in SW480 and HT-29 cells.
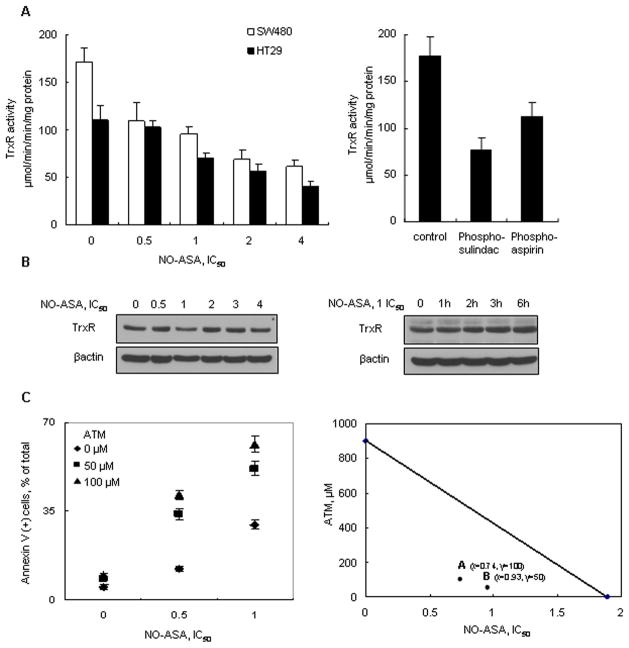
Following treatment with NO-ASA for 1 h, TrxR activity was decreased concentration-dependently in SW480 and HT-29 cells (Fig. 5A). The other three compounds also reduced the activity of TrxR. However, the protein level of TrxR was not changed by NO-ASA, even when the incubation period was prolonged to 6 h or when high concentrations of NO-ASA (up to 4xIC50) were used. Thus changes in TrxR levels are an unlikely explanation for its reduced activity.
Figure 5. Anticancer agents decrease TrxR activity and a TrxR inhibitor potentiates cell death.
A: SW480 and HT-29 cells were treated with NO-ASA for 1 h as indicated, harvested and their TrxR activity was measured as in Methods (left). HT-29 cells were treated for 1 h with the other three anticancer agents, each used alone at its respective IC50. B: The expression of TrxR was determined in SW480 cells treated with various concentrations of NO-ASA for 1 h (left panel) or with 1xIC50 for up to 6 h (right panel). C: SW480 cells were treated with various concentrations of NO-ASA for 16 h, following 24 h pretreatment with or without the TrxR inhibitor aurothiomalate (ATM). The percentage of annexin V (+) cells is shown. ATM enhanced greatly the NO-ASA-induced cell death. The isobologram on the right, based on the results of cell death by annexin V staining, evaluated potential synergy between NO-ASA and ATM. The additivity line connects the IC50 value of each compound when used alone. A and B represent two different dose pairs of each compound (the respective concentrations of each are shown next to each point). Values are mean±SEM.
To further evaluate the effect of oxidized Trx-1 on oxidative stress-induced cell death, we used the TrxR inhibitor aurothiomalate (ATM), which prevents the conversion of oxidized Trx-1 to its reduced form (28). Cells pretreated with 50 μM or 100 μM ATM for 24 h were exposed to NO-ASA at its 0.5xIC50 concentration for 16 h. ATM increased cell death from 12% (NO-ASA alone) to 34% or 41% at 50 μM or 100 μM ATM, respectively. At a higher NO-ASA concentration (1xIC50), ATM increased cell death from 29% (NO-ASA alone) to 52% or 62% at 50 μM or 100 μM ATM, respectively. Examined by isobolograms (23), the effects of ATM on cell death represent pharmacological synergy (Fig. 5C).
Combined with the siRNA results, these findings strongly suggested that the oxidized form of Trx-1 provides a critical contribution to cell death in response to anticancer agents that share the ability to induce RONS.
DISCUSSION
Our data demonstrate that four anticancer agents generate in human colon cancer cell lines a state of oxidative stress, which involves at least four individual RONS. This effect is accompanied by changes in two critical cell signaling cascades, NF-κB and MAPK, both responsive to RONS, and culminates in apoptotic or necrotic cell death, with the specific type of cell death depending on RONS levels. The Trx system plays a pivotal role in the induction of oxidative stress, as Trx-1 and TrxR are altered in a way that prevents them from countering the prooxidant effect of RONS.
The four anticancer agents that we used are structurally diverse. Three of them have demonstrated anticancer properties in preclinical models, while the fourth, arsenic trioxide, is the drug of choice for the treatment of promyelocytic leukemia. Their IC50s for cell growth are similar, ranging between 10 and 90 μM. Our data establish that their common property is the induction of RONS in two colon cancer cell lines, documented by the response of the general RONS probe DCFDA. In the case of NO-ASA, which was studied in greater detail, the induction of RONS involved at least four individual species, NO, H2O2, ONOO− and . These compounds encompass a wide range of reactivities and biological behavior. The mitochondria demonstrated the most notable production of . The reaction of with NO may be the sole source of the very reactive (and damaging) ONOO−. The ultimate result of the generation of RONS was cell death; the etiologic relationship between the two was underscored by the abrogation of cell death by NAC, which blocked the generation of RONS by NO-ASA. A remarkable finding was the clear relationship between RONS levels and the type of cell death-apoptosis at lower RONS concentrations and necrosis at higher levels.
Contrary to expectations, DPI potentiated the generation of RONS and consequently the degree of cell death. In fact, DPI synergized with NO-ASA, as was formally demonstrated by our data analysis. DPI, considered predominantly as an antioxidant, is also known to have prooxidant properties and this may indeed be the case here. It is unclear what determined the type of response to DPI.
RONS are increasingly appreciated as significant signaling molecules, a role distinct from their potentially catastrophic effects, which occur at high concentrations and represent the situation conventionally described as oxidative stress (29). NO-ASA activated MAPKs, a signaling pathway also known to be redox-responsive (25). NAC prevented to a large extent the activation of these pathways, establishing a potential cause-and-effect relationship with RONS. In addition, all four compounds inhibited NF-κB activation. In each case RONS appear to have been the responsible signaling molecule, as NAC abrogated their effect on NF-κB and DPI, which unexpectedly increased RONS levels, inhibited further the activity of NF-κB (which was already inhibited by NO-ASA). An important proximal signaling molecule in this cascade is ASK1, which, as mentioned, is physically associated with reduced Trx-1. Our data confirmed this association and also showed that NO-ASA which oxidized Trx-1 released ASK1, while reduction of Trx-1 by the reducing agent DTT favored formation of the Trx-1-ASK1 complex.
The redox status of the cell regulates NF-κB activity, but the specific response (NF-κB inhibition or activation) appears to be dependent on cell type and perhaps on the individual RONS involved (24, 30). Direct interactions of RONS with the NF-κB subunits or with proteins in the NF-κB regulatory cascade have been described. Cysteine residue(s) of NF-κB subunits are involved in its recognition of, and binding to, DNA, such as the redox-sensitive Cys62 residue of p50, whereas the redox control mechanism mediated by Trx may regulate NF-κB-dependent gene expression (31, 32). The most reasonable explanation of our data is that the four anti-cancer agents induced RONS, which interacted either directly or indirectly with NF-κB, inhibiting its ability to bind to its cognate DNA recognition sequence (inhibition of NF-κB activation). The effects of NAC and DPI strongly support this notion.
The Trx system, along with GSH, is currently thought to be at the heart of the antioxidant response of a mammalian cell. In the face of high RONS levels in response to NO-ASA, it is easy to anticipate that Trx-1 with its wide substrate specificity was extensively oxidized as it engaged in the reduction of its many oxidized client proteins. Here again, the degree of Trx-1 oxidation paralleled the amount of RONS and such oxidation was prevented by pretreatment with NAC and exacerbated by DPI. At the highest NO-ASA concentrations, the oxidation of Trx-1 appeared complete. Trx-1 seemed to play a central role in the growth inhibitory effect of NO-ASA. In particular, when its expression was knocked down by siRNA, the proapoptotic effect of NO-ASA and of the other three compounds was abrogated; this effect was nearly complete (except for the 50% reduction by arsenic trioxide).
TrxR, the enzyme that reduces oxidized Trx-1, was significantly inhibited by these compounds and this explains, at least partially, the persistence of the oxidized form of Trx-1. That the interaction between Trx-1 and its reductase is important in these cells was further substantiated by the finding that a TrxR inhibitor actually synergized with NO-ASA to inhibit cell death. It is, therefore, likely that the Trx system suffered a dual assault consisting of a) inhibition of TrxR by these agents and b) oxidation of Trx-1, as it had to reduce its client proteins that were extensively oxidized by RONS. The end result was the oxidation of Trx-1, which in its extreme form became complete and culminated in massive cell death (Fig. 6).
Figure 6. The redox status of Trx-1 modulates cell survival: Dependence on intracellular RONS levels.
This diagram depicts the relationship between Trx-1, TrxR and RONS. As RONS levels increase, Trx-1 changes from its completely reduced form (Trx-1red) to partially oxidized (Trx-1ox-I) to its fully oxidized form (Trx-1ox-II). TrxR, which reverses the oxidation of Trx-1, was also inhibited by the compounds we studied. At low RONS concentrations, when Trx-1 is in its reduced form, cell death is minimal. As RONS concentrations increase cell death is evident, becoming massive at the highest RONS concentrations. The predominance of apoptosis at intermediate RONS levels is replaced by necrosis at higher RONS levels.
The exact mechanism by which oxidized Trx-1 participates in the induction of cell death by these compounds is not fully delineated. It is, however, clear that NF-κB and MAPKs are modulated in our experimental system. Both pathways a) have been previously documented to be critical for the growth inhibitory effect of NO-ASA, (13, 33) and b) depend on Trx for their oxidation status that determines their biological activity. Based on the known role of Trx-1 in the regulation of transcription factors, it is likely that the oxidation of Trx-1 is proximal to NF-κB and MAPKs. Indeed, in the case of MAPK activation the release of ASK1 from its complex with Trx-1 represents a convincing mechanistic link between the two.
In conclusion, our data demonstrate the significant regulatory role of the Trx system in the induction of cell death by four diverse anticancer compounds. It is reasonable to consider that the mechanism presented here may be shared by more compounds in addition to those studied by us and thus it deserves further assessment as a candidate mechanism for the pharmacological control of cancer.
Supplementary Material
Figure 1. The structures of NO-donating aspirin, phospho-aspirin and phospho-sulindac.
HT-29 cells were treated for 1 h with the indicated compounds, stained with the general RONS probe DCFDA and their fluorescence intensity was detected as in Methods. All compounds increased intracellular RONS levels, except for conventional aspirin, which was used at the high concentration of 5 mM (lower panel).
Acknowledgments
Financial support: NIH grants R01 CA10101902 and R01 CA092423 and a grant from the Emmanuel Foundation
We thank Gerardo Mackenzie for technical assistance and Onika Murray for helpful comments.
References
- 1.Kaimul AM, Nakamura H, Masutani H, Yodoi J. Thioredoxin and thioredoxin-binding protein-2 in cancer and metabolic syndrome. Free Radic Biol Med. 2007;43(6):861–8. doi: 10.1016/j.freeradbiomed.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 2.Arner ES, Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16(6):420–6. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Maulik N, Das DK. Emerging potential of thioredoxin and thioredoxin interacting proteins in various disease conditions. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbagen.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Lillig CH, Holmgren A. Thioredoxin and related molecules--from biology to health and disease. Antioxid Redox Signal. 2007;9(1):25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 5.Berndt C, Lillig CH, Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2007;292(3):H1227–36. doi: 10.1152/ajpheart.01162.2006. [DOI] [PubMed] [Google Scholar]
- 6.Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. The EMBO journal. 1998;17(9):2596–606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rigas B, Sun Y. Induction of oxidative stress as a mechanism of action of chemopreventive agents against cancer. Br J Cancer. 2008;98(7):1157–60. doi: 10.1038/sj.bjc.6604225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rigas B. The use of nitric oxide-donating nonsteroidal anti-inflammatory drugs in the chemoprevention of colorectal neoplasia. Curr Opin Gastroenterol. 2007;23(1):55–9. doi: 10.1097/MOG.0b013e32801145b0. [DOI] [PubMed] [Google Scholar]
- 9.Gao J, Liu X, Rigas B. Nitric oxide-donating aspirin induces apoptosis in human colon cancer cells through induction of oxidative stress. Proc Natl Acad Sci U S A. 2005;102(47):17207–12. doi: 10.1073/pnas.0506893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rigas B, Kozoni V. The novel phenylester anticancer compounds: Study of a derivative of aspirin (phoshoaspirin) Int J Oncol. 2008;32(1):97–100. [PubMed] [Google Scholar]
- 11.Sekeres MA. New data with arsenic trioxide in leukemias and myelodysplastic syndromes. Clin Lymphoma Myeloma. 2007;8 (Suppl 1):S7–S12. doi: 10.3816/clm.2007.s.027. [DOI] [PubMed] [Google Scholar]
- 12.Penning TD, Talley JJ, Bertenshaw SR, et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib) J Med Chem. 1997;40(9):1347–65. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 13.Williams JL, Ji P, Ouyang N, Liu X, Rigas B. NO-donating aspirin inhibits the activation of NF-kappaB in human cancer cell lines and Min mice. Carcinogenesis. 2008;29(2):390–7. doi: 10.1093/carcin/bgm275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson WH, Pohl J, Montfort WR, et al. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. The Journal of biological chemistry. 2003;278(35):33408–15. doi: 10.1074/jbc.M211107200. [DOI] [PubMed] [Google Scholar]
- 15.Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983;130(4):1910–7. [PubMed] [Google Scholar]
- 16.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chemical research in toxicology. 1992;5(2):227–31. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 17.Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13(3):789–94. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 18.Bubici C, Papa S, Pham CG, Zazzeroni F, Franzoso G. The NF-kappaB-mediated control of ROS and JNK signaling. Histology and histopathology. 2006;21(1):69–80. doi: 10.14670/HH-21.69. [DOI] [PubMed] [Google Scholar]
- 19.de Carvalho DD, Sadok A, Bourgarel-Rey V, et al. Nox1 downstream of 12-lipoxygenase controls cell proliferation but not cell spreading of colon cancer cells. International journal of cancer. 2008;122(8):1757–64. doi: 10.1002/ijc.23300. [DOI] [PubMed] [Google Scholar]
- 20.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer research. 2007;67(22):10823–30. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 21.Probin V, Wang Y, Zhou D. Busulfan-induced senescence is dependent on ROS production upstream of the MAPK pathway. Free radical biology & medicine. 2007;42(12):1858–65. doi: 10.1016/j.freeradbiomed.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albini A, D’Agostini F, Giunciuglio D, Paglieri I, Balansky R, De Flora S. Inhibition of invasion, gelatinase activity, tumor take and metastasis of malignant cells by N-acetylcysteine. International journal of cancer. 1995;61(1):121–9. doi: 10.1002/ijc.2910610121. [DOI] [PubMed] [Google Scholar]
- 23.Tallarida RJ. Drug synergism: its detection and applications. The Journal of pharmacology and experimental therapeutics. 2001;298(3):865–72. [PubMed] [Google Scholar]
- 24.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochemical pharmacology. 2006;72(11):1493–505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 25.McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxidants & redox signaling. 2006;8(9–10):1775–89. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 26.Takeda K, Noguchi T, Naguro I, Ichijo H. Apoptosis signal-regulating kinase 1 in stress and immune response. Annual review of pharmacology and toxicology. 2008;48:199–225. doi: 10.1146/annurev.pharmtox.48.113006.094606. [DOI] [PubMed] [Google Scholar]
- 27.Lu J, Chew EH, Holmgren A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc Natl Acad Sci U S A. 2007;104(30):12288–93. doi: 10.1073/pnas.0701549104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omata Y, Folan M, Shaw M, et al. Sublethal concentrations of diverse gold compounds inhibit mammalian cytosolic thioredoxin reductase (TrxR1) Toxicol In Vitro. 2006;20(6):882–90. doi: 10.1016/j.tiv.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Frein D, Schildknecht S, Bachschmid M, Ullrich V. Redox regulation: a new challenge for pharmacology. Biochem Pharmacol. 2005;70(6):811–23. doi: 10.1016/j.bcp.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Byun MS, Jeon KI, Choi JW, Shim JY, Jue DM. Dual effect of oxidative stress on NF-kappakB activation in HeLa cells. Exp Mol Med. 2002;34(5):332–9. doi: 10.1038/emm.2002.47. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi T, Ueno Y, Okamoto T. Oxidoreductive regulation of nuclear factor kappa B. Involvement of a cellular reducing catalyst thioredoxin. J Biol Chem. 1993;268(15):11380–8. [PubMed] [Google Scholar]
- 32.Matthews JR, Botting CH, Panico M, Morris HR, Hay RT. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 1996;24(12):2236–42. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hundley TR, Rigas B. Nitric oxide-donating aspirin inhibits colon cancer cell growth via mitogen-activated protein kinase activation. The Journal of pharmacology and experimental therapeutics. 2006;316(1):25–34. doi: 10.1124/jpet.105.091363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. The structures of NO-donating aspirin, phospho-aspirin and phospho-sulindac.
HT-29 cells were treated for 1 h with the indicated compounds, stained with the general RONS probe DCFDA and their fluorescence intensity was detected as in Methods. All compounds increased intracellular RONS levels, except for conventional aspirin, which was used at the high concentration of 5 mM (lower panel).