Abstract
As a result of shared routes of transmission, coinfection with hepatitis C virus (HCV) is common in human immunodeficiency virus (HIV)–infected patients. The prevalence of HIV/HCV coinfection is particularly high among persons who have used injection drugs; however, more recently, sexual transmission of HCV has been recognized among HIV-infected men who have sex with men (MSM). Over the past decade, the effectiveness of HIV treatment improved substantially, leading to a substantial reduction in HIV/AIDS-related deaths; in this context, liver disease due to HCV infection has emerged as major concern for co-infected patients. Over the same period, treatment of HCV remained stagnant, with pegylated interferon alfa (PegIFN) plus ribavirin (RBV; PegIFN/RBV) entrenched as the standard treatment for HCV infection for co-infected patients, who have the greatest risk for liver disease. However, the effectiveness of HCV treatment in this population has been disappointing because of low rates of treatment initiation and success. In 2011, novel HCV NS3/4A PIs (PIs), telaprevir and boceprevir, were approved for use in combination with PegIFN/RBV for the treatment of HCV genotype 1 infection; at the time of approval, important questions regarding the efficacy, safety, and potential for drug interactions with telaprevir and boceprevir had not been answered. More recently, data from drug-interaction studies and 2 small, phase II clinical trials indicate that these HCV treatment regimens may lead to higher rates of HCV eradication in HIV/HCV-coinfected patients, with manageable toxicity and pharmacologic interactions with antiretroviral drugs. As such, these HCV PI–based regimens have emerged as the standard for the treatment of HCV genotype 1 infection in carefully selected HIV-infected patients.
Keywords: hepatitis C, HCV, HIV, peginterferon, ribavirin, boceprevir, telaprevir
As a result of shared modes of transmission, the prevalence of chronic hepatitis C virus (HCV) infection is high among human immunodeficiency virus (HIV)–infected patients, with HCV infecting approximately 25% of patients in some regions [1–3]. Over the past 15 years, treatment of HIV infection has improved substantially, with greater access to effective antiretroviral therapy (ART) for many patients, leading to substantial reductions in HIV-related mortality [3]. Over the same period, treatment of HCV has been largely unchanged, with pegylated interferon alfa (PegIFN) plus ribavirin (RBV; PegIFN/RBV) in use since 1998 [4]. HCV treatment with interferon alfa or longer-acting PegIFN/RBV has been recommended for HIV/HCV-coinfected patients who are at the greatest risk for liver disease. However, the effectiveness of HCV treatment in this population has been disappointing [5, 6]. The reasons for the limited impact of HCV therapy include low rates of treatment initiation, high prevalence of relative and absolute contraindications to the drugs, and, among those treated, low rates of sustained virologic response (SVR), especially among individuals coinfected with HCV genotype 1 and among African Americans [7–9]. As such, direct-acting antivirals (DAAs) for HCV infection are urgently needed and could overcome host factors such as immunodeficiency that impair the efficacy of many drugs. Challenges to the use of DAAs in patients with HIV/HCV co-infection include the potential for interactions between different drugs, additional drug toxicities, and the need for therapy with interferon alfa. Despite these challenges, limited data indicate that the HCV protease inhibitors (PIs) telaprevir and boceprevir, in combination with PegIFN/RBV, may increase the SVR rate, with manageable toxicity and drug-drug interactions.
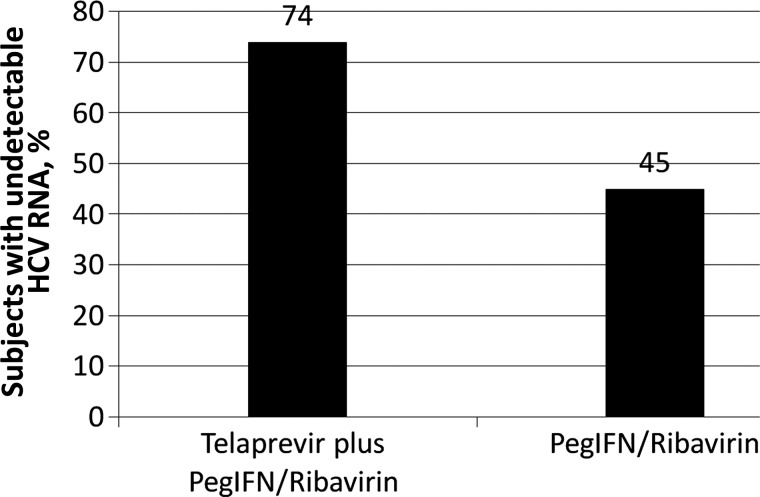
Figure 1.
Sustained virologic response at 12 weeks after stopping therapy, based on an analysis of a phase IIa study of telaprevir plus combined pegylated interferon alfa (PegIFN) and ribavirin for the treatment of hepatitis C virus (HCV) infection in human immunodeficiency virus–infected patients
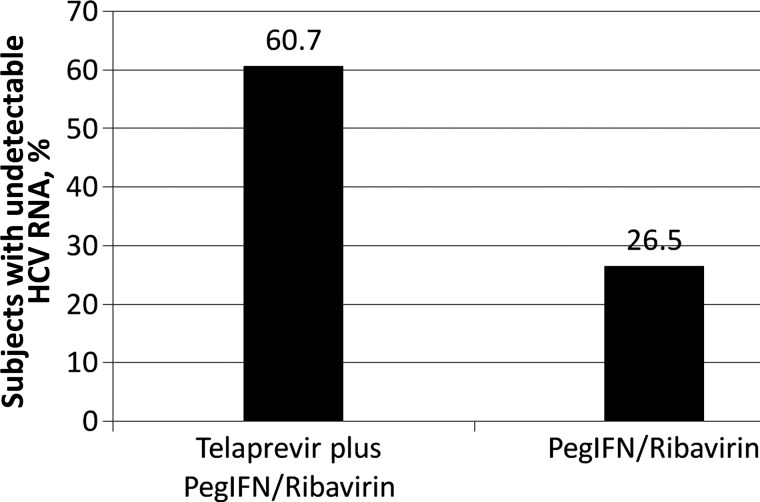
Figure 2.
Sustained virologic response at 12 weeks after stopping therapy, based on an analysis of a phase IIa study of boceprevir plus combined pegylated interferon alfa (PegIFN) and ribavirin for the treatment of hepatitis C virus (HCV) infection in human immunodeficiency virus–infected patients.
IMPACT OF HCV CO-INFECTION IN HIV-INFECTED PATIENTS
With increased effectiveness of treatment for HIV infection, coinfection with HCV has emerged as a major cause of morbidity and mortality in this population [10–12]. The emergence of HCV infection as a clinically important disease in HIV-infected patients is the result of at least 3 factors. First, the prevalence of HCV infection in this population is relatively high. Because of shared modes of transmission, up to 30% of HIV-infected patients in the United States are co-infected with HCV [1, 13, 14]. While injection drug use remains the primary mode of HCV acquisition for many HIV-infected persons, sexual transmission of this pathogen has been increasingly recognized among homosexual males without percutaneous risk factors for HCV infection, often in the setting of traumatic sexual practices [15–17]. Second, compared with HCV-monoinfected persons, those with HIV/HCV coinfection are at increased risk for progressive liver disease, including end-stage liver disease, hepatocellular carcinoma, and death. In a study by Ly et al of deaths due to hepatitis C in the United States, coinfection was HIV was associated with an approximately 2-fold increased risk for HCV-related mortality [18]. Although ART and the prevention or reversal of HIV-related immunosuppression have been strongly associated with decreased risk of severe liver-related outcomes or death, the adverse impact of HIV coinfection on HCV disease does not appear to be completely ameliorated by the treatment of HIV infection [19, 20]. Third, alcohol use disorders are common among HIV/HCV-coinfected adults (prevalence, 30%–50%) [21, 22]. Alcohol is strongly linked to HCV disease progression, including death. As such, harmful alcohol exposure is likely to be an important contributor to the HCV-related morbidity and mortality observed in HIV-infected patients. On the basis of these 3 considerations, efforts to minimize the impact of HCV disease in HIV-infected patients should be focused on the following: (1) education aimed at preventing newly acquired HCV infections; (2) provision of ART to all HIV/HCV-coinfected patients, regardless of CD4+ T-cell count; and (3) detection and treatment of alcohol use disorders [14, 23, 24].
Although these measures are important steps in the management of HIV/HCV-coinfected patients, chronic HCV infection is capable of causing life-threatening liver disease in the absence of HIV coinfection and alcohol abuse; accordingly, control of these cofactors alone is likely to be insufficient for many patients. Treatments aimed at eradication of chronic HCV infection will also be required. Sustained virologic response following HCV treatment has been associated with decreased risk of liver-specific and all-cause mortality in patients with HCV monoinfection and HIV/HCV coinfection. In the Veterans Affairs population, sustained virologic response has been associated with a marked reduction in all-cause mortality [25]. Similarly, HIV/HCV-coinfected patients in the Johns Hopkins HIV/HCV cohort who underwent HCV treatment and achieved transient suppression (viral relapse) of HCV RNA to undetectable levels or SVR did not experience any clinical outcomes, including patients with advanced fibrosis or cirrhosis [20]. While the clinical benefit of HCV eradication is increasingly recognized, the effectiveness of HCV treatment in HIV/HCV-coinfected patients has been very low in many settings [26].
The effectiveness of HCV treatment in this population has been limited by relatively low rates of treatment initiation in many settings and, among those who initiate treatment, by low rates of SVR. Low uptake of treatment has been associated with comorbid medical and psychiatric conditions in this population that do not permit the safe administration of PegIFN/RBV; with uncontrolled HIV disease; and with active substance abuse, which limits the ability to take and adhere to complex HCV treatment regimens [7, 8]. However, in HIV-infected patients for whom such barriers to the use of interferon alfa–based therapy are overcome and treatment is initiated, the observed SVR rate ranges from 15% to 70%, depending on HCV genotype, IL28B haplotype, and HCV RNA level. In the recent large, multicenter, North American study of PegIFN/RBV (the PARADIGM study), only approximately 25% of white patients and approximately 15% of African American patients with HIV/HCV genotype 1 coinfection achieved SVR with PegIFN/RBV therapy [6]. Thus, measures to improve the rate of HCV eradication must focus both on improving treatment uptake among HIV/HCV-coinfected patients and on improving the safety, tolerability, and efficacy of the available HCV treatment regimens, including DAAs, such as the HCV N3/4A PIs telaprevir and boceprevir.
HCV TREATMENT WITH PegIFN/RBV and HCV NS3/4A PIs
In May 2011, the US Food and Drug Administration approved 2 HCV NS3/4A PIs, telaprevir and boceprevir, for the treatment of chronic HCV genotype 1 infection in combination with PegIFN/RBV [27–30]. The basis for this approval were findings from large, randomized controlled trials that demonstrated substantially higher SVR rates in HCV-monoinfected patients who had never been treated for HCV infection and in those who had not responded to previous PegIFN/RBV therapy. Following the approval of these DAAs, the American Association for the Study of Liver Disease published updated guidelines for the treatment of chronic HCV genotype 1 infection that recommended the use of HCV PIs in most patients treated with PegIFN/RBV [31]. However, at the time of FDA approval and the drafting of these treatment guidelines, the safety and efficacy of these PI-based regimens had not been established in HIV/HCV-coinfected patients, leading to lack of consensus about their use in this population [32].
Telaprevir
On 5 March 2012, the results of a phase IIa study of telaprevir or placebo in combination with PegIFN/RBV for the treatment of chronic HCV genotype 1 infection in HIV-infected adults was presented at the 19th Conference on Retroviruses and Opportunistic Infections (CROI) [33]. In this study, 60 HIV/HCV-coinfected patients were randomly assigned to receive treatment with 12 weeks of combination therapy of telaprevir (n = 38) or placebo (n = 22) plus PegIFN/RBV, followed by 36 weeks of PegIFN/RBV alone. While response-guided therapy to deliver a shorter duration of therapy (total, 24 weeks) to patients who achieve and maintain an undetectable HCV RNA level during telaprevir therapy is the standard approach for patients with HCV monoinfection, HIV/HCV-coinfected patients in this study were treated for a total of 48 weeks. On the basis of previously conducted drug-drug interaction studies, co-infected patients were required to be receiving no antiviral therapy (n = 13) or one of the following HIV treatment regimens: atazanavir/ritonavir plus tenofovir/emtricitabine (n = 23), or efavirenz plus tenofovir/emtricitabine (n = 24). Notably, patients receiving efavirenz-based ART were treated with higher telaprevir doses (1125 mg every 8 hours) to offset the reduction in telaprevir levels by efavirenz; other patients received the standard telaprevir dose (750 mg every 8 hours) [34].
The majority of patients were male (67%–100%), white (29%–87%), and infected with genotype 1, subtype a (43%–80%). The median CD4+ T-cell count was high (514–675 cells/mm3 [range, 254–1189 cells/mm3]) in all patient groups. The SVR-12 rate, defined as no detectable HCV RNA at 12 weeks after discontinuing treatment, was 74% (28 of 38) in patients treated with telaprevir plus PegIFN/RBV, compared with 45% (10 of 22) in patients treated with PegIFN/RBV alone. Although this study included a small number of patients, there was no difference in HCV virologic outcomes among patients receiving no antiretroviral agents, atazanavir/ritonavir plus either tenofovir/emtricitabine or lamivudine, or a fixed-dose combination of efavirenz with tenofovir/emtricitabine.
During treatment, virologic breakthrough was observed in 3 telaprevir-treated patients (8%), and after stopping therapy, virologic relapse was reported in 1 telaprevir-treated patient (3%). Adverse events were common among patients who received telaprevir, with a greater frequency of pruritus, dizziness, headache nausea, rash, and anemia as compared to those who received placebo plus PegIFN/RBV; however, discontinuation of study drugs because of adverse events was uncommon in both groups (telaprevir, 3 patients; placebo, 0 patients). Importantly, no adverse impact on HIV disease parameters (ie, HIV RNA level and CD4+ T-cell count) was observed, and the steady-state pharmacokinetic profiles of the antiretroviral drugs (efavirenz and atazanavir/ritonavir) and telaprevir were acceptable.
Overall, the authors concluded that the safety, tolerability, and efficacy profile of telaprevir for 12 weeks in combination with PegIFN/RBV for 48 weeks was similar to that previously reported in HCV genotype 1–infected patients without HIV coinfection. As a result of these observations, larger, phase III studies of telaprevir plus PegIFN/RBV are underway to evaluate the effectiveness of this regimen in HIV/HCV-coinfected patients who have not been previously treated for HCV infection and in those who have not responded to past treatment with PegIFN/RBV (ClinicalTrials.gov identifiers: NCT01335529 and NCT01482767). In addition to providing confirmatory data regarding safety and efficacy in co-infected patients, these studies will assess the administration of telaprevir as 1125 mg twice daily (except with efavirenz) and the use of response guided therapy with 24 weeks of PegIFN/RBV for patients who achieve an extended rapid virologic response. The studies are estimated to be completed in 2014.
Boceprevir
On 5 March 2012, the results of a phase IIa study of boceprevir or placebo in combination with PegIFN/RBV for the treatment of chronic HCV genotype 1 infection in HIV-infected adults was presented at the 19th CROI [35]. In this study, 98 HIV/HCV-coinfected patients were randomly assigned to receive treatment with 4 weeks of PegIFN/RBV (the lead-in phase), followed by 44 weeks of combination therapy of boceprevir 800 mg every 8 hours (n = 64) or placebo (n = 34) plus PegIFN/RBV. On the basis of previously conducted drug-drug interaction studies in healthy volunteers, patients receiving efavirenz or other nonnucleoside reverse transcriptase inhibitors (NNRTIs) were not enrolled. Patient receiving ritonavir-boosted HIV type 1 (HIV-1) PIs, including darunavir, atazanavir, and lopinavir, were enrolled in the phase IIa study. However, after enrollment into the treatment trial, drug-drug interaction studies conducted in healthy volunteers demonstrated significant bidirectional interactions leading to lower concentrations of the HIV-1 PIs and boceprevir; the drug interaction findings from the healthy-volunteer studies were also presented at the 19th CROI and communicated directly to healthcare professionals via letter [36]. Pharmacokinetics of the HIV-1 PIs and other antiretroviral agents were not assessed in this phase IIa study.
The majority of patients were male (65%–72%), white (81%–82%), and infected with genotype 1, subtype A (65%–66%). The median CD4+ T-cell count was relatively high in all patient groups (577–586 cells/mm3 [range, 187–1539 cells/mm3]). The SVR-12 rate was 60.7% (37 of 61 patients) in the group treated with boceprevir plus PegIFN/RBV, compared with 26.5% (9 of 34) among those treated with PegIFN/RBV alone. In addition, 3 patients treated with boceprevir plus PegIFN/RBV had an undetectable HCV RNA level at 4 weeks after treatment but had not yet reached the 12-week posttreatment assessment. During treatment, HCV virologic failure was observed in 6 boceprevir-treated patients (9%), and, after stopping therapy, HCV virologic relapse was reported in 2 boceprevir-treated patients (5%).
Although this study included a small number of patients, there was no difference in HCV virologic response among patients treated with ritonavir-boosted HIV PIs, despite the anticipated interaction of these drugs with boceprevir leading to lower levels of the HCV PI. Similarly, despite the anticipated lowering of exposure of HIV PIs by boceprevir, HIV breakthrough was not observed more frequently among patients receiving boceprevir (3 of 64) as compared to those receiving placebo (4 of 34). Plasma levels of the HIV PIs were not measured in this study; as such, the magnitude of the drug interaction of these agents with boceprevir in co-infected patients is not known. Studies are underway to carefully assess the impact of boceprevir on antiretroviral drugs, including HIV PIs and efavirenz, in patients with HIV/HCV coinfection (ClinicalTrials.gov identifier: NCT01482767). Adverse events were common among patients who received boceprevir, with a greater frequency of anemia, neutropenia, pyrexia, decreased appetite, diarrhea, vomiting, and dysgeusia as compared to those who received placebo plus PegIFN/RBV. Discontinuation of study drugs because of adverse events was also more common in boceprevir-treated patients (12 [20%]), compared with placebo-treated patients (3 [9%]).
Overall, the authors concluded that the safety, tolerability, and efficacy profile of PegIFN/RBV for 4 weeks (the lead-in period), followed by boceprevir in combination with PegIFN/RBV for 44 weeks, was similar to the profile previously reported in HIV-uninfected patients with HCV genotype 1 infection. As a result of these observations, larger, phase III studies of boceprevir plus PegIFN/RBV are underway to evaluate the effectiveness of this regimen in HIV/HCV-coinfected patients who have not been previously treated for HCV infection and in those who have not responded to previous treatment with PegIFN/RBV (ClinicalTrials.gov identifiers: NCT01513941 and NCT01467479). In addition to providing confirmatory data regarding safety and efficacy in co-infected patients, the study conducted by the AIDS Clinical Trials Group will carefully assess the pharmacokinetics of boceprevir and antiretroviral drugs, including HIV PIs, raltegravir, and efavirenz. In addition, response-guided therapy with boceprevir plus PegIFN/RBV, with a shorter therapy duration for patients who achieve rapid virologic response, will be assessed. The studies are estimated to be completed in 2015.
Drug Interactions With ART
In the current era, most HIV-infected patients are receiving combination ART; as such, a thorough understanding of the potential interactions between HCV and HIV PIs is essential prior to concurrent use of these agents. Both telaprevir and boceprevir also interact with CYP3A as inhibitors and substrates, raising the potential for interactions with drugs that are metabolized through this pathway. Boceprevir is metabolized primarily by aldo-keto reductase and is a strong inhibitor of and partially metabolized by CYP3A4. Similarly, telaprevir is an inhibitor and substrate of CYP3A4. These effects on CYP3A suggest that drugs that are metabolized by this enzyme may have increased concentrations and that drugs that induce this enzyme may lower telaprevir and boceprevir concentrations. There are limited studies that assess interactions between the HCV PIs and antiretroviral agents [37, 38].
Telaprevir
In studies of healthy volunteers, telaprevir was combined with ritonavir-boosted HIV PIs, including atazanavir, darunavir, fosamprenavir, and lopinavir. Telaprevir led to significant reductions in the concentrations of darunavir (the area under concentration curve [AUC] decreased by 40%) and fosamprenavir (the AUC decreased by 47%), but there was less impact with lopinavir (the AUC was unchanged) and atazanavir (the AUC decreased by 17%). Conversely, the HIV PIs also led to significant reductions in telaprevir concentrations (the AUC decreased by 20%–54%), with the smallest impact observed with atazanavir. On the basis of these studies, atazanavir boosted with ritonavir was permitted in the phase II study of telaprevir in HIV-infected patients; other HIV PIs were not allowed. With respect to the NNRTI efavirenz, coadministration with this agent led to a 20% reduction in the AUC of telaprevir. This effect was offset by the administration of a higher dose of telaprevir, 1125 mg every 8 hours. More recently, similar healthy-volunteer studies demonstrated no significant interactions between telaprevir and the HIV integrase inhibitor raltegravir or the HIV NNRTIs etravirine and rilpivirine. On the basis of the pharmacokinetic data from healthy volunteers and the patients treated in the phase IIa study, the use of telaprevir can be considered for HCV/HIV-coinfected individuals who are not receiving ART and for those who are receiving ART that includes 2 nucleos(t)ide reverse-transcriptase inhibitors plus either raltegravir, efavirenz, atazanavir/ritonavir, etravirine, or rilpivirine. Patients receiving other ART regimens may be candidates for telaprevir if they are able to switch to an acceptable regimen for the treatment of their HIV infection. However, such alterations in HIV treatment must be carefully considered, taking into account prior antiretroviral exposures, HIV genotype, and anticipated tolerability.
Boceprevir
In healthy-volunteer studies, when boceprevir was coadministered with efavirenz, the concentration of efavirenz (an inducer of CYP3A) increased, and the concentration of boceprevir was reduced. Similar interactions were observed with the HIV PI ritonavir, an inhibitor of CYP3A4. In the phase IIa clinical trial, patients receiving efavirenz and other NNRTIs were not enrolled. On the basis of the predictions regarding the absence of clinically significant drug interactions, the phase IIa trial did enroll co-infected patients receiving ritonavir-boosted HIV PIs. However, on 8 February 2012, the Food and Drug Administration notified healthcare professionals and patients that drug interactions between boceprevir and ritonavir-boosted atazanavir, lopinavir, and darunavir could potentially reduce the effectiveness of these medicines when they are used together. This warning was based on the findings of drug interaction studies in healthy volunteers treated with boceprevir and these 3 HIV PIs. The coadministration of boceprevir resulted in reduced mean concentrations of ritonavir-boosted atazanavir, lopinavir, and darunavir by 49%, 43%, and 59%, respectively. Similar reductions in AUC and peak concentration of atazanavir, lopinavir, and darunavir were also observed. While the coadministration of ritonavir-boosted atazanavir did not alter the exposure of boceprevir, the coadministration of lopinavir/ritonavir and ritonavir-boosted darunavir decreased the AUC of boceprevir by 45% and 32%, respectively. Thus, on the basis of current data, the use of boceprevir should be limited to HIV/HCV-coinfected patients who are not receiving concurrent ART and to those receiving raltegravir in combination with nucleoside reverse-transcriptase inhibitors (NRTIs). Patients receiving other ART regimens may be candidates for boceprevir if they are able to switch to raltegravir plus NRTIs for the treatment of their HIV infection. However, such alterations in HIV treatment must be carefully considered, taking into account prior antiretroviral exposures, HIV genotype, and anticipated tolerability.
APPROACH TO THE TREATMENT OF HCV IN HIV-COINFECTED PATIENTS
Although the data regarding the safety and efficacy of telaprevir or boceprevir in combination with PegIFN/RBV for the treatment of HCV infection in HIV-infected patients is limited, the phase IIa studies provide evidence that the addition of HCV PIs is associated with a clinically meaningful increase in SVR rate as compared to placebo control. Furthermore, the adverse effects of these HCV drugs were similar in co-infected patients and monoinfected patients and, while drug interactions with antiretrovirals must be taken into account, healthy-volunteer studies have led to the identification of several antiretroviral regimens that are compatible with HCV PIs. Telaprevir or boceprevir plus PegIFN/RBV should be considered for HIV/HCV-coinfected patients for whom the medical need for HCV treatment is high and for whom deferral of HCV treatment may present clinical risk of liver outcomes, although such use is off-label. For most co-infected patients, hepatic fibrosis stage is predictive of the risk of subsequent clinical outcomes, with the lowest risk observed in patients with no or minimal hepatic fibrosis [20]. In light of rapid development of additional DAAs for the treatment of HCV infection, including interferon alfa–free combination DAA regimens, the decision to treat or defer therapy with telaprevir or boceprevir plus PegIFN/RBV should be based on a careful assessment of the individual patient's medical need for HCV treatment. Specifically, on the basis of the expected introduction of novel HCV treatment regimens by 2014, some clinicians recommend deferral of HCV treatment in HIV/HCV-coinfected patients with minimal hepatic fibrosis.
CONCLUSIONS
Approximately 30% of HIV-infected persons in the United States and other parts of the world are co-infected with HCV. In addition to these prevalent cases, new HCV infections continue to occur as the result of sexual (among HIV-infected men who have sex with men) and/or parenteral exposure to the pathogen. In the era of effective HIV therapy, chronic HCV infection is a leading cause of liver disease and mortality in HIV-infected patients. Although treatment of HIV infection with ART is associated with lower risk of HCV disease progression, HIV/HCV-coinfected patients remain at greater risk for HCV-related liver disease and death, compared with HCV-monoinfected patients. Accordingly, the development and delivery of effective HCV treatment is a priority for the management of co-infected patients. The use of telaprevir or boceprevir plus PegIFN/RBV for the treatment of HCV infection in HIV-infected patients presents multiple challenges, including significant side effects and drug interactions with some antiretroviral drugs. Nonetheless, 2 small, phase IIa studies have demonstrated high rates of SVR in HIV-infected patients with HCV genotype 1 infection treated with telaprevir or boceprevir in combination with PegIFN/RBV, supporting the off-label use of these regimens in carefully selected coinfected patients.
Notes
Financial support. This work was supported by National Institute on Drug Abuse (grants K24DA034621 and R01DA16065), the National Center for Research Resources (grant UL1RR025005), and the National Institutes of Health Roadmap for Medical Research.
Potential conflicts of interest. M. S. S. has received consulting fees and research grants from Abbott Laboratories, Bristol-Myers Squibb, Boehringer Ingelheim Pharmaceuticals, Gilead, Janssen, Merck, Roche/Genentech, and Vertex.
The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–7. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 2.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002;288:199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- 3.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–50. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Torres M, Slim J, Bhatti L, et al. Peginterferon alfa-2a plus ribavirin for HIV-HCV genotype 1 coinfected patients: a randomized international trial. HIV Clin Trials. 2012;13:142–52. doi: 10.1310/hct1303-142. [DOI] [PubMed] [Google Scholar]
- 7.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–33. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta SH, Lucas GM, Mirel LB, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20:2361–9. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DL. The challenge of hepatitis C in the HIV-infected person. Annu Rev Med. 2008;59:473–85. doi: 10.1146/annurev.med.59.081906.081110. [DOI] [PubMed] [Google Scholar]
- 10.Smith C. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 11.Monto A, Schooley RT, Lai JC, et al. Lessons from HIV therapy applied to viral hepatitis therapy: summary of a workshop. Am J Gastroenterol. 2010;105:989–1004. doi: 10.1038/ajg.2009.726. [DOI] [PubMed] [Google Scholar]
- 12.Pineda JA, Gonzalez J, Ortega E, et al. Prevalence and factors associated with significant liver fibrosis assessed by transient elastometry in HIV/hepatitis C virus-coinfected patients. J Viral Hepat. 2010;17:714–9. doi: 10.1111/j.1365-2893.2009.01229.x. [DOI] [PubMed] [Google Scholar]
- 13.Staples CT, Jr., Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29:150–4. doi: 10.1086/520144. [DOI] [PubMed] [Google Scholar]
- 14.Wandeler G, Gsponer T, Bregenzer A, et al. Hepatitis C virus infections in the Swiss HIV Cohort Study: a rapidly evolving epidemic. Clin Infect Dis. 2012;55:1408–6. doi: 10.1093/cid/cis694. [DOI] [PubMed] [Google Scholar]
- 15.van de Laar T, Pybus O, Bruisten S, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136:1609–17. doi: 10.1053/j.gastro.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urbanus AT, van de Laar TJ, Stolte IG, et al. Hepatitis C virus infections among HIV-infected men who have sex with men: an expanding epidemic. AIDS. 2009;23:F1–F7. doi: 10.1097/QAD.0b013e32832e5631. [DOI] [PubMed] [Google Scholar]
- 17.Danta M, Brown D, Bhagani S, et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21:983–91. doi: 10.1097/QAD.0b013e3281053a0c. [DOI] [PubMed] [Google Scholar]
- 18.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 19.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22:1979–91. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 20.Limketkai BN, Mehta SH, Sutcliffe CG, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012;308:370–8. doi: 10.1001/jama.2012.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsui JI, Saitz R, Cheng DM, et al. Awareness of hepatitis C diagnosis is associated with less alcohol use among persons co-infected with HIV. J Gen Intern Med. 2007;22:822–5. doi: 10.1007/s11606-007-0147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell JV, Hagan H, Latka MH, et al. High prevalence of alcohol use among hepatitis C virus antibody positive injection drug users in three US cities. Drug Alcohol Depend. 2006;81:259–65. doi: 10.1016/j.drugalcdep.2005.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 24.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Ward JW. Hepatitis C Virus Testing of Persons Born during 1945 to 1965: recommendations From the Centers for Disease Control and Prevention. Ann Intern Med. 2012;157:817–22. doi: 10.7326/0003-4819-157-9-201211060-00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–16. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Kramer JR, Kanwal F, Richardson P, Mei M, El-Serag HB. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012;56:320–5. doi: 10.1016/j.jhep.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Poordad F, McCone J, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 29.Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–28. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 30.Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–17. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–44. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas DL, Bartlett JG, Peters MG, Sherman KE, Sulkowski MS, Pham PA. Provisional guidance on the use of hepatitis C virus protease inhibitors for treatment of hepatitis C in HIV-infected persons. Clin Infect Dis. 2012;54:979–83. doi: 10.1093/cid/cir882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.on behalf of the Study 110 Team. Dieterich DT, Soriano V, Sherman K, Girard P-M, Rockstroh J, Adiwijaya B, McCallister S, Adda N, Mahnke L, Sulkowski MS. Telaprevir in combination with pegylated interferon-a-2a+RBV in HCV/HIV-co-infected patients: a 24-week treatment interim analysis. 2012 March 5-8-2012, [abstract #46] Conference on Retroviruses and Other Opportunistic Infections, Seattle, WA. [Google Scholar]
- 34.Garg V, Chandorkar G, Yang Y. The effect of CYP3A inhibitors and inducers on the pharmacokinetics of telaprevir in healthy volunteers. Br J Clin Pharmacol. 2013;75:431–9. doi: 10.1111/j.1365-2125.2012.04345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sulkowski MS, Pol S, Cooper C, Fainboim H, Slim J, Rivero A, Laguno M, Thompson S, Wahl J, Greaves W. Boceprevir + pegylated interferon + ribavirin for the treatment of HCV/HIV-coinfected patients: end of treatment (week 48) interim results. Conference on Retroviruses and Other Opportunistic Infections; Seattle, WA. 2012. March 5-8 2012, [Abstract #47]. , [Google Scholar]
- 36.Wilby KJ, Greanya ED, Ford JA, Yoshida EM, Partovi N. A review of drug interactions with boceprevir and telaprevir: implications for HIV and transplant patients. Ann Hepatol. 2012;11:179–85. [PubMed] [Google Scholar]
- 37.Seden K, Back D, Khoo S. New directly acting antivirals for hepatitis C: potential for interaction with antiretrovirals. J Antimicrob Chemother. 2010;65:1079–85. doi: 10.1093/jac/dkq086. [DOI] [PubMed] [Google Scholar]
- 38.Naggie S, Sulkowski MS. Management of patients coinfected with HCV and HIV: a close look at the role for direct-acting antivirals. Gastroenterology. 2012;142:1324–34. doi: 10.1053/j.gastro.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]