Abstract
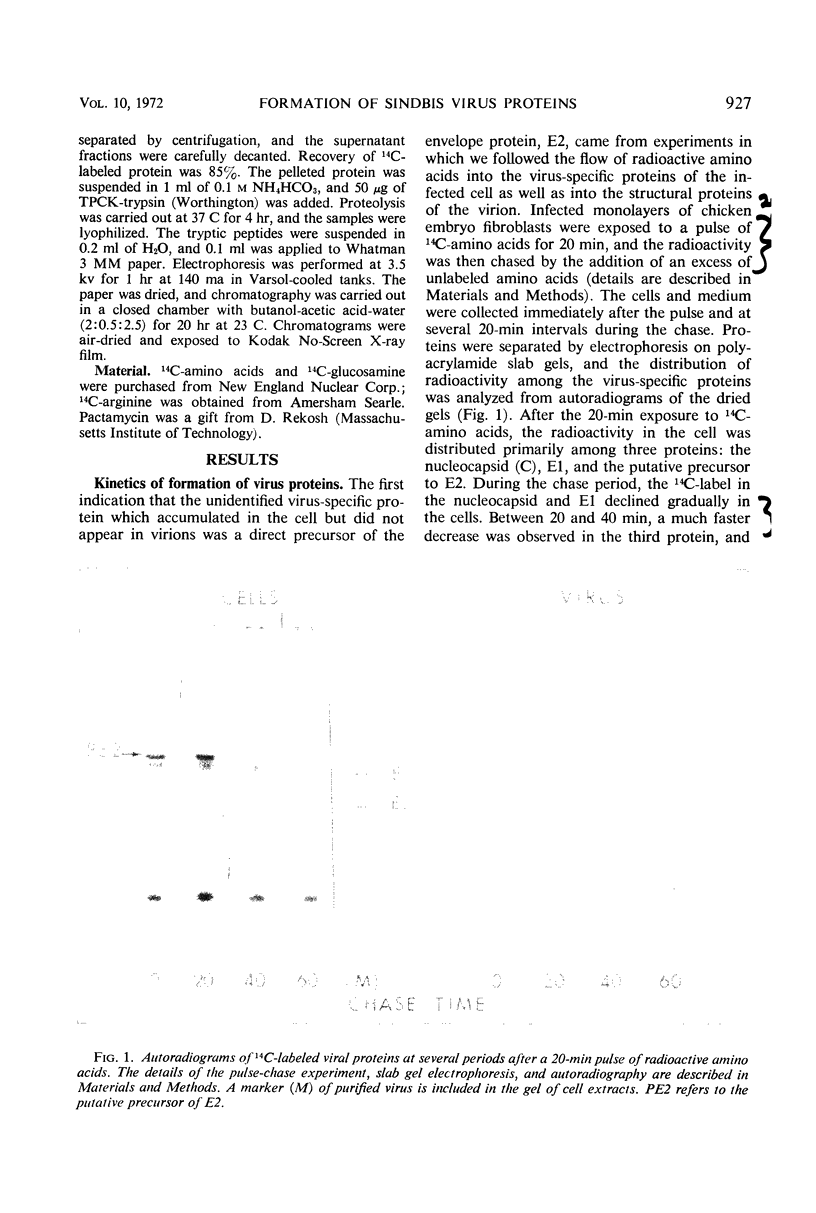
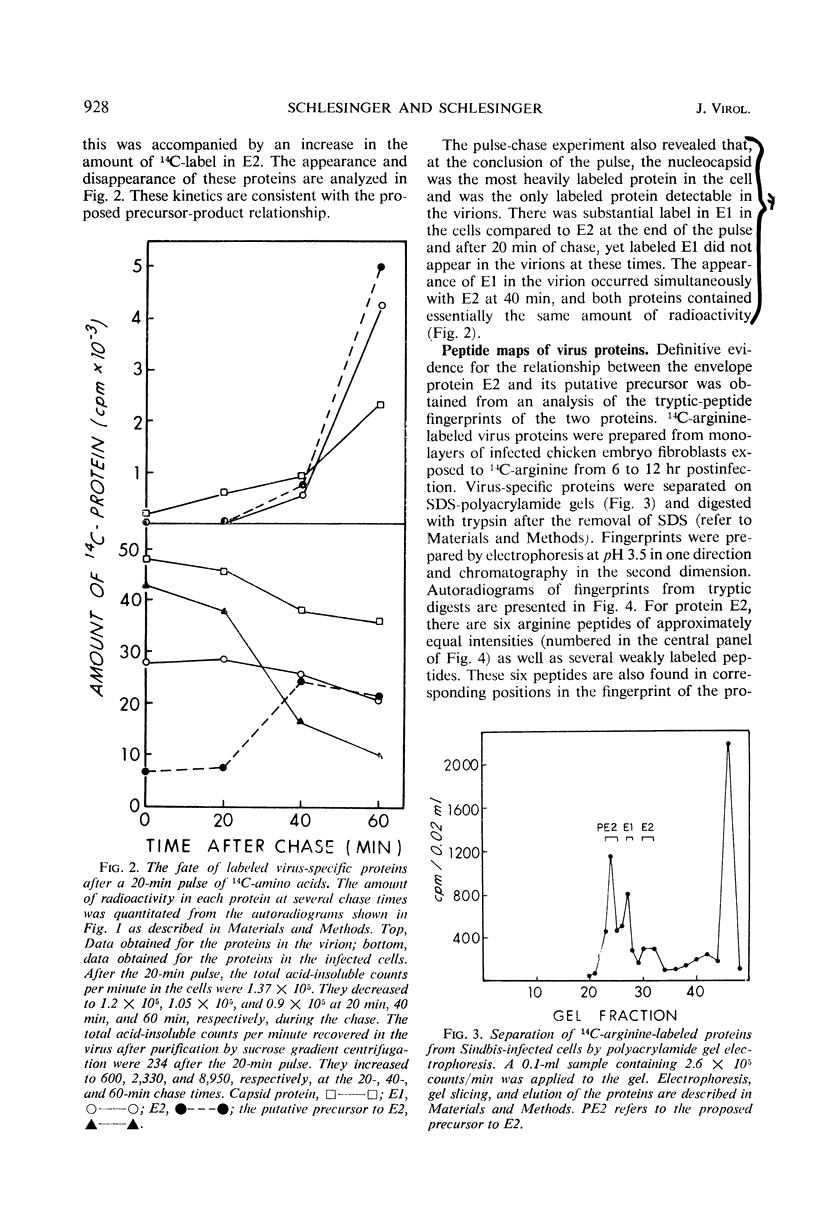
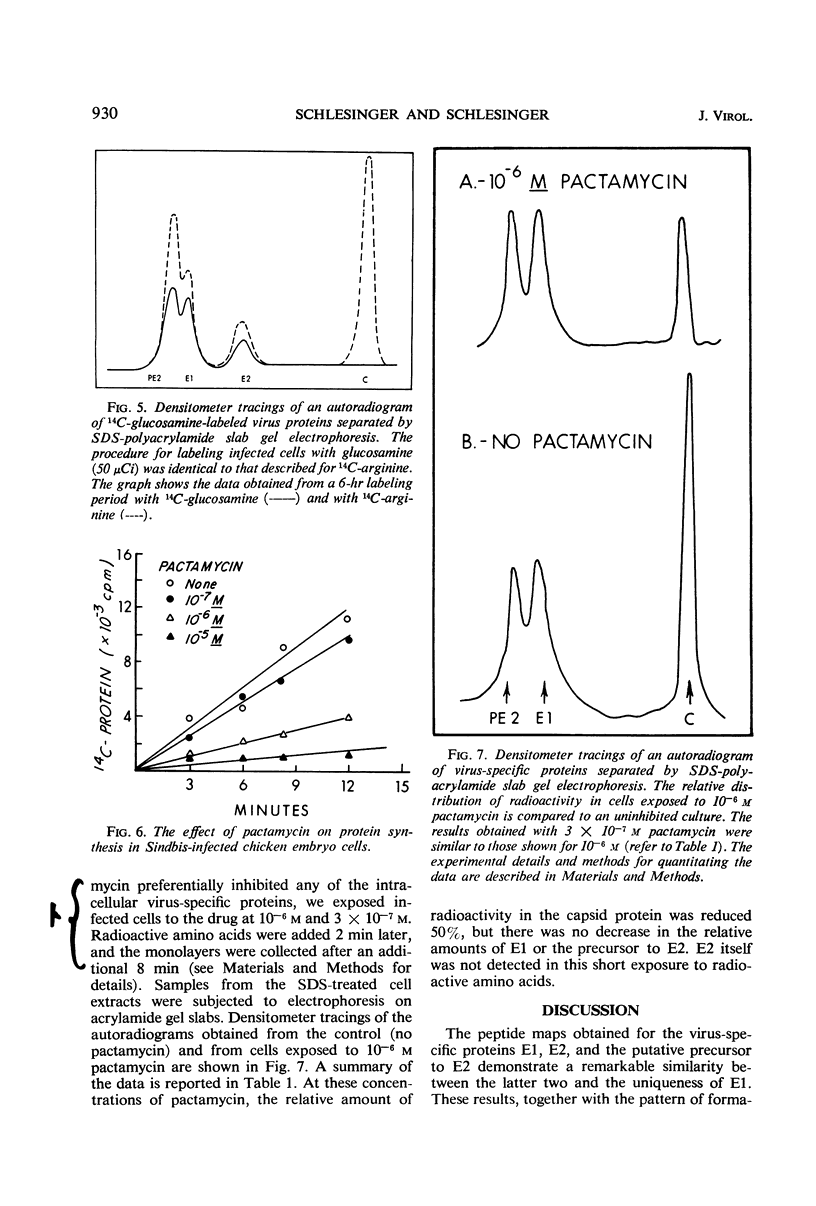
Exposure of Sindbis virus-infected chicken embryo cells to a short pulse of radioactive amino acids revealed the formation of primarily three proteins: the nucleocapsid (C) of the virus, one of the viral envelope proteins (E1), and a glycoprotein that did not appear in the virion. This third protein (PE2) has now been identified as a precursor of the other viral envelope protein (E2) on the basis of two observations: (i) the simultaneous disappearance of radioactive PE2 and appearance of labeled E2 in pulse-chase experiments, and (ii) the identity of 14C-arginine tryptic peptides in fingerprints of the two proteins. The nucleocapsid was the most heavily labeled protein in the cell and appeared in the virus during the short pulse. The two 14C-labeled envelope proteins, although having different kinetics of labeling in the cell, appeared simultaneously in the virus only after the chase. Addition of pactamycin, a drug inhibiting initiation of protein synthesis, preferentially inhibited the formation of capsid protein Assuming that Sindbis virus proteins are formed initially as a single polypeptide, our studies locate the nucleocapsid at the amino-terminal end of the polypeptide chain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bose H. R., Brundige M. A. Selective association of Sindbis virion proteins with different membrane fractions of infected cells. J Virol. 1972 May;9(5):785–791. doi: 10.1128/jvi.9.5.785-791.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Herner A. E., Goldberg I. H. Inhibition by pactamycin of the initiation of protein synthesis. Binding of N-acetylphenylalanyl transfer ribonucleic acid and polyuridylic acid to ribosomes. Biochemistry. 1969 Apr;8(4):1312–1326. doi: 10.1021/bi00832a004. [DOI] [PubMed] [Google Scholar]
- Dobos P., Faulkner P. Molecular weight of Sindbis virus ribonucleic acid as measured by polyacrylamide gel electrophoresis. J Virol. 1970 Jul;6(1):145–147. doi: 10.1128/jvi.6.1.145-147.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macdonald J. S., Goldberg I. H. An effect of pactamycin on the initiation of protein synthesis in reticulocytes. Biochem Biophys Res Commun. 1970 Oct 9;41(1):1–8. doi: 10.1016/0006-291x(70)90460-2. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. PURIFICATION AND PARTIAL CHEMICAL ANALYSIS OF SINDBIS VIRUS. Virology. 1963 Jul;20:433–445. doi: 10.1016/0042-6822(63)90092-8. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Boyle M. K. Selective inhibition of the synthesis of Sindbis virion proteins by an inhibitor of chymotrypsin. J Virol. 1972 Jan;9(1):187–188. doi: 10.1128/jvi.9.1.187-188.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Kinetics of incorporation of structural proteins into Sindbis virions. J Virol. 1969 Apr;3(4):369–375. doi: 10.1128/jvi.3.4.369-375.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Virus-specific proteins synthesized in cells infected with RNA+ temperature-sensitive mutants of Sindbis virus. J Virol. 1970 Mar;5(3):329–337. doi: 10.1128/jvi.5.3.329-337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Determination of the gene sequence of poliovirus with pactamycin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2852–2856. doi: 10.1073/pnas.68.11.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber R., Rekosh D., Baltimore D. Effect of pactamycin on synthesis of poliovirus proteins: a method for genetic mapping. J Virol. 1971 Oct;8(4):395–401. doi: 10.1128/jvi.8.4.395-401.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]





