Abstract
Objectives
Currently, there are few options other than cystectomy for the management of BCG refractory non-muscle invasive bladder cancer. We report our experience with intravesical combination chemotherapy using gemcitabine and MMC in such patients.
Materials and methods
We identified all patients with non-muscle invasive bladder cancer who were BCG refractory or intolerant and had been treated with intravesical gemcitabine and MMC at our institution. Patients were treated with a combination of intravesical gemcitabine (1000 mg in 50 ml sterile water) followed sequentially by intravesical MMC (40 mg in 20 ml sterile water) every week for 6 weeks (induction). Induction therapy was followed by a maintenance regimen using the same dose of gemcitabine and MMC once a month for 12 months. Data regarding patient demographics and disease information such as previous intravesical therapy, previous cystoscopy, cytology results, time to recurrence, and side effect profile were collected.
Results
A total of 10 patients (6 male and 4 female) aged 48 to 85 years (median 67 years) underwent treatment with a median follow-up of 26.5 months (4–34 months). Six patients were recurrence free and have maintained their response at a median of 14 months (4–34 months). Four patients had biopsy proven recurrence. Median time to recurrence was 6 months (range 4–13 months). The therapy was well tolerated in all patients. There were no major complications. Two patients experienced irritative lower urinary tract symptoms, which did not require cessation of therapy and one experienced a maculopapillary rash that improved with benadryl.
Conclusions
In patients with recurrent BCG refractory bladder cancer, intravesical combination chemotherapy with gemcitabine and MMC appears to be well tolerated and yields a response in a good proportion number of patients.
Keywords: Bladder cancer, Intravesical chemotherapy, Mitomycin C, Gemcitabine, BCG-refractory
1. Introduction
After prostate cancer, bladder cancer is the most common urologic malignancy and fifth most common overall in the United States [1]. It has been estimated that in 2008 there will be 69,000 new cases of bladder cancer in the United States and 14,000 deaths [2]. Intravesical bacillus Calmette-Guerin (BCG) remains the most effective intravesical agent for non-muscle invasive bladder cancer and is generally used as first line therapy. Despite the overall effectiveness of BCG, 30% to 40% of those treated do not respond [3,4]. Of the patients who initially responded, 30% to 40% of those will recur before 5 years [3,4]. Two important meta-analyses by Huncharek and colleagues [5,6] found that a failure to control for previous chemotherapy regimens may have overestimated the effectiveness of BCG to prevent tumor recurrence and progression.
Typically, patients with non-muscle invasive bladder cancer refractory to BCG are offered cystectomy. If the patient is a poor surgical candidate or refuses cystectomy, a second line regimen is given, such as BCG with interferon, mitomycin C (MMC), or gemcitabine. Gemcitabine (2′,2′-difluoro-2′-deoxycytidine; Gemzar, Eli Lilly and Co., Indianapolis, IN) is a nucleotide analog with a broad spectrum of antitumor activity. Phase I and II trials of intravesical gemcitabine in BCG refractory bladder cancer patients yielded initially promising results; however, the response has not been durable [7,8]. Dalbagni and colleagues reported a 50% complete response rate in 30 patients; the 1 year recurrence-free survival was 10%. The therapy was well tolerated with minimal bladder irritation and myelosuppression [7].
MMC is produced from Streptomycete caespitosus. Upon entry into the cell, it cross-links DNA and inhibits DNA synthesis. MMC can be administered as a one time dose after transurethral resection of bladder tumor or instilled weekly for 6 to 8 weeks. A recent meta-analysis found a 38% reduction in tumor recurrence with intravesical MMC, less than that of BCG [5].
Our hypothesis is that gemcitabine and MMC sequential therapy can prevent recurrent disease in BCG refractory or intolerant patients for whom cystectomy was not an option due to medical reasons, or was offered but refused.
2. Materials and methods
The Internal Review Board-approved urologic oncology database was searched from October 2005 to January 2008. Patients with non-muscle invasive bladder cancer who were BCG refractory or intolerant and treated with gemcitabine and MMC were identified. Patients were considered BCG-refractory if they had a positive biopsy after 2 courses of induction BCG. Criteria for treatment included patients who were unfit for cystectomy or refused cystectomy. All but 1 patient had failed at least 1 course of intravesical BCG and 8/10 patients had failed at least one additional course of intravesical BCG + interferon α2b. One patient with highly recurrent Ta disease was not offered BCG, but failed MMC therapy. Patients who discontinued intravesical BCG due to toxicity were also offered sequential intravesical gemcitabine and MMC. Information regarding patient demographics, previous intravesical therapy regimen, previous cystoscopy and cytology results, time to recurrence, and side effect profile was obtained by chart review.
All patients underwent a 6-week induction regimen followed by a 12-month, once monthly maintenance regimen if they were deemed to have responded to the induction course. Induction consisted of intravesical gemcitabine followed sequentially by MMC every week for 6 weeks. Gemcitabine was given first because, when dissolved in water, it carries a pH of 2.4 and MMC degrades in an acidic environment. Gemcitabine is a non-vesicant while MMC is a vesicant. One gram of gemcitabine in 50 ml of sterile water was retained for 90 minutes and drained out. MMC 40 mg in 20 cc of sterile water was instilled and retained for 90 minutes and then drained [9]. This was followed by the maintenance regimen using the same dose of gemcitabine and MMC once a month for 12 months. Complete response was defined as normal cytology, cystoscopy and/or biopsy at 6 weeks after completion of a full course of induction therapy. Follow-up evaluations consisting of cytology and cystoscopy were performed at 3-month intervals after the first negative post-induction evaluation. Upper tract evaluation with CT urography was performed annually. All patients in the series had a complete transurethral resection (TUR) prior to initiation of therapy. Then, after gemcitabine/MMC, each patient had random bladder biopsies or if cystoscopically visible disease was present, a repeat TUR was performed prior to cystectomy. Patients with biopsy proven recurrent disease after induction course of gemcitabine/MMC therapy were advised to undergo cystectomy.
Statistical analyses were mainly descriptive (mean, median, and distributions). Log rank analysis was performed using the Prism 4.0 (GraphPad software, San Diego, CA) to generate a Kaplan Meier curve of recurrence free survival.
3. Results
A total of 10 patients (6 male and 4 female) aged 48 to 85 years (median 67 years) underwent instillation of intravesical gemcitabine and MMC. Details regarding patient characteristics are given in Table 1. All patients underwent random bladder biopsy after induction, except 1 who refused. This patient was BCG intolerant and initially failed single agent MMC before undergoing sequential gemcitabine and MMC. Currently, he has no evidence of disease based on cystoscopy, cytology, and FISH with a follow-up of 27 months. Of the 9 patients who underwent biopsy, 5 had a negative first post-induction evaluation and were deemed responders. Of those who responded to therapy, all remained free of recurrence at a median of 14 months (range 4 to 34 months) (Fig. 1). All 6 responders have had a recent negative follow-up cystoscopy and benign urinary cytology. Four patients had biopsy proven disease recurrence of whom 3 were detected at first post-induction biopsy whereas 1 patient had a negative post-induction biopsy and a positive biopsy 13 months after completion of induction. Median time to recurrence was 6 months (4 to 13 months). Only 1 of these patients who failed the gemcitabine/MMC therapy experienced stage progression to muscle invasive disease (cT2NOMx) but continues to refuse a cystectomy and has received chemotherapy and radiation. Table 2 lists the subsequent disease course after gemcitabine/MMC failure. The 2 patients who underwent cystectomy had pT0N0Mx disease on final pathology. Of note, 1 patient who underwent cystectomy had previously undergone a nephroureterectomy for upper tact TCC 5 years earlier. No patient has developed evidence of metastatic disease or has died. The median number of recurrences before initiation of therapy was 4 (2–12), patients who responded had a median of 4 recurrences and failures had a median of 5 recurrences. There were no major complications. Minor complications were observed in 2 patients, neither of whom required cessation of therapy. One of the patients experienced severe dysuria and another patient experienced severe bladder spasms and a maculopapular rash that resolved with oral benadryl.
Table 1.
Patient characteristics (n = 10)
| Characteristic | Number of patients |
|---|---|
| Age | 48–85 (median = 67) |
| Sex | |
| M | 6 |
| F | 4 |
| Highest stage | |
| Low grade Ta w/o CIS | 1 |
| Low grade Ta w/ CIS | 1 |
| High grade Ta w/ CIS | 4 |
| High grade T1 w/o CIS | 1 |
| High grade T1 w/ CIS | 3 |
| Prior therapy | |
| BCG ×2 | 1 |
| BCG × 1, BCG + interferon ×1 | 2 |
| BCG × 2, BCG + interferon ×1 | 4 |
| BCG × 2, BCG + interferon ×2 | 2 |
| Mitomycin C* | 1 |
| Response | 6 |
| Failure | 4 |
| Number (median) of recurrences prior to Gemcitabine/MMC |
5 (2–14) |
| Responders | 4 recurrences |
| Failures | 5 recurrences |
| Median time to recurrence (n = 4) | 6 months (4–13 months) |
| Follow-up median | 26.5 months (4–34 months) |
cis = carcinoma in situ; BCG = bacille Calmette-Guerin.
This patient had highly recurrent low-grade Ta disease. BCG therapy was not utilized because it is reserved for high grade or T1 disease.
Fig. 1.
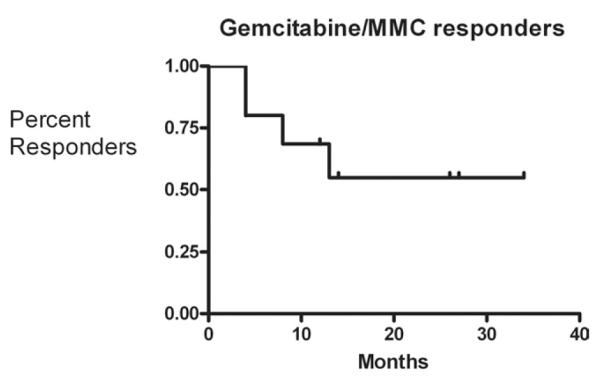
Kaplan-Meier curve of responders to gemcitabine/MMC therapy censured for follow-up time.
Table 2.
Histology of failures and subsequent therapy
| Histology of failures | Subsequent treatment | Cystectomy stage |
|---|---|---|
| High grade Ta w/ CIS |
Cystectomy w/ neobladder, now NED. Patient had history of upper tract TCC treated with nephroureterectomy 5 years prior |
T0N0MX |
| High grade Ta w/ CIS |
Phase 2 GM-CSF trial, now NED |
|
| High grade Ta w/ CIS |
Cystectomy w/ Indiana Pouch, now NED |
T0N0MX |
| High grade T1 w/ CIS |
Systemic chemotherapy/ radiation, recent TURBT yielded pT2a dz |
4. Discussion
Treatment options can be limited in patients with recurrent non-muscle invasive bladder cancer who refuse cystectomy, are unsuitable for surgery, or in those who fail or are intolerant of BCG intravesical therapy. In this case series, gemcitabine and MMC were administered intravesically and produced a complete and durable response in 60% of patients with a median overall follow-up of 14 months (4–34 months). This response rate was achieved in a group of patients at high risk for recurrence and progression. Most patients in our series had high-grade disease or carcinoma in situ (9/10) and a median of 5 recurrences (2–14) prior to gemcitabine/MMC therapy. Interestingly, the 2 patients who went on to have a cystectomy had no evidence of carcinoma in their bladder after resection.
Overall, the complications were minor and the incidence low (20%). This rate of minor complications was similar to single agent intravesical gemcitabine from phase I and II studies that were approximately 27% and 29%, respectively[7,8]. Similarly, in a recent study of intravesical epirubicin, the rate of minor chemical cystitis was 25% [10]. While local toxicity from MMC alone was 30% [11].
Gemcitabine has been administered in clinical trials as a single agent to treat non-muscle invasive bladder cancer. In 2002, Dalbagni and colleagues reported minimal toxicity in a phase I trial of gemcitabine in the treatment of BCG refractory TCC of the bladder [8]. Patients experienced some minor grade 2/3 dysuria and minimal systemic nausea and vomiting. A complete response was observed in 39% of patients (7/18). In 2006, the same group published phase II trial results [7]. Thirty patients with BCG refractory TCC who refused cystectomy were given gemcitabine twice weekly for 3 weeks. Surveillance was performed at 8 weeks and then every 3 months. While complete response was achieved in 50% on first surveillance, the 1-year recurrence-free survival was only 10% (95% CI, 0%–21%).
MMC therapy is often administered as a single post-operative dose after transurethral resection of bladder tumor or on a weekly basis for 6 to 8 weeks. Malmstrom and colleagues found only 4 of 21 BCG-refractory patients treated with MMC were disease-free at 3 years [12]. Combining MMC with BCG does not appear to increase the regimen’s effectiveness [13,14].
Another intravesical agent that has been employed to treat BCG refractory bladder cancer is valrubicin (N-trifluoroacetyladriamycin-14-valerate, AD 32), an analogue of doxorubicin [15]. Phase I trials demonstrated the agent has minimal toxicity [15]. Steinberg and colleagues reported a multi-institutional open label noncomparative trial on the use of intravesical valrubicin in patients with BCG refractory non-muscle invasive bladder cancer [16]. Patients received 6 weekly instillations of 800 mg of valrubicin. Of 90 patients, 19 (21 %) had a complete response at 6 months and 7 (8%) at 30 months.
Hyperthermia combined with MMC for treatment of non-muscle invasive bladder cancer has shown promise[17,18]. Hyperthermia produces increased cellular permeability, increased MMC cellular distribution and increased MMC reaction with DNA [19]. In one study, compared to BCG (64%) and passive MMC (58%), electromotive MMC (31%) had reduced recurrence rates at 6 months after therapy [20].
The main findings of this study are that a few select patients who have failed intravesical immunotherapy may be salvageable by sequential intravesical gemcitabine and MMC. This therapy may offer a potential alternative to cystectomy particularly for those patients with high risk non-muscle invasive disease who refuse or are unfit for cystectomy. The treatment appears to be well tolerated and the responses durable. More long-term follow-up is necessary before we can assess the effect of such treatment on local or systemic progression. These data do appear encouraging enough to perhaps warrant further investigation of this approach. Combination chemotherapy is used routinely for treatment of metastatic bladder cancer or in a neoadjuvant or adjuvant setting in those with muscle invasive disease. Single agent therapy has not been efficacious in these settings. Hence it is logical to assume that the same may be true in the case of intravesical chemotherapy for bladder cancer.
The data from our series corroborate that of an earlier report by Maymi and colleagues who demonstrated efficacy of sequential intravesical chemotherapy with gemcitabine and MMC used as third line therapy in patients with recurrent non-muscle invasive disease [21]. They compared patients with non-muscle invasive urothelial carcinoma who had failed multiple intravesical agents treated with gemcitabine (n = 12) versus gemcitabine and MCC (n = 27). Interestingly, the 12 patients treated with single agent gemcitabine failed at a median five months (range 2–27). While, 15/27 (55%) patients treated with a combination of gemcitabine and MMC remained disease-free with median follow up of seven months (range 2–24). Overall, the median disease-free survival was 6.5 months with gemcitabine alone vs. 20 months with combination therapy. While the median follow-up in the study is short and no biopsy or cystectomy data are presented, their response rates are similar to the present series and underline the potential benefit of sequential therapy.
Recent data has demonstrated the ability of maintenance intravesical therapy to increase recurrence-free rates compared with short-term administration [22]. Friedrich and colleagues randomized 495 patients with intermediate to high risk non-muscle invasive disease to either short-term BCG, short-term MMC, or MMC long-term administered monthly for 3 years [22]. The long-term regimen had significantly lower 3-year recurrence-free rates (86.1%) compared with short-term BCG (65.5%) and short-term MMC (68.6%). The 12-month maintenance regimen the patients in the present series underwent likely has contributed to their disease-free rates.
The present study is limited by the small number of patients and the short duration of follow-up. Longer follow-up is needed in the responders to determine if the treatment effect is durable. A prospective, multi-center Phase II trial is needed to fully evaluate the validity of this approach in patients with BCG refractory bladder cancer as well as to determine its ability to limit progression and enhance disease specific survival.
5. Conclusion
In patients with recurrent bladder cancer refractory to conventional intravesical therapy who refuse cystectomy or are unfit for surgery, sequential intravesical chemotherapy with gemcitabine and MMC appears to be well tolerated and yielded a complete response in a good proportion of patients. The present results serve as impetus for larger studies with longer follow-up to determine the overall efficacy of the regiment in preventing recurrence and progression.
Acknowledgments
The authors thank Dr. Michael O’Donnell for providing them with his protocol for intravesical gemcitabine+mitomycin instillation.
References
- [1].Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- [3].Lamm DL, Blumenstein BA, Crawford ED, et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guerin for transitional-cell carcinoma of the bladder. N Engl J Med. 1991;325:1205–9. doi: 10.1056/NEJM199110243251703. [DOI] [PubMed] [Google Scholar]
- [4].Joudi FN, O’Donnell MA. Second-line intravesical therapy vs. cystectomy for bacille Calmette-Guerin (BCG) failures. Curr Opin Urol. 2004;14:271–5. doi: 10.1097/00042307-200409000-00005. [DOI] [PubMed] [Google Scholar]
- [5].Huncharek M, Kupelnick B. Impact of intravesical chemotherapy vs. BCG immunotherapy on recurrence of superficial transitional cell carcinoma of the bladder: Meta-analytic re-evaluation. Am J Clin Oncol. 2003;26:402–7. doi: 10.1097/01.COC.0000026911.98171.C6. [DOI] [PubMed] [Google Scholar]
- [6].Huncharek M, Kupelnick B. The influence of intravesical therapy on progression of superficial transitional cell carcinoma of the bladder: A meta-analytic comparison of chemotherapy vs. bacillus Calmette-Guerin immunotherapy. Am J Clin Oncol. 2004;27:522–8. doi: 10.1097/01.coc.0000135570.37287.7f. [DOI] [PubMed] [Google Scholar]
- [7].Dalbagni G, Russo P, Bochner B, et al. Phase II trial of intravesical gemcitabine in bacille Calmette-Guerin-refractory transitional cell carcinoma of the bladder. J Clin Oncol. 2006;24:2729–34. doi: 10.1200/JCO.2005.05.2720. [DOI] [PubMed] [Google Scholar]
- [8].Dalbagni G, Russo P, Sheinfeld J, et al. Phase I trial of intravesical gemcitabine in bacillus Calmette-Guerin-refractory transitional-cell carcinoma of the bladder. J Clin Oncol. 2002;20:3193–8. doi: 10.1200/JCO.2002.02.066. [DOI] [PubMed] [Google Scholar]
- [9].Au JL, Badalament RA, Wientjes MG, et al. Methods to improve efficacy of intravesical mitomycin C: Results of a randomized phase III trial. J Natl Cancer Inst. 2001;93:597–604. doi: 10.1093/jnci/93.8.597. [DOI] [PubMed] [Google Scholar]
- [10].Hendricksen K, Witjes WP, Idema JG, et al. Comparison of three schedules of intravesical epirubicin in patients with non-muscle invasive bladder cancer. Eur Urol. 2007 doi: 10.1016/j.eururo.2007.12.033. [DOI] [PubMed] [Google Scholar]
- [11].Shelley MD, Wilt TJ, Court J, et al. Intravesical bacillus Calmette-Guerin is superior to mitomycin C in reducing tumor recurrence in high-risk superficial bladder cancer: A meta-analysis of randomized trials. BJU Int. 2004;93:485–90. doi: 10.1111/j.1464-410x.2003.04655.x. [DOI] [PubMed] [Google Scholar]
- [12].Malmstrom PU, Wijkstrom H, Lundholm C, et al. Five-year follow-up of a randomized prospective study comparing mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinoma. Swedish-Norwegian Bladder Cancer Study Group. J Urol. 1999;161:1124–7. [PubMed] [Google Scholar]
- [13].Rintala E, Jauhiainen K, Kaasinen E, et al. Alternating mitomycin C and bacillus Calmette-Guerin instillation prophylaxis for recurrent papillary (stages Ta to T1) superficial bladder cancer. Finn Bladder Group. J Urol. 1996;156:56–9. Discussion 59–60. [PubMed] [Google Scholar]
- [14].Witjes JA, Caris CT, Mungan NA, et al. Results of a randomized phase III trial of sequential intravesical therapy with mitomycin C and bacillus Calmette-Guerin vs. mitomycin C alone in patients with superficial bladder cancer. J Urol. 1998;160:1668–71. Discussion 71–2. [PubMed] [Google Scholar]
- [15].Greenberg RE, Bahnson RR, Wood D, et al. Initial report on intravesical administration of N-trifluoroacetyladriamycin-14-valerate (AD 32) to patients with refractory superficial transitional cell carcinoma of the urinary bladder. Urology. 1997;49:471–5. doi: 10.1016/s0090-4295(96)00621-8. [DOI] [PubMed] [Google Scholar]
- [16].Steinberg G, Bahnson R, Brosman S, et al. Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol. 2000;163:761–7. [PubMed] [Google Scholar]
- [17].Moskovitz B, Meyer G, Kravtzov A, et al. Thermo-chemotherapy for intermediate or high-risk recurrent superficial bladder cancer patients. Ann Oncol. 2005;16:585–9. doi: 10.1093/annonc/mdi124. [DOI] [PubMed] [Google Scholar]
- [18].Paroni R, Salonia A, Lev A, et al. Effect of local hyperthermia of the bladder on mitomycin C pharmacokinetics during intravesical chemotherapy for the treatment of superficial transitional cell carcinoma. Br J Clin Pharmacol. 2001;52:273–8. doi: 10.1046/j.0306-5251.2001.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Herman TS, Teicher BA, Jochelson M, et al. Rationale for use of local hyperthermia with radiation therapy and selected anticancer drugs in locally advanced human malignancies. Int J Hyperthermia. 1988;4:143–58. doi: 10.3109/02656738809029305. [DOI] [PubMed] [Google Scholar]
- [20].Di Stasi SM, Giannantoni A, Stephen RL, et al. Intravesical electromotive mitomycin C vs. passive transport mitomycin C for high risk superficial bladder cancer: A prospective randomized study. J Urol. 2003;170:777–82. doi: 10.1097/01.ju.0000080568.91703.18. [DOI] [PubMed] [Google Scholar]
- [21].Maymi JLSN, O’Donnell MA. Intravesical sequential gemcitabinemitomycin chemotherapy as salvage treatment for patients with refractory superficial bladder cancer; Proceedings of the American Urologic Association National Meeting; Atlanta (GA). May, 2006. [Abstract 840] [Google Scholar]
- [22].Friedrich MG, Pichlmeier U, Schwaibold H, et al. Long-term intravesical adjuvant chemotherapy further reduces recurrence rate compared with short-term intravesical chemotherapy and short-term therapy with bacillus Calmette-Guerin (BCG) in patients with non-muscle invasive bladder carcinoma. Eur Urol. 2007;52:1123–9. doi: 10.1016/j.eururo.2007.02.063. [DOI] [PubMed] [Google Scholar]


