Abstract
BACKGROUND
The milk-derived peptides isoleucine–proline–proline (IPP) and valine–proline– proline (VPP) have been shown to reduce systolic blood pressure (SBP). This decrease is convincingly shown in subjects of Asian origin, but less consistent results have been obtained in European populations.
METHODS
A meta-analysis was conducted in accord with the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) requirements, to assess the effect of IPP and VPP on SBP in Europeans, and to explore some determinants of this effect.
RESULTS
Ninety-one publications on the effect of IPP and VPP on SBP in Europeans were identified, and 14 trials with 15 sets of data (n = 1,306) met the inclusion criteria for the meta-analysis. A random-effects model (using the restricted maximum likelihood (REML) estimator) was used for the analysis. Although not all individual trials showed a statistically significant effect of IPP or VPP in reducing SBP, the combination of all data for the two peptides yielded a statistically significantly greater effect for IPP/VPP than for placebo. The decrease in SBP with IPP/VPP was 1.28mm Hg (95% CI, –2.09 to –0.48, P = 0.0017) and the decrease in diastolic BP (DBP) was 0.59mm Hg (95% CI, –1.18 to –0.01, P = 0.047). There was no evidence in the meta-analysis of any publication bias or of heterogeneity (P = 0.13). Among other features, a significant effect was seen for age, with each additional year of age reducing the effect on SBP by 0.09mm Hg. This might be related to isolated systolic hypertension, a condition often encountered in the elderly, who may be poorly responsive to first-line treatments for hypertension.
CONCLUSION
The peptides IPP and VPP are effective in moderately reducing SBP in European subjects, as is known for Asian populations. These two peptides could therefore have a role in controlling blood pressure (BP), a prospect that merits their further study.
Keywords: blood pressure, hypertension, IPP, VPP, meta-analysis.
Hypertension can be prevented with lifestyle measures, such as physical activity, maintaining a normal body weight, and adopting a healthy diet.1 Besides this, several randomized trials and meta-analyses have shown that some peptides derived from milk proteins, such as isoleucine–proline–proline (IPP) and valine–proline–proline (VPP), decrease systolic blood pressure (SBP).2–4 The precise mechanisms responsible for this antihypertensive effect are still unknown, but it may involve the inhibition of angiotensin-converting enzyme (ACE),5,6 production of vasodilators,7,8 or an effect on sympathetic nervous activity.9 Although previous meta-analyses of the effect of VPP or IPP have estimated decreases in SBP of –4.8mm Hg (95% CI, –6.0 to –3.7)3 or –3.73mm Hg (95% CI, –6.70 to –1.76]),4 some heterogeneity existed among the studies on which these analyses were done, and the effect of IPP/VPP on SBP appeared stronger in Asian subjects (–6.93mm Hg) than in European subjects (–1.17mm Hg).4 Indeed, effective dosages of antihypertensive drugs vary according to ethnicity.10 Thus, for example, the same dosage of perindopril decreases SBP more effectively in Japanese patients than in European patients (P = 0.001).11 However, it is also possible that the smaller and marginally nonsignificant effect of IPP/VPP (P = 0.07) observed in European populations is linked to the small number of studies of European subjects that were included in our previous meta-analysis.4 This may have led to an insufficient statistical power to detect an effect in Europeans, in whom the effects of IPP/VPP were likely to be smaller than in Japanese subjects. Several studies have been published since our previous analysis, and are included in the meta-analysis described in the present report of the effect of IPP/VPP on SBP in European subjects. The objectives of this meta-analysis were to estimate the size of the change in SBP after IPP/VPP intake and to identify the roles of the various characteristics of subjects and studies in this change.
METHODS
This meta-analysis was done according to the recommendations of the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) statement12 (see the supplementary digital content SDC Table 1).
Search strategy and selection criteria
Potentially relevant published studies from the Medline and Cochrane databases were identified through a search limited to human adults as study subjects. The retrieved publications were screened independently by two nutritional scientists with doctoral degrees, who resolved potential conflicts in the selection of studies through detailed discussions of the full text of each study. Reviews, studies not involving humans, or studies that were not randomized, double-blinded, and placebo-controlled were excluded from the meta-analysis, as were studies in which no measure of SBP or no intervention with IPP/VPP was reported. Additionally, studies enrolling non-European populations or drug-treated hypertensive patients, or those testing IPP/VPP for less than 1 week or at high dosages (above 12mg/d), which are unlikely to be easily available in everyday life, were excluded (SDC Table 2).
A data extraction sheet was developed for the collection of data. The extracted data were independently checked by two trained statisticians, with disagreements resolved by discussion. Authors of seven publications were contacted for further information. All responded, and five provided the level of precision required. Adequate data could not be retrieved for two studies,13 for one of which we used the same data as used in a previous meta-analysis,4 and for the second of which14 we extrapolated the analyzed data from a graphical presentation in the published paper.
Information was extracted from each included trial on: (i) characteristics of participants (mean age, blood pressure (BP) status, country of origin); (ii) characteristics of the study, including its design, duration of IPP/VPP administration, and year of publication; and (iii) primary outcome measure (change from baseline to endpoint office SBP), secondary outcome measure (change in office diastolic BP (DBP)), number of subjects for which study data were analyzed, mean effect, and variability measures (SD or SEM). Office measurement was chosen for the evaluation of SBP because it was the component of BP measured (in accord with good practice) in each study, whereas ambulatory BP was assessed in only a few centers.
According to the study-selection criteria for the meta-analysis, only placebo-controlled, double-blinded, and randomized trials were included, hence reducing the risk of bias within each individual study. The hypothesis that quality of data retrieval could have an impact on the effect size observed in each study was tested in exploratory analyses of heterogeneity.
Statistical analysis
We used the R system statistical software version 2.13.115 and the Metafor package, version 1.4–016, for all computations in our statistical analysis of data. All of the analyses that were done were preplanned and described in a statistical analysis plan. The outcome measure was the mean difference between groups receiving IPP/VPP and those receiving placebo in the change from baseline to endpoint office SBP (primary outcome) and office DBP (secondary outcome). The retained endpoint for each study was the reading at the final evaluation visit if there was only one post-baseline evaluation, or the evaluation that was done most closely to week 8 if several evaluations were done.
Without adequate adjustment, errors in units of analysis may arise with data from crossover and parallel-group trials that have multiple arms. Four of the studies included in our meta-analysis used crossover designs,13,14,17,18 and three other studies had either multiple active-treatment arms, used different daily doses of IVP/IPP,19,20 or used different types of IPP/VPP.21
A carryover effect was reported as significant only in the study conducted by Tuomilehto et al.17 Consequently, only results from the first period of that study were used in our meta-analysis. In the case of parallel-group studies with multiple active-treatment arms, we pooled the dara for all active-treatment groups to avoid including the placebo group more than once in the same meta-analysis. This is unlikely to introduce any major bias, as we did not previously identify4 any effect of the dose or matrix of an IPP/VPP product on its effect on SBP.
In calculating the mean pooled effect of IPP/VPP and its 95% CI, we estimated both fixed and random effects. Because we suspected heterogeneity on the basis of inconsistencies in treatment effects in the literature and in a previous meta-analysis,4 we used a random-effects model meta-analysis as our primary analysis, with the REML (REstricted Maximum Likelihood) estimator.16 Studies were weighted according to the inverse of their variance. Between-study heterogeneity was quantified by calculating tauÂ2, IÂ2 and HÂ2 statistics and by computing the Cochran’s Q test statistic.22 In investigating the risk of bias across studies, we examined the potential for publication bias by means of a funnel plot (SE of effect versus estimate of effect size for each study) and by computing the Kendall rank correlation test statistic (Kendall’s tau) for the size of a standardized effect vs. the SE values of the effect.23
Heterogeneity was explored in meta-regressions and subgroup analyses. The influence of each of the following characteristics was investigated: year of publication, country of origin of the patients (three groups: Nordic, Mediterranean, and others), BP status (normotensive, prehypertensive, or hypertensive), study design (parallel group or crossover), duration of administration of IPP/VPP, and assessment of “quality of data retrieval.” The influence of each individual study on the overall results was analyzed by omitting one study at a time. Influence effects were also computed, using studentized residuals, Cook’s distance, and hat value.
RESULTS
Characteristics of included studies
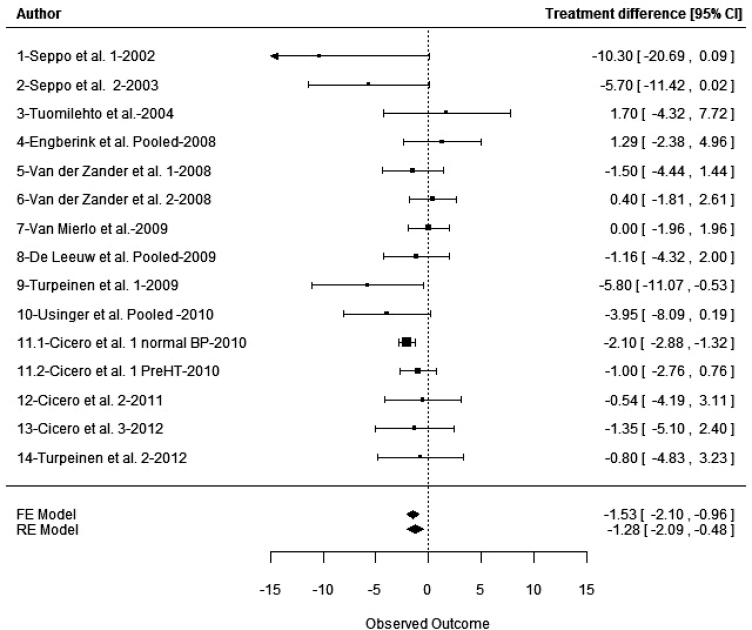
A total of 91 potentially relevant publications was identified. On the basis of the criteria described above, 77 publications were discarded (see SDC Table 2) and 14 studies, including 1 that was analyzed according to 2 subgroups,18 totaling 15 different series, were included (Tables 1 and 2). All of the studies were published in English in international, peer-reviewed journals (except for one study24), and as full papers (except for one study14 that was published as a research letter). Together, the 15 reported series that were analyzed included 1,465 treatment periods (830 with IPP/VPP and 635 with placebo) in 1,277 subjects (188 subjects in crossover trials). All of the studies were randomized and double-blinded. However, only 6 of the 14 studies used SBP as the primary criterion for the calculation of statistical power.13,19–21,25,26 The assumptions made about effect size or SD values, often not mentioned, seemed to overestimate the effect size, which was expected to be between –3 and –8mm Hg, and to underestimate the SD, thus potentially leading to insufficient sample sizes. Estimations of study quality (Jadad score) and data-retrieval quality indicated that the risk of bias within studies was minor. For the primary outcome (change from baseline to endpoint in office SBP) among individual studies, the mean difference between IPP/VPP and placebo varied from –10.3 (± 5.3 SE) mm Hg in favor of IPP/VPP to +1.7 (± 1.07 SE) mm Hg in favor of placebo (Tables 2 and Figure 2). Data related to DBP are provided in SDC Table 3.
Table 1.
Characteristics of studies included in meta-analysis of randomized controlled trials of the effect of isoleucine–proline–proline and valine–proline–proline on systolic blood pressure
| Intervention | Population | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study number | Reference no. | Jadad score | IPP/VPP dose in mg/d and (vehicle)a | Design | Country | Duration (weeks) | n (analyzed) | BP status | Mean age |
| 1 | 24 | 3 | 5.6 (fermented milk) | Parallel | Finland | 8 | 17 | HT | 47.76 |
| 2 | 28 | 3 | 5.3 (fermented milk) | Parallel | Finland | 21 | 39 | HT | 49.59 |
| 3 | 17 | 4 | 5.1 (fermented milk) | Crossover | Finland | 10 | 59 | HT | 52.73 |
| 4 | 21 | 5 | 10.0, 10.2, 10.4 (fermented milk) | Parallel | The NL | 8 | 135 | HT | 57.92 |
| 5 | 14 | 3 | 8.7 (powered fermented milk) | Crossover | The NL | 4 | 40 | PreHT/ HT | NA |
| 6 | 25 | 3 | 10.2 (powder in yoghurt) | Parallel | The NL | 8 | 271 | HT | 60.00 |
| 7 | 13 | 4 | 4.6, 10.2 (powder in yoghurt) | Crossover | Scotland | 4 | 162 | HT | 61.70 |
| 8 | 19 | 5 | 2.3, 4.6, 9.0 (powder in yoghurt) | Parallel | The NL | 8 | 166 | HT | 58.73 |
| 9 | 29 | 3 | 4.2 (powder in spread) | Parallel | Finland | 10 | 58 | HT | 48.00 |
| 10 | 20 | 4 | 1.8, 3.6 (fermented milk) | Parallel | Denmark | 8 | 94 | PreHT /HT | 53.33 |
| 11 | 18 | 3 | 6.0 (powder in fruit juice) | Crossover | Italy | 4 | 55 | Normal/ PreHT | 40.27 |
| 12 | 27 | 4 | 3.0 (powder in fruit juice) | Parallel | Italy | 6 | 52 | PreHT/ HT | 41.00 |
| 13 | 40 | 5 | 3.0 (powder in fruit juice) | Parallel | Italy | 6 | 164 | PreHT/ HT | 43.85 |
| 14 | 26 | 3 | 4.2 (powder in spread) | Parallel | Finland | 10 | 104 | HT | 49.50 |
aPlacebo products were indistinguishable from verum and were either the vehicle food (yoghurt or fruit juice or spread), or milk fermented with bacteria unable to produce IPP/VPP.
Abbreviations: BP, blood pressure; HT, hypertension; IPP, isoleucine–proline–proline; NA, not available; preHT, pre-hypertension; VPP, valine–proline–proline.
Table 2.
Effect of isoleucine–proline–proline and valine–proline–proline on systolic blood pressurea
| Treated group | Placebo group | Effect size | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Change in SBP (mm Hg) | Change in SBP (mm Hg) | |||||||||
| Series number | Study reference | n | Mean | SD | n | Mean | SD | Mean difference between groups | SE | Quality of data retrieval |
| 1 | 24 | 10 | –15.1 | 10 | 7 | –4.8 | 11.9 | –10.3 | 5.3 | 3 |
| 2 | 28 | 22 | –13.5 | 9.85 | 17 | –7.7 | 7.83 | –5.7 | 2.92 | 3 |
| 3 | 17 | 30 | –10.8 | 12.9 | 29 | –12.5 | 10.5 | 1.7 | 3.07 | 3 |
| 4 | 21 | 102 | –3.02 | 9.61 | 31 | –4.3 | 7.24 | 1.29 | 1.87 | 3 |
| 5 | 14 | 40 | NA | NA | 40 | NA | NA | –1.5 | 1.5 | 1 |
| 6 | 25 | 134 | –1.9 | 9.26 | 137 | –2.3 | 9.36 | 0.4 | 1.13 | 3 |
| 7 | 13 | 64 | –4.7 | 8 | 64 | –4.7 | 8 | 0 | 1 | 2 |
| 8 | 19 | 125 | –3.36 | 8.96 | 41 | –2.2 | 8.96 | –1.16 | 1.61 | 3 |
| 9 | 29 | 30 | –5.8 | 9.22 | 28 | 0 | 11.07 | –5.8 | 2.69 | 3 |
| 10 | 20 | 60 | –7.75 | 9.3 | 30 | –3.8 | 9.6 | –3.95 | 2.11 | 3 |
| 11.1 | 18 | 33 | –0.4 | 2.2 | 33 | 1.7 | 1.7 | –2.1 | 0.4 | 3 |
| 11.2 | 18 | 22 | –1.7 | 2.2 | 22 | –0.7 | 2 | –1 | 0.9 | 3 |
| 12 | 27 | 25 | –4.85 | 8.74 | 25 | –4.31 | 9.28 | –0.54 | 1.86 | 3 |
| 13 | 40 | 82 | –2.73 | 12.58 | 82 | –1.38 | 11.92 | –1.35 | 1.91 | 3 |
| 14 | 26 | 51 | –2.30 | 10.20 | 49 | –1.50 | 10.36 | –0.80 | 2.06 | 3 |
The quality of data retrieval was rated as 3 if adequate information had been found in the publication or obtained from the authors; as 2 if some information was retrieved from the previously published meta-analysis by Cicero et al4; and as 1 if any numerical information had to be extrapolated from a graphical presentation in the publication.
Figure 2.
Forest plot of treatment effects of isoleucine–proline–proline/valine–proline–proline (IPP/VPP) in the meta-analysis of 15 series of findings of its effect on SBP in European subjects.
Heterogeneity and publication bias
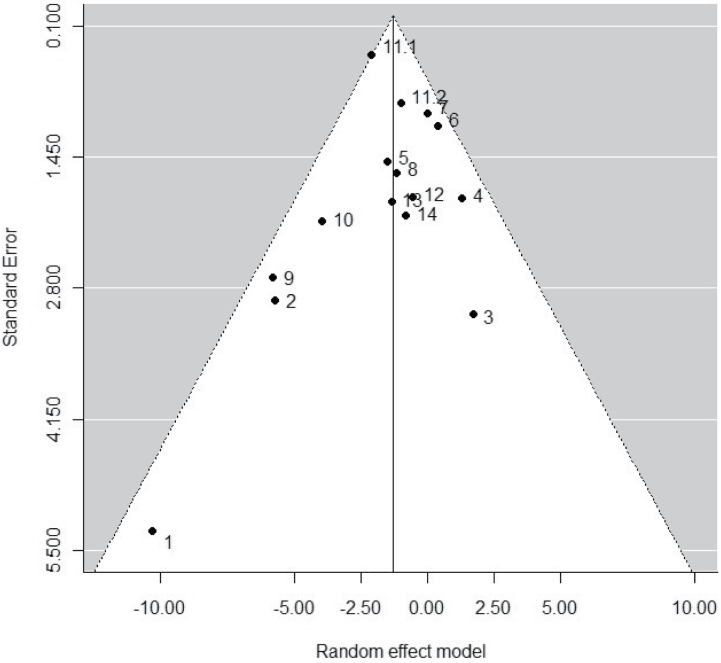
Between-series heterogeneity (IÂ2 = 20.6%, tauÂ2 = 0.44) was not statistically significant (Q = 20.1, P = 0.13). However, we further explored heterogeneity, as discussed subsequently in this paper, because it provides relevant information about the factors that can affect data. In documenting the potential for publication bias, the funnel plot did not show asymmetry, although the study by Seppo et al.24 had a large effect size and SE (Figure 1). The Kendall rank correlation test between of standardized effect sizes vs. their SEs was also nonsignificant (Kendall’s tau = –0.28, P = 0.17). Consequently, there was no evidence of any publication bias in the studies used in the meta-analysis.
Figure 1.
Funnel plot used in assessing for publication bias in the meta-analysis of 15 studies for the effect of isoleucine–proline–proline/valine–proline–proline (IPP/VPP) on systolic blood pressure (SBP).
Effect of isoleucine–proline–proline/valine–proline–proline on blood pressure
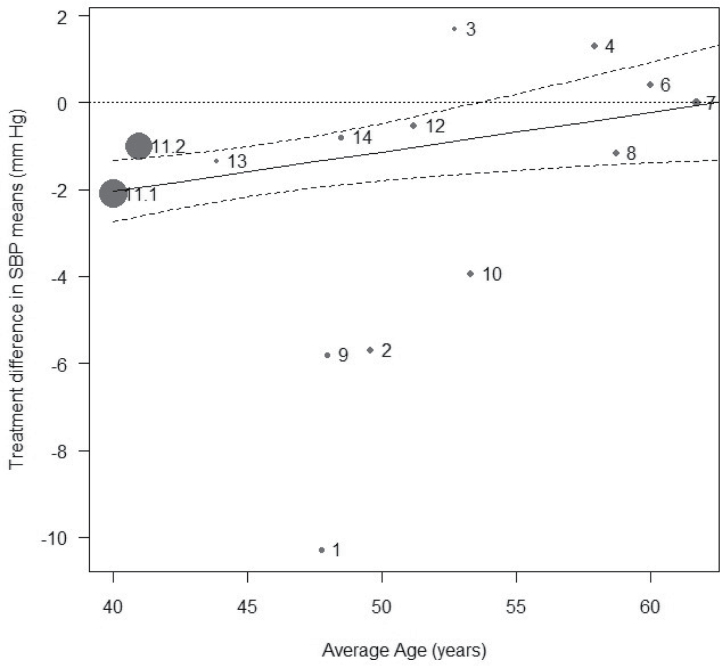
The results of the primary meta-analysis done with the random-effect model showed a statistically significantly greater effect of IPP/VPP over placebo on SBP in European patients, of a magnitude of –1.28mm Hg (95% CI, –2.09 to –0.48, P = 0.0017) (Figure 2). The effect estimate, determined with the fixed-effect model, was slightly larger (–1.53mm Hg (95% CI, –2.10 to –0.96). The decrease in DBP was marginal (–0.59mm Hg (95% CI, –1.18 to –0.01)) but significant (P = 0.047)(SDC Table 3). Exploration of heterogeneity, performed through meta-regressions and subgroup analyses of SBP data (see SDC Table 4 for corresponding data on DBP), provided evidence for the following effects: (i) A substantial effect of age (Figure 3), with a decrease in treatment effect on SBP with increasing age (0.09mm Hg/year of age (95% CI, 0.02 to 0.16), P = 0.011) without heterogeneity (P = 0.33), and the older the subjects in the study, the smaller the effect on SBP. The LTPs antihypertensive effect seems to be inversely related to the age of the subjects enrolled in the trials. Two series,18 with subjects of young average age and an intermediate treatment effect, tended to lower the regression slope of age on treatment effect. Without these two series, the relationship between age and treatment effect would have been even stronger. (ii) A significant influence of country on the IPP/VPP-mediated reducton of SBP (P < 0.001). Subgroup analyses done on groups of countries showed no significant heterogeneity in any group, although showing a significant effect (P = 0.010) of IPP/VPP on SBP in Nordic countries (–3.31mm Hg (95% CI, –5.84 to –0.78)) and a significant effect (P < 0.0001) of IPP/VPP on SBP in Italy (–1.77mm Hg (95% CI, –2.60 to –0.94)), but virtually no effect in other countries (–0.15mm Hg (95% CI, –1.30 to 1.00, P = 0.80)). (iii) A significant influence of study design (P = 0.015). Subgroup analyses showed a significant effect for IPP/VPP on SBP, with a decrease of –1.26mm Hg (95% CI, –2.33 to –0.19, P = 0.021) in crossover studies and of –1.43mm Hg (95% CI, –2.85 to –0.011, P = 0.048) in parallel-group studies. (iv) A significant influence of BP status (P < 0.0001), with subgroup analyses showing a decrease of –1.88 mm Hg (95% CI, –2.55 to –1.22) in subjects without overt hypertension and a nonsignificant (P = 0.3593) effect of –0.52 mm Hg (95% CI, –1.64 to 0.59) in subjects with hypertension.
Figure 3.
Meta-regression plot of the effect on SBP of treatment with isoleucine–proline–proline/valine–proline–proline (IPP/VPP) vs. mean age.
Heterogeneity was nonsignificant
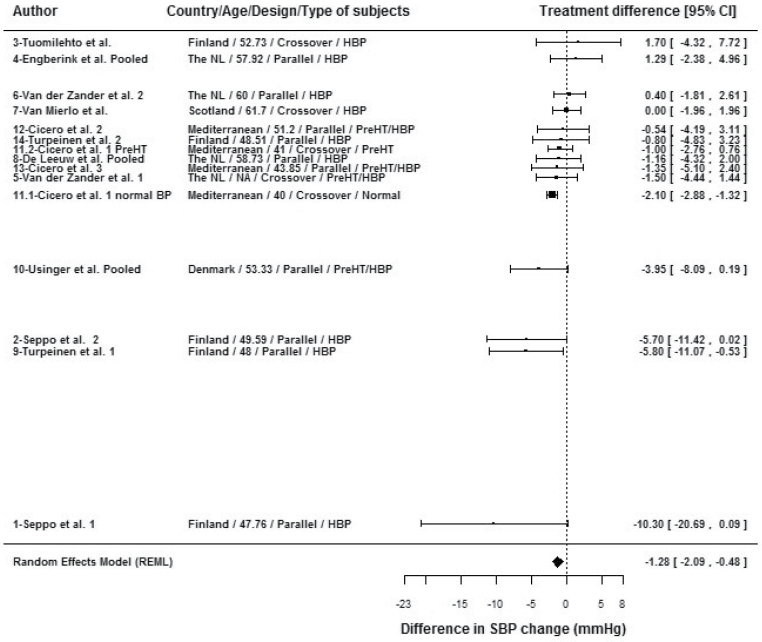
No significant influence of year of publication, duration of treatment, or quality of data retrieval was seen on the estimate of the reduction in SBP. The influence of the different covariates on the results is illustrated in the forest plot of all the studies, sorted by effect size (Figure 4). A potential confounding effect of country with age is visible, showing a clear association between younger age, Nordic countries, and important-effect size. A confounding effect of country with study design is also apparent: all Nordic studies showing a large effect size were parallel-design studies, whereas the only Nordic study showing a negative effect had a crossover design. The heterogeneity of the effect in studies of hypertensive subjects is also apparent: studies of hypertensive subjects are positioned both at the top (no effect) and bottom (stronger effect) parts of the Figure 4, whereas studies of subjects without overt hypertension are shown in the intermediate area of the figure. A potential confounding effect of age with BP status must also be reported. Among the seven series whose subjects had a mean age above 50 years,13,17,19–21,25,27 four, all of which involved overtly hypertensive subjects, showed no effect of IPP/VPP on SBP, whereas two showed moderate effects and one20 showed a large effect. Among the seven series whose subjects had a mean age below 50 years,24,26,28,29 four series, all of which involved hypertensive subjects, showed a large effect on SBP (up to –10.3mm Hg). The influence of each individual series was analyzed by omitting one study at a time. Omission of series 11.1 (the normal BP subgroup in the study done by Cicero et al.18) decreased the estimate of the effect of IPP/VPP to –0.89mm Hg (95% CI, –1.72 to –0.05), which nevertheless remained statistically significant (P = 0.037). The omitted series 11.1 had a large standardized residual of 2.08, indicating that the observed effect for this series deviated from the predicted effect under the fitted meta-analytic model. This same series had an increased Cook’s distance and hat value (and weight), and hence had an important influence on the model. Decreases in tauÂ2 and Q upon the removal of Cicero and colleagues’18 series 11.1 (normal BP subgroup) from the model indicated that it introduced residual heterogeneity into the model. Whichever study was omitted, the results of the meta-analysis of the remaining studies consistently showed a statistically significant result favoring IPP/VPP over placebo.
Figure 4.
Forest plot of treatment effects of isoleucine–proline–proline/valine–proline–proline (IPP/VPP) in the meta-analysis of 15 series of findings of its effect on SBP in European subjects, with the series sorted by effect size and study characteristics.
DISCUSSION
The meta-analysis of 14 European studies, done with the preplanned random-effect model, gave a statistically significant estimate of the effect of IPP/VPP on SBP of –1.28mm Hg (95% CI, –2.09 to –0.48, P = 0.0017), without significant heterogeneity among studies and without evidence of publication bias. Bias within individual studies was minimized by limiting the selection of studies for meta-analysis to randomized, placebo-controlled, double-blind studies and excluding studies with “extreme” conditions, such as very high doses of IPP/VPP, very short duration of intake, and populations consisting of subjects with treated hypertension. This also contributed to limiting the heterogeneity among the studies used for meta-analysis. The quality of data retrieval was good for most of the studies selected, and this was confirmed by the absence of an influence of the quality of data retrieval on the results of the analysis. This is to our knowledge the first meta-analysis restricted to European subjects, and it confirms that although Japanese populations may be better responders to IPP/VPP than are European populations, this peptide combination can be a useful means for helping to maintain a normal SBP in European subjects.
The magnitude of the decrease in SBP with IPP/VPP is moderate, and might not be relevant at the individual level, but can make sense at the population level. Indeed, several recommended and efficient lifestyle changes, such as moderate alcohol consumption30 or dietary sodium restriction,31,32 do not translate into very substantial decreases in SBP at the population level. Intake of IPP/VPP might be used, in addition to lifestyle changes, to better control BP.
Most of the individual studies included in our meta-analysis had a small population-sample size and are likely to be statistically underpowered for detecting small effects. Retrospective power calculations, with the observed SDs and size effect found in the present meta-analysis, indicate that around 1,000 subjects would be needed to reach a power of 80% with a 5% alpha risk. In this context of small studies, nonsignificant results do not necessarily mean an absence of effect. By increasing the statistical power for demonstrating an effect of IPP/VPP in reducing BP, our meta-analysis provides convincing evidence of such an effect, whereas individual studies failed to do so.
Some limitations of our study should, however, be acknowledged. Despite the lack of evidence of publication bias in the studies used in our meta-analysis, some relevant studies might still have remained unpublished. Moreover, the meta-analysis was done on summary results reported in publications, rather than on individual data. The effect of individual covariates (e.g. age or baseline BP) has consequently not been assessed at an individual level.
Meta-regression approaches and subgroup meta-analyses in our study highlighted some features that can help in identifying the optimal conditions for IPP/VPP to exert their effect of reducing SBP. Among these features, the significant influence of age (P = 0.011) may deserve further attention, with a trend toward a stronger effect in younger subjects. When baseline mean BP values are carefully examined (see SDC Table 4), it can be seen that several studies are likely to have enrolled subjects with isolated systolic hypertension, whose mean age was also higher.13,19,21,25 In these studies, IPP/VPP seemed to be less effective in lowering SBP, which is an expected observation because isolated systolic hypertension in the elderly is often relatively resistant to first-line antihypertensive treatments.33 Indeed, specific treatment guidelines are proposed for subjects with isolated hypertension,34,35 who should probably not have been considered as a population responsive to IPP/VPP, and whose inclusion may therefore have produced heterogeneity in the meta-analysis. In addition, the effect of age combines with BP status and country, with the results being a significant influence of BP status of subjects, a significant reduction of –1.88mm Hg in subjects without overt hypertension, and a nonsignificant reduction in SBP of –0.52mm Hg in patients with hypertension. In addition to the isolated systolic hypertension mentioned above, hypertensive patients are most often older than prehypertensive or normotensive individuals. Similarly, unlike studies in other countries, studies in Nordic countries, with a greater size effect, usually include younger subjects, in whom the effect of IPP/VPP is less than in other subjects. Although the analyses that we used do not provide a formal statistical demonstration of this influence, it can be hypothesized that the age of subjects is a key factor for the efficiency of IPP/VPP in lowering SBP. In most of the studies included in our meta-analysis, it appears that patients were not selected on the basis of variability in SBP before randomization, as is usually done when antihypertensive compounds are developed.36 This probably explains the large SD of the change in SBP often observed in many of the studies included in our meta-analysis. The study by Cicero et al.,18 which contributes substantially to the results of our meta-analysis, reports the lowest SD of changes in SBP because healthy subjects have per se a smaller excursion of BP than unhealthy ones, and because subjects whose SBP changed by less than 7mm Hg from their run-in visit to their randomization visit were targeted by preselection.
In conclusion, our findings indicate that the milk-derived peptides IPP and VPP can significantly decrease office SBP in European subjects, as previously shown in Japanese subjects. The estimated effect is small but significant, both statistically and clinically, when compared with other lifestyle interventions for reducing BP, and may well yield a significant decrease in the risk of coronary and cerebrovascular disease at the population level.37 Meta-regressions suggest that the effect could be more significant in middle-aged adults with only slightly elevated values of SBP, suggesting that IPP/VPP is potentially of interest preventively, in that treating prehypertension is associated with the effective prevention of frank hypertension.38,39 Consequently, IPP and VPP could have a role in the treatment of hypertension, although further studies are still needed to evaluate their antihypertensive efficacy in everyday life.
DISCLOSURE
Arrigo Cicero and Claudio Borghi declare that they have no conflicts of interest in the work described in this paper. Francois Aubin and Veronique Azais-Braesco have received fees from Calpis, Inc., of Tokyo, Japan, for performing the meta-analysis described in the paper and participating in the writing of the paper.
Supplementary Material
REFERENCES
- 1. US Department of Health, Education, and Welfare. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2004; 43: 1–3v. [DOI] [PubMed] [Google Scholar]
- 2. Pripp AH. Effect of peptides derived from food proteins on blood pressure: a meta-analysis of randomized controlled trials. Food Nutr Res 2008; 52 103402/fnrv52i01641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu JY, Qin LQ, Wang PY, Li W, Chang C. Effect of milk tripeptides on blood pressure: a meta-analysis of randomized controlled trials. Nutrition 2008; 24: 933–940. [DOI] [PubMed] [Google Scholar]
- 4. Cicero AF, Gerocarni B, Laghi L, Borghi C. Blood pressure lowering effect of lactotripeptides assumed as functional foods: a meta-analysis of current available clinical trials. J Hum Hypertens 2011; 25: 425–436. [DOI] [PubMed] [Google Scholar]
- 5. Nakamura Y, Yamamoto N, Sakai K, Takano T. Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme. J Dairy Sci 1995; 78: 1253–1257. [DOI] [PubMed] [Google Scholar]
- 6. Pina AS, Roque AC. Studies on the molecular recognition between bioactive peptides and angiotensin-converting enzyme. J Mol Recognit 2009; 22: 162–168. [DOI] [PubMed] [Google Scholar]
- 7. Hirota T, Nonaka A, Matsushita A, Uchida N, Ohki K, Asakura M, Kitakaze M. Milk casein-derived tripeptides, VPP and IPP induced NO production in cultured endothelial cells and endothelium-dependent relaxation of isolated aortic rings. Heart Vessels 2011; 26: 549–556. [DOI] [PubMed] [Google Scholar]
- 8. Yamaguchi N, Kawaguchi K, Yamamoto N. Study of the mechanism of antihypertensive peptides VPP and IPP in spontaneously hypertensive rats by DNA microarray analysis. Eur J Pharmacol 2009; 620: 71–77. [DOI] [PubMed] [Google Scholar]
- 9. Usinger L, Ibsen H, Linneberg A, Azizi M, Flambard B, Jensen LT. Human in vivo study of the renin-angiotensin-aldosterone system and the sympathetic activity after 8 weeks daily intake of fermented milk. Clin Physiol Funct Imaging 2010; 30: 162–168. [DOI] [PubMed] [Google Scholar]
- 10. Johnson JA. Ethnic differences in cardiovascular drug response. Circulation 2008; 118: 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arima H, Anderson C, Omae T, Liu L, Tzourio C, Woodward M, Macmahon S, Neal B, Rodgers A, Chalmers J. PROGRESS Collaborative Group. Perindopril-based blood pressure lowering reduces major vascular events in Asian and Western participants with cerebrovascular disease: the PROGRESS trial. J Hypertens 2010; 28: 395–400. [DOI] [PubMed] [Google Scholar]
- 12. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Mierlo LA, Koning MM, van der Zander K, Draijer R. Lactotripeptides do not lower ambulatory blood pressure in untreated whites: results from 2 controlled multicenter crossover studies. Am J Clin Nutr 2009; 89: 617–623. [DOI] [PubMed] [Google Scholar]
- 14. van der Zander K, Jakel M, Bianco V, Koning MM. Fermented lactotripeptides- containing milk lowers daytime blood pressure in high normal-to-mild hypertensive subjects. J Hum Hypertens 2008; 22: 804–806. [DOI] [PubMed] [Google Scholar]
- 15.R, version 2.13.1 (2011-07-08), Copyright ©2011 The R Foundation for Statistical Computing, ISBN 3-900051-07-0
- 16. Viechtbauer W. Conducting meta-analyses in R with the Metafor package. J Statistical Software 2010; 36: 1–48. [Google Scholar]
- 17. Tuomilehto J, Lindström J, Hyyrynen J, Korpela R, Karhunen ML, Mikkola L, Jauhiainen T, Seppo L, Nissinen A. Effect of ingesting sour milk fermented using Lactobacillus helveticus bacteria producing tripeptides on blood pressure in subjects with mild hypertension. J Hum Hypertens 2004; 18: 795–802. [DOI] [PubMed] [Google Scholar]
- 18. Cicero AF, Rosticci M, Veronesi M, Bacchelli S, Strocchi E, Melegari C, Grandi E, Borghi C. Hemodynamic effects of lactotripeptides from casein hydrolysate in Mediterranean normotensive subjects and patients with high-normal blood pressure: a randomized, double- blind, crossover clinical trial. J Med Food 2010; 13: 1363–1368. [DOI] [PubMed] [Google Scholar]
- 19. de Leeuw PW, van der Zander K, Kroon AA, Rennenberg RM, Koning MM. Dose- dependent lowering of blood pressure by dairy peptides in mildly hypertensive subjects. Blood Press 2009; 18: 44–50. [DOI] [PubMed] [Google Scholar]
- 20. Usinger L, Jensen LT, Flambard B, Linneberg A, Ibsen H. The antihypertensive effect of fermented milk in individuals with prehypertension or borderline hypertension. J Hum Hypertens 2010; 24: 678–683. [DOI] [PubMed] [Google Scholar]
- 21. Engberink MF, Schouten EG, Kok FJ, van Mierlo LA, Brouwer IA, Geleijnse JM. Lactotripeptides show no effect on human blood pressure: results from a double-blind randomized controlled trial. Hypertension 2008; 51: 399–405. [DOI] [PubMed] [Google Scholar]
- 22. Cochran WO. The combination of estimates from different experiments. Biometrics 1954; 10: 101–29. [Google Scholar]
- 23. Begg CB, Mazumbdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 24. Seppo L, Kerojoki O, Suomalainen T, Korpela R. The effect of Lactobacillus helveticus LBK-16H fermented milk on hypertension—a pilot study on humans. Milchwissenschaft 2002; 57: 124–127. [Google Scholar]
- 25. van der Zander K, Bots ML, Bak AA, Koning MM, de Leeuw PW. Enzymatically hydrolyzed lactotripeptides do not lower blood pressure in mildly hypertensive subjects. Am J Clin Nutr 2008; 88: 1697–1702. [DOI] [PubMed] [Google Scholar]
- 26. Turpeinen AM, Ikonen M, Kivimaki AS, Kautiainen H, Vapaatalo H, Korpela R. A spread containing bioactive milk peptides Ile-Pro-Pro and Val-Pro-Pro, and plant sterols has antihypertensive and cholesterol-lowering effects. Food Funct 2012; 3: 621–627. [DOI] [PubMed] [Google Scholar]
- 27. Cicero AF, Rosticci M, Gerocarni B, Bacchelli S, Veronesi M, Strocchi E, Borghi C. Lactotripeptides effect on office and 24-h ambulatory blood pressure, blood pressure stress response, pulse wave velocity and cardiac output in patients with high-normal blood pressure or first-degree hypertension: a randomized double-blind clinical trial. Hypertens Res 2011; 34: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 28. Seppo L, Jauhiainen T, Poussa T, Korpela R. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr 2003; 77: 326–330. [DOI] [PubMed] [Google Scholar]
- 29. Turpeinen A-M, Kumpu M, Rönnback M, Seppo L, Kautiainen H, Jauhiainen T, Vapaatalo H, Korpela R. Antihypertensive and cholesterol-lowering effects of a spread 390containing bioactive peptides IPP and VPP and plant sterols. J Functional Foods 2009; 1: 260–265. [Google Scholar]
- 30. Ueshima H, Ogihara T, Baba S, Tabuchi Y, Mikawa K, Hashizume K, Mandai T, Ozawa H, Kumahara Y, Asakura S. The effect of reduced alcohol consumption on blood pressure: a randomised, controlled, single blind study. J Hum Hypertens 1987; 1: 113–119. [PubMed] [Google Scholar]
- 31. He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004; CD004937. [DOI] [PubMed] [Google Scholar]
- 32. Jurgens G, Graudal NA. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride. Cochrane Database Syst Rev 2003; CD004022. [DOI] [PubMed] [Google Scholar]
- 33. Casiglia E, Tikhonoff V, Pessina AC. Hypertension in the elderly and the very old. Expert Rev Cardiovasc Ther 2009; 7: 659–665. [DOI] [PubMed] [Google Scholar]
- 34. Chobanian AV. Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med 2007; 357: 789–796. [DOI] [PubMed] [Google Scholar]
- 35. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Kjeldsen SE, Erdine S, Narkiewicz K, Kiowski W, Agabiti- Rosei E, Ambrosioni E, Cifkova R, Dominiczak A, Fagard R, Heagerty AM, Laurent S, Lindholm LH, Mancia G, Manolis A, Nilsson PM, Redon J, Schmieder RE, Struijker-Boudier HA, Viigimaa M, Filippatos G, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Kiowski W, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Viigimaa M, Waeber B, Williams B, Zamorano JL. 2007; Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007; 28: 1462–1536. [DOI] [PubMed] [Google Scholar]
- 36. Lee SE, Kim YJ, Lee HY, Yang HM, Park CG, Kim JJ, Kim SK, Rhee MY, Oh BH. Investigators. Efficacy and tolerability of fimasartan, a new angiotensin receptor blocker, compared with losartan (50/100mg): a 12-week, phase III, multicenter, prospective, randomized, double-blind, parallel-group, dose escalation clinical trial with an optional 12-week extension phase in adult Korean patients with mild-to-moderate hypertension. Clin Ther 2012; 34: 552–568, 568.e1–9. Epub 2012 Mar 3 [DOI] [PubMed] [Google Scholar]
- 37. Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet 2007; 370: 591–603. [DOI] [PubMed] [Google Scholar]
- 38. Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, Black HR, Grimm RH, Messerli FH, Jr, Oparil S, Schork MA. Trial of Preventing Hypertension (TROPHY) Study Investigators. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 2006; 354: 1685–1697. [DOI] [PubMed] [Google Scholar]
- 39. Lüders S, Schrader J, Berger J, Unger T, Zidek W, Böhm M, Middeke M, Motz W, Lübcke C, Gansz A, Brokamp L, Schmieder RE, Trenkwalder P, Haller H, Dominiak P. PHARAO Study Group. The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal blood pressure: a prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens 2008; 26: 1487–1496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.