Abstract
Rationale
Human genetics have implicated the 5- lipoxygenase (5-LO) enzyme in the pathogenesis of cardiovascular disease and an inhibitor of the 5-LO activating protein (FLAP) is in clinical development for asthma.
Objective
Here we determined whether FLAP deletion modifies the response to vascular injury.
Methods and Results
Vascular remodeling was characterized 4 weeks after femoral arterial injury in FLAP knockout (FLAP KO) mice and wild type (WT) controls. Both neointimal hyperplasia and the intima/media ratio of the injured artery were significantly reduced in the FLAP KOs while endothelial integrity was preserved. Lesional myeloid cells were depleted and vascular smooth muscle cell (VSMC) proliferation, as reflected by bromodeoxyuridine (BrdU) incorporation, was markedly attenuated by FLAP deletion. Inflammatory cytokine release from FLAP KO macrophages was depressed and their restricted ability to induce VSMC migration ex vivo was rescued with leukotriene B4 (LTB4). FLAP deletion restrained injury and attenuated upregulation of the extracellular matrix protein, tenascin C (TNC), which affords a scaffold for VSMC migration. Correspondingly, the phenotypic modulation of VSMC to a more synthetic phenotype, reflected by morphological change, loss of α-smooth muscle cell actin and upregulation of vascular cell adhesion molecule (VCAM) -1 was also suppressed in FLAP KO mice. Transplantation of FLAP replete myeloid cells rescued the proliferative response to vascular injury.
Conclusion
Expression of lesional FLAP in myeloid cells promotes LTB4 dependent VSMC phenotypic modulation, intimal migration and proliferation.
Keywords: Restenosis, vascular injury, leukotrienes, inflammation, angioplasty and stenting, smooth muscle cell, animal model of human disease remodeling
INTRODUCTION
Leukotrienes (LTs) are derived from arachidonic acid (AA) and are involved in a variety of inflammatory diseases, including asthma, arthritis and psoriasis. 5-lipoxygenase (5-LO) activating protein (FLAP) binds to 5-LO and facilitates its translocation to the nuclear membrane, where 5-LO binds to AA, triggering formation of LTA4 which is further metabolized to LTB4 and cysLTs (i.e. LTC4, LTD4, and LTE4) 1. Genetic disruption of FLAP suppresses LT synthesis 2.
Several key proteins within the LT cascade are expressed in human atherosclerotic plaques, including 5-LO, FLAP, two downstream enzymes (LTA4 hydrolase and LTC4 synthase) and four LT receptors, suggesting an active, pro-inflammatory circuit in the diseased vasculature 3–4. Preliminary evidence indicates that antagonism of the LTB4 receptor 1 (BLT1) or pharmacological inhibition of either FLAP or 5-LO restrains cholesterol absorption and augments to the hypolipidemic effects of a statin in mice and hamsters 5. LTB4 induces migration and proliferation of humancoronary artery vascular smooth muscle cells in vitro 6–7 and inhibition of LT biosynthesis decreases neutrophil deposition at sites of arterial injury in pigs 8. Polymorphismsin the FLAP gene cosegregate with risk of myocardialinfarction, stroke 9–11 and restenosis 12.
Endothelial cell disruption by vascular injury results in increased permeability and recruitment of inflammatory cells and platelets 13. This is accompanied by the modulation of vascular smooth muscle (VSMCs) to a more “synthetic” phenotype 14. VSMCs, which normally reside in the media, proliferate and migrate to the intima. They extend lamellipodia toward attractant mediators via actin polymerization, detach their trailing edges by degrading focal contacts, and generate force via myosin II to propel themselves forward 15–16. Inflammatory lipid mediators contribute to the initiation and progression of VSMC migration and proliferation 17.
Here, we show that FLAP expression is evident in lesional myeloid cells during the proliferative response to vascular injury. FLAP deletion attenuates the augmented expression of tenascin C (TNC) and vascular cell adhesion molecule (VCAM-1), impairing the migratory response of VSMCs by suppressing macrophage release of inflammatory cytokines, LTB4 and cysLTs. Reconstitution of knockout mice with FLAP replete myeloid cells rescues this phenotype, implicating directly myeloid cell FLAP in the vascular response to injury.
METHODS
Animals
All animal studies were performed according to protocols approved by the Institute for Animal Care and Use Committee at the University of Pennsylvania. FLAP knockout (FLAP KO) mice have been fully backcrossed onto C57BL-6J background and were a kind gift from Merck Research Laboratories.
Experimental model
Male FLAP KO and C57BL-6J wild type (WT) mice, aged 12 weeks, were subject to injury of the right femoral artery by passage of a 0.38mm diameter angioplasty guide wire, as previously described 1. Briefly, mice were anaesthetized with ketamine and acepromazine after which the right femoral artery was isolated through an incision in the right hind limb. Transluminal arterial injury was induced by insertion of a wire into the right femoral artery for 1 minute to induce endothelial damage. Sham surgeries were performed on the left femoral artery of the same mice. Mice were sacrificed 2 hours, 1, 2 or 4 weeks after the surgery for immunohistochemistry.
Bromodeoxyuridine (BrdU) (0.7mg/day for 28 days, Sigma, St. Louis, MO) was administrated to the mice via mini osmotic pumps (ALZET, Cupertino, CA) subcutaneously placed through a midback incision for each mouse.
Mice were sacrificed 4 weeks after the surgery for BrdU quantification, histology and morphometric analysis.
Histology and morphometric analysis
Femoral arteries were perfusion-fixed with 4% paraformaldehyde and processed for paraffin embedding. Serial clusters of 6-μm-thick sections were cut 80-μm apart from each other along the length of the femoral artery. One section from each cluster was stained with hemotoxylin-eosin (Sigma): sections with the most severe lesions from each femoral artery were selected for analysis using computerized morphometry (Image Pro Plus software, Media Cybernetics, Bethesda, MD). Measurements included luminal area, medial area and intimal area both at baseline and four weeks after wire injury. The intima to media (I/M) ratio was calculated as previously described 19. The percentage of stenosis was calculated as the ratio of the intimal area to the area inside the external elastic lamina.
Immunohistochemistry and BrdU quantification
Representative sections were immunohistochemically stained for FLAP (1:300, a kind gift from Jilly Evans), α-smooth muscle cell actin (α-SMC actin, 0.6 μg/ml, Abcam, Cambridge, MA), BrdU (5μg/ml, Hybridoma Bank, Iowa City, IA), TNC (10ug/ml, Chemicon International Inc., Temecula, CA), VCAM-1 (10μg/ml, Southern Biotech, Birmingham, AL), CD45 (2.5μg/ml, BD Pharmingen, San Jose, CA) and Von Willebrand factor (VWF, 0.5μg/ml, Sigma). All sections were deparaffinized and pretreated with specific antigen retrieval buffers. For FLAP immunostaining, sections were incubated with primary antibody and then incubated with a secondary antibody conjugated to horseradish peroxidase. FLAP positive cells were quantified using Image Pro Plus software. For immunoflourescence staining, Alex Fluor 488 was used as a secondary antibody for FLAP and Alexa Fluor 568 for CD45.
For TNC staining, sections were treated with Pronase (Boehringer Mannheim/Roche, Indianapolis, IN) for 10 minutes. For Brdu staining, sections were treated with 0.05% Trypsin (Sigma) in 0.1M Tris for 10 minutes. For VCAM-1 staining, antigen retrieval was accomplished by microwaving tissue for 15 minutes in unmasking solution (H-3300, Vecto r Laboratory Inc., Burlingame, CA). Endogenous peroxidase was then blocked with 3% hydrogen peroxide for 10 min. All sections were then incubated with primary antibody and species specific secondary antibody and developed with a Vectastain Elite ABC kit (Vector Laboratory Inc.). For VWF staining, sections were incubated with primary antibody overnight at 4 oC and then incubated with Alexa Fluor 568 secondary antibody for 1 hour at 25oC. Vector M.O.M. kit was used for α-SMC actin staining following manufacture instructions. α-SMC actin and VCAM-1 staining were quantified as the ratio of stain positive area to intimal area using Image Pro Plus software. VWF staining was quantified as ratio of positive length to lumen circumferences using Image Pro Plus software.
BrdU-positive cells and total cells in the intimal area were manually counted in a double blind manner and proliferation index was calculated as a percentage of the ratio between BrdU stained nuclei over the total number of nuclei in the intimal area.
Cell culture
Peritoneal macrophages were collected as previously described 20. Cells were treated with calcium ionophore, A23187 (Sigma) 10μM for 20mins and cell culture media were collected for cell migration studies and detection of 5-Hydroxyeicosatetraenoic acid (5-HETE), LTB4, LTC4, LTD4 and LTE4. Cell culture media were collected for measurements of cytokines from macrophages stimulated with lipopolysaccharide (LPS) (5μg/ml, Sigma) for 12 hours. Primary VSMCs from the aorta of WT or FLAP KO mice were cultured as previously described 21. Briefly, mouse aortas were isolated and placed under cell culture coverslips (Nalge Nunc International, Rochester, NY) on tissue culture plates in Dulbecco Eagle’s Medium/Ham aortas were isolated and placed under cell culture coverslips (Nalge Nunc International, Rochesterow for 7 days. Cells were then routinely passaged and used from passages two to four.
5-ethynyl-2′-deoxyuridine (EdU) cell proliferation assay
EdU incorporation assay using Click-It EdU Imaging Kits (Invitrogen, C10339) was performed to evaluate new DNA synthesis in WT and FLAP KO mVSMC. 2.5×103 cells in 12-well dish were seeded in DMEM/F12-10% FBS. After 24 hours, cells were incubated in DMEM/F12-1mg/ml BSA for 48 hours. Then, the cells were incubated in DMEM/F12-10% FBS with 10 μM solution of EdU for 24, 48 and 72 hours. At each time-point, the cells were formaldehyde-fixed and permeabilized with 0.5% Triton x-100 in PBS for 20 min.
Mass spectrometric analysis of 5-HETE and LTB4
Macrophage production of 5(S)-HETE and LTB4 was measured by mass spectrometry (MS). Briefly, 1 ml of cell culture media was spiked with stable isotope labeled internal standards: d4-LTB4 and d8-5(S)-HETE (Cayman Chemical). Samples were acidified with formic acid, extracted with ethyl acetate, dried and stored in 80μl acetonitrile until analysis, when 120μl water was added. A TSQ Quantum Ultra mass spectrometer interfaced with a heated electrospray probe and an Accela UHPLC solvent delivery system (Thermo Scientific) was used with Hypersil Gold 200 × 2.1 mm with 1.9μ particle size columns (Thermo Scientific). The mobile phase was generated from (A) water and (B) acetonitrile:methanol (95:5), each containing 0.005% acetic acid and adjusted to pH 5.7 with ammonium hydroxide. The gradient consisted of a 2 min isocratic segment at 40% B followed by a linear increase to 50% B at 3 min, then a linear increase to 70% B at 20 min. After each sample, the column was washed for two minutes with 100% B and equilibrated at 40% B for 10 min. The flow rate was 350μl/min. Transitions monitored were m/z 319 → 301 and m/z 327 → 309, collision energy 12 v, for 5(S)-HETE and d8-5-HETE, and m/z 335 → 195 and 339 → 197, collision energy 16 v, for LTB4 and d4-LTB4. Retention times were approximately 7.7 min for LTB4 and 15.2 min for 5(S)-HETE. Quantitation was by peak area ratios and normalized by media protein concentration.
Boyden Chamber assay
Aliquots of 10,000 primary VSMCs were added to the top wells of Costar Transwell modified Boyden chambers (6.5-mm-diameter tissue culture-treated polycarbonate membranes containing 8-μm pores, Corning, NY) and grow confluent. Cells were primed with tumor necrosis factor- alpha (TNF-α) (20ng/mL, Sigma) for 24 hours in RPMI with 2% serum. Culture media from stimulated peritoneal macrophages were then added in the bottom wells. In other studies, medium containing exogenous LTB4 (100nM, Cayman Chemical, Ann Arbor, MI) and LTD4 (80nM, Cayman Chemical) were added into the bottom wells. Six replicates were used for each condition. After 12 hours, cells remained in the top wells were scraped off using cotton swabs from three replicates of each condition, leaving the other three untouched serving as cell loading controls. All remaining cells were stained with 0.1% crystal violet (Sigma) for 20 minutes. Stained cells in all six replicates were washed off by 1% deoxycholic acid (Sigma) solution and absorbance was measured at 595nm.
Enzyme-Linked ImmunoSorbent Assay (ELISA) for determination of cytokines
Supernatants collected from LPS (5μg/ml) stimulated macrophage cells were measured for cytokines: TNF-α, interleukin (IL)-6, IL-1 β and IL-10 by Mouse ELISA Kits (Thermo Scientific, Madison WI) following the manufacturer’s instructions.
Bone marrow transplantation
Bone marrow transplantation experiments were performed with methods similar to those previously described 22. Bone marrow cells were flushed out from the femurs and tibias of WT (CD45.1+) and FLAP KO (CD45.2+) mice with cell culture medium (RPMI 1640). Bone marrow cells were meshed and cleared of erythrocytes by treating with ACK buffer. Recipient mice, WT, and FLAP KO mice, were lethally irradiated with two 525-rad doses spaced three hours apart (totally 1050 rads). Bone marrow cells were injected into recipient mice through the tail vein (1 × 107 cells per mouse). Repopulation of the immune system was monitored by flow cytometric analysis of the blood cells using FITC anti-mouse 45.2 and PE mouse anti-mouse 45.1. In the chimeric mice, more than 95% of the myeloid cells were derived from donor bone marrow. Chimeric mice were subjected to femoral artery injury and intimal hyperplasia was quantified as described above.
Statistical analysis
Data were subject to ANOVA with post-hoc pairwise comparisons as appropriate. Nonparametric approaches were employed when the variable distributions departed from normality or if the sample sizes were low (<6 per group). One-sided or two sided tests were performed, as appropriate. Tukey’s multiple comparison corrections were used for post-hoc comparisons if the multiple-testing corrected ANOVA p-values were significant at the 0.05 level.
RESULTS
FLAP expression was markedly increased in response to vascular injury
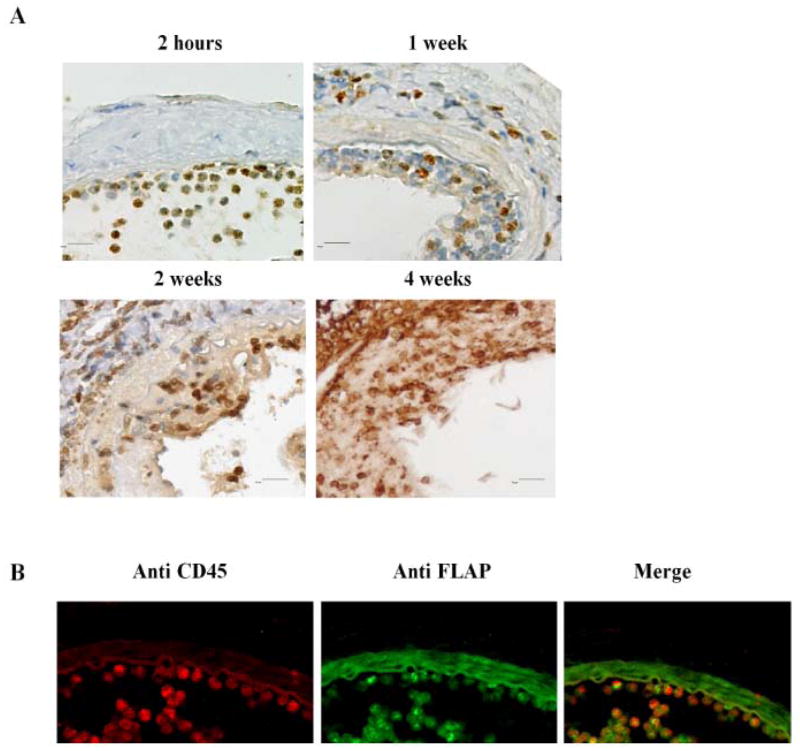
Lesional FLAP expression appeared increased at 2 hours, 1, 2 and 4 weeks after vascular injury (Figure 1A) and FLAP positive cells were co-localized with those staining for the leukocyte marker, CD45+ (Figure 1B). This increase in FLAP expression was most marked in the acute inflammatory phase 2 hours after injury and declined comparatively by 1 week as the lesions had become relatively depleted of inflammatory cells. During the early phase of the response (2 hours), the denuded luminal surface was covered with a monolayer of leukocytes positive for both CD45+ and FLAP (Figure 1B). Augmented FLAP expression was detectable in the neointimal lesions persisted at one, two and four weeks after vascular injury (Figure 1A). However, insufficient antibody was available to quantitate expression precisely 2 and 4 weeks after injury.
Figure 1. FLAP expression is upregulated in the neointima after vascular injury.
A: Representative staining of FLAP of mice femoral arteries 2 hours, 1 week, 2 weeks and 4 weeks after wire injury, from WT mice FLAP positive cells show brown staining. B: Co-staining of FLAP and CD45 positive cells of mice femoral arteries 2 hours after wire injury, from WT mice. CD45 positive cells show red staining; FLAP positive cells show bright green staining; the dull green staining in the vessel is nonspecific background staining.
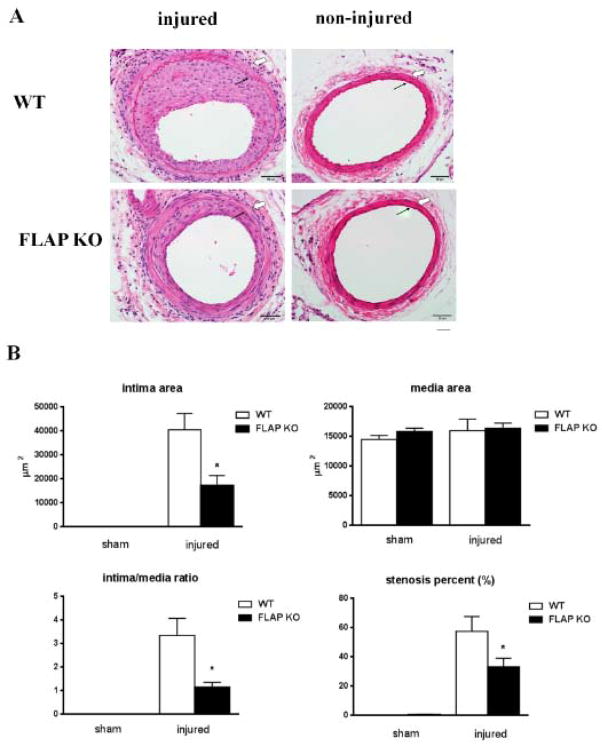
FLAP deletion protects against the intimal hyperplastic response to injury
FLAP KO mice and controls were subjected to femoral artery wire injury. Lesion formation in response to injury four weeks later was markedly reduced in FLAP KO mice (Figure 2A). The mean intimal and medial area, intima/media ratio and the percentage vascular stenosis did not differ between WT and FLAP KO at baseline (Figure 2B). The mean intimal area, intima/media ratio and percentage vascular stenosis of FLAP KO mice were reduced on average by 57% (40436 ± 6842 versus 17244 ± 4066 μm2, P< 0.05), 66% (3.34 ± 0.73 versus 1.15± 0.2, P<0.05) and 42% (57.3 ± 10.1 versus 33.1 ± 5.9 percent, P<0.05) respectively, compared with WT mice at four weeks after wire injury (Figure 2B). The medial area, by contrast, did not differ between genotypes (15937 ± 1953 versus 16334 ± 873 m 2, P>0.05) (Figure 2B).
Figure 2. FLAP deficiency is associated with a decreased intimal hyperplastic response to injury.
A, Hematoxylin eosin staining of representative sections of mice femoral arteries 28 days after wire injury, from WT(n=6) or FLAP KO mice (n=8). Solid arrowheads: internal elastic lamina; Open arrowheads, external elastic lamina, defining the borders of intima and the media. B, Quantification of intimal and medial areas. The ratio of intima to media is shown. Measurements were taken at baseline and four weeks after wire injury in both WT and FLAP KO mice. *P<0.05 *P<0.01, WT vs. FLAP KO. Scale bar 50μm.
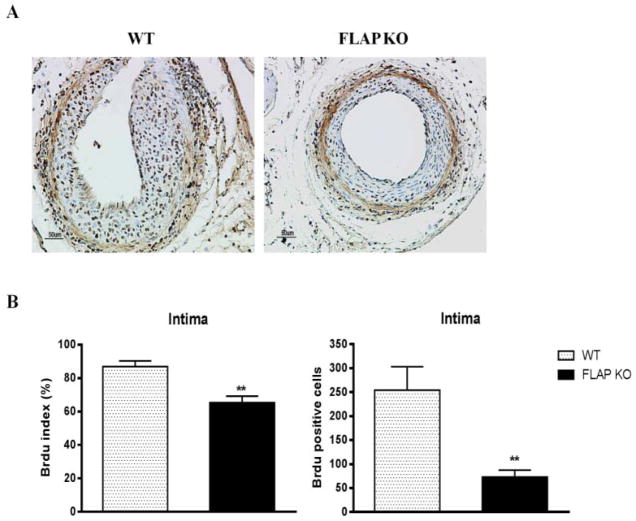
FLAP deficiency results in decreased VSMC proliferation
The response to vascular injury is believed to involve proliferation of VSMCs and their migration into the neointima. FLAP KO mice displayed a significant decrease VSMC proliferation as reflected by BrdU staining (Figure 3A). The BrdU index, calculated as a percentage of the ratio of BrdU positive nuclei over total number of cells in the neoinitma, was significantly depressed in FLAP KO mice compared to WTmice (87 ±3.3 versus 65.3 ± 3.8 percent, P<0.01) (Figure 3B). The absolute number of BrdU positive cells was also significantly reduced in the FLAP KOs (254 ± 48 versus 73 ± 14 percent, P<0.01) (Figure 3B).
Figure 3. FLAP deficiency results in decreased VSMC proliferation.
A, Representative staining of BrdU of mice femoral arteries 28 days after wire injury, from WT (n=6) or FLAP KO mice (n=8). (B) BrdU index was calculated as percentage of the ratio between BrdU-stained nuclei over the total number of cells in the intimal lesion. Absolute BrdU positive cells of each group were also compared. **P<0.01 Scale bar 50μm.
FLAP deficiency suppresses VSMC phenotype transition and attenuates TNC deposition while preserving endothelial integrity
While VSMCs continue to predominate in the intima, their loss of α-SMC actin after injury was attenuated in FLAP KO mice (Supplemental Figure IA). Moreover, the transformation of VSMC from elongated spindle-shaped cells, aligned perpendicular to the blood vessel lumen to a more disordered orientation and morphology was prominent in WT mice after injury, but was suppressed in FLAP KO mice. The injury induced upregulation of medial and neointimal VCAM-1 and TNC was also markedly attenuated in VSMCs from FLAP KO mice (Supplemental Figure IB and D). Despite its effects on VSMC proliferation, FLAP deficiency did not affect endothelial integrity, as reflected by staining with an antibody directed against VWF (Supplemental Figure IC), or endothelial function, as assessed by measurement of isometric tension in aorica rings (Supplemental Figure II A and B). Endothelium dependent relaxation in response to either acetylcholine or sodium nitroprusside was not different between WT and FLAP KO mice., Moreover, there was no significant difference in systolic and diastolic blood pressure between WT and FLAP KO mice (Supplemental Figure III A and B).
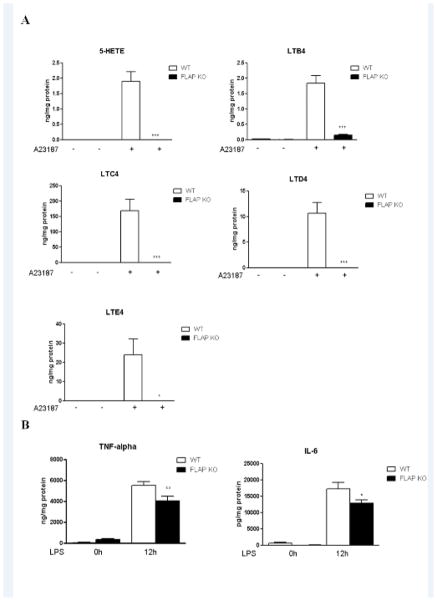
FLAP deficiency decreased macrophage leukotriene and pro-inflammatory cytokine production
FLAP deficiency disrupted LT synthesis as measured by 5-HETE, LTB4, LTC4, LTD4 and LTE4 production in peritoneal macrophages (Figure 4A). LPS induced macrophage generation of TNF-α and IL-6 was also attenuated by FLAP deletion (Figure 4B). IL-1β and IL-10 levels were not altered by FLAP deficiency (Supplemental Figure IV).
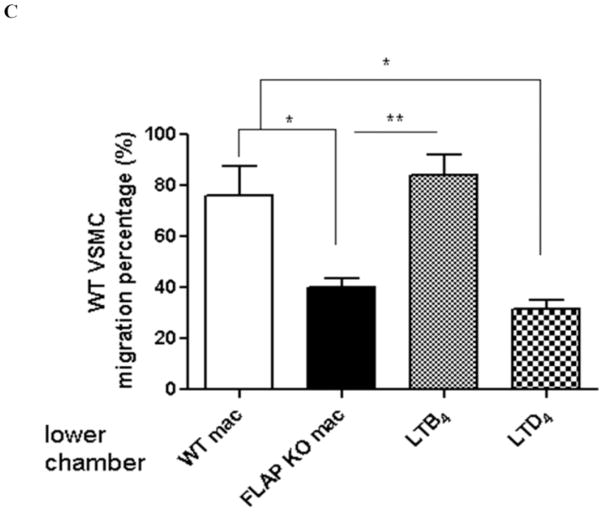
Figure 4. FLAP deficiency attenuates VSMC migration by disruption of LTB4 and proinflammatory cytokine synthesis.
A, Peritoneal macrophages from WT or FLAP KO mice were cultured and stimulated with A23187 (10μM) for 20 minutes or LPS (5μg/ml) overnight. 5HETE, LTB4, LTC4, LTD4 and LTE4were measured using cell culture supernatant. **P<0.01, n=5. B, Macrophage cell culture supernatants were collected at 0 hour and 12 hours after LPS stimulation. TNF-α and IL-6 levels were measured by ELISA. * p<0.05, n=5–10. C, Vascular SMCs isolated from the aortas of WT were placed in modified Boyden chambers and chemotaxis assessed to stimulated peritoneal macrophage medium harvested from either WT or FLAP KO mice as indicated. As noted, LTB4 or LTD4 are added into the lower wells. * p<0.05, **P<0.01, n=3.
Effect of FLAP deficiency on VSMC migration and proliferation
Culture medium derived from FLAP KO macrophages was considerably less effective than that from WT VSMCs in inducing VSMC migration in a Boyden Chamber assay (75.9% versus 40.1% respectively, p<0.05). Replacing the upper chamber with exogenous LTB4, but not LTD4, restores VSMC migration (Figure 4C).
Exogenous LTB4 and LTD4, but not LTC4 or LTE4, stimulate WT VSMC proliferation in a dose dependent manner (Supplemental Figure VA). We also observed a small decrease in the proliferative ability - as measured by EdU incorporation - of VSMCs from FLAP KOs compared to those from WT mice (49.7%versus 40.5% after 48 hours, (P<0.01) and 67.9% versus 45.7% at 72 hours (P<0.01) respectively) (Supplemental Figure VB). Both LTB4 (10nM) and LTD4 (10nM) fully or partially restored this proliferation defect in FLAP KO VSMCs (Supplemental Figure VA, P<0.001).
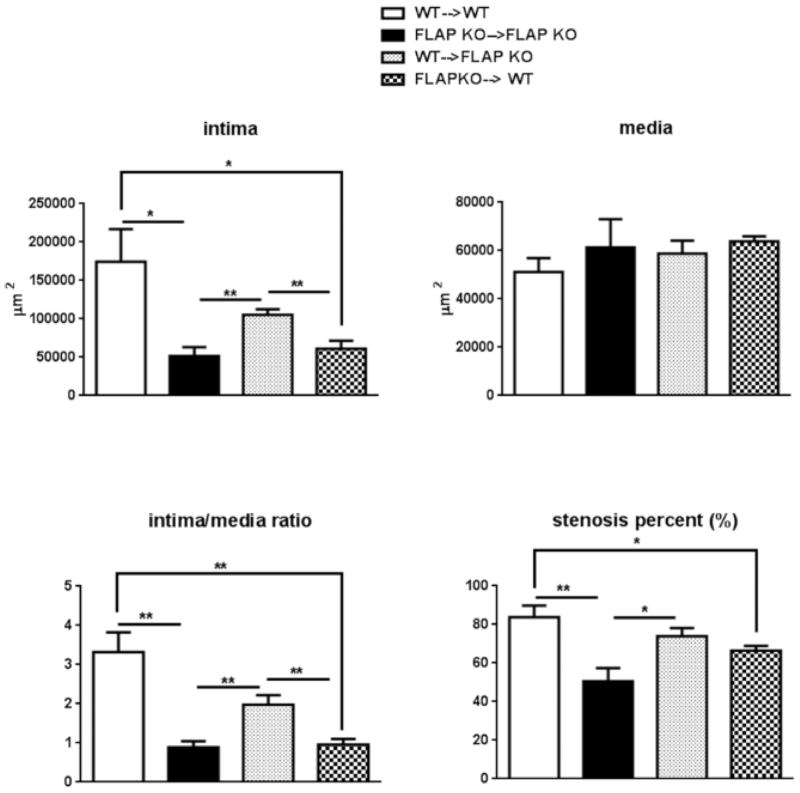
Bone marrow cell derived FLAP regulates neointima formation
Bone marrow was transplanted from WT or FLAP KO donors into WT or FLAP KO recipient mice. After repopulation, 95% of the bone marrow cells were shown to derive from donor mice (Supplemental Figure VI). The intimal hyperplastic response was significantly reduced in FLAP KO→FLAP KO (FLAP KO donor into FLAP KO recipient) mice comparing to that in WT→WT (WT donor into WT recipient) mice, consistent with what we observed previously, when comparing global FLAP KO mice with WT controls (Figure 5). This intimal proliferative phenotype was rescued by restoring FLAP in bone marrow cells as observed in WT→FLAP KO (WT donor into FLAP KO recipient) mice. The intimal hyperplastic response was again attenuated if WT bone marrow cells were replaced by FLAP deficient myeloid cells, as observed in FLAP KO→WT (FLAP KO donor into WT recipient) mice (Figure 5). No difference in intimal formation was detected between WT→WT and WT→FLAP KO as well as between FLAP KO→FLAP KO and FLAP KO→WT mice. FLAP KO→WT mice displayed a significantly lower vascular proliferative response compared to WT→FLAP KO mice (Figure 5).
Figure 5. Bone marrow derived FLAP regulate neointima formation.
Four groups of bone marrow transplanted mice were used in wire injury experiments: WT→WT (n=7), FLAP KO→ FLAP KO (n=5), WT→ FLAP KO (n=13) and FLAP KO→WT mice (n=9). Quantification of intima, media, intima/media ratio, stenosis percent were performed in arteries harvested 28 days after wire injury. *P<0.05 **P<0.01,***P<0.001. I/M ratio: intima/media ratio.
DISCUSSION
Here we report a striking impact of FLAP deletion on the proliferative response to vascular injury. Pharmacological inhibitors of either 5- LO or FLAP, both of which disrupt formation of LTB4, are being developed for use in asthma, a condition in which antagonists of receptors for the sulphidopeptideleukotrienes, LTC4, LTD4 and LTE4, already have established clinical utility 23–24.
The functional importance of LTs in cardiovascular disease is more controversial. Variations in genes relevant to LT biosynthesis, including FLAP, have cosegregated with cardiovascular events in humans 9–12 and expression of genes relevant to synthesis of LTB4 has been detected in human atherosclerotic plaques3.
Antagonism of BLT1 attenuates the intimal hyperplastic response to vascular injury in both rats and rabbits 7,25. However, trials of drugs that perturb the LT synthesis/response pathway have yet to be reported to influence cardiovascular outcomes in patients. Furthermore, attempts to study the impact of 5-LO deletion on atherogenesis in rodents have yielded conflicting results 26–28.
While FLAP inhibition doesn’t influence atherogenesis induced in hyperlipidemic mice with a high fat diet, it does have an impact when atherogenesis is accelerated in such models by additional manipulation like COX-2 deletion or T cell activation 7,29. Thus, although it may be unimportant as a phenotypic modulator when atherosclerosis is accelerated purely by dyslipidemia, additional proinflammatory manipulations appear to render mice susceptible to a contribution from FLAP dependent pathways. It is always difficult to extrapolate the relative importance of a particular pathway from experiments in mice to the human condition. The clear impact of FLAP disruption here does not preclude a similar phenotypic response to disruption of an apparently unconnected biological pathway. However, such data as ours do frame hypotheses which can then be addressed in clinical trials where the issue of potential functional redundancy will become apparent.
Following vascular injury, platelets and leukocytes are activated and adhere to the damaged vessel wall 30. Activated leukocytes release LTs, which may induce leukocyte chemotaxis, vasoconstriction and an increase in vascular permeability 1. Infiltrated leukocytes persist in the vessel wall long after experimental balloon induced vascular injury 31–32. Release of LTB4 by activated leukocytes would be presumed to promote further recruitment of inflammatory cells, production of pro-inflammatory cytokines and activation of VSMCs. Mature, fully differentiated “contractile” SMCs express four actin isoforms, the most abundant of which is α-SMA 33. VSMCs modulate their phenotypes in response to injury coincident with downregulation of contractile proteins, such as α-SMA, and an increase of VCAM-1expression 34–35. They gain the ability to proliferate and to upregulate extracellular matrix (ECM) proteins, including TNC, a large o1igomeric ECM glycoprotein, that plays an important role during the early cellular events of restenosis and provides a milieu that facilitates cell migration and proliferation of VSMC 36.
Here, we show that FLAP deletion disrupts several elements of this process, restraining the intimal proliferation consequent to vascular injury. Firstly, detection of FLAP is consistent with its involvement in the early margination of CD45+ leukocytes. Secondly, FLAP is fundamental to the capacity of lesional leukocytes to elaborate both LTB4 and the pro-inflammatory cytokines, TNF-α and IL-6. Deletion of FLAP impairs these properties and LTB4 rescues the phenotype. Thirdly, FLAP deletion restrains both the phenotypic transition of VSMCs from a contractile to a more synthetic phenotype and the upregulation of TN-C in response to injury. TN-C is upregulated during neointimal hyperplasia and is associated with the synthetic proliferative phenotype of VSMCs after vascular injury 37–38, in pulmonary hypertension39 and after vascular engraftment 40. It provides a scaffold along which proliferating VSMCs can migrate to form the neointima13. Thus, these effects are mechanistically congruent with the marked effect of FLAP deletion on both VSMC migration, evoked either by LPS stimulated macrophage culture media or by authentic LTB4 and on the intimal proliferative response to femoral vascular injury in vivo. Finally, we provide evidence from transplantation experiments that implicate myeloid cell FLAP as the primary influence on VSMC migration and proliferation. Transplantation of FLAP deficient myeloid cells recapitulates the impact of global FLAP deletion on the response to vascular injury while delivery of FLAP replete myeloid cells rescues the impact of global FLAP deletion in this model.
While myeloid FLAP predominates, these results do not exclude a minor contribution from FLAP deficiency in other cells to the phenotype observed in FLAP KO mice. For example, we noticed a small decrease in cycling of FLAP KO cells relative to WT (Supplemental Figure VB) which could be due to trace amounts of FLAP in VSMCs as detected by RT-PCR (Supplemental Figure VC), albeit that we failed to detect FLAP protein. Alternatively, it may be a compensatory effect. 5-LO has been detected in human pulmonary artery endothelial cells 41, human VSMCs 42, and cultured mouse VSMCs43. Although we find minimal expression of FLAP message in VSMCs and are unable to detect FLAP protein, deletion of FLAP reduces VSMC proliferation (Figure 3A and 3B). This could contribute to the reduction of the neointimal hyperplasia and the intima/media ratio observed in FLAP KOs after vascular injury.
In summary, we have shown that deletion of myeloid cell FLAP markedly attenuates the proliferative response to vascular injury, while preserving endothelial integrity. Disruption of the LT synthesis/response pathway consequent to FLAP deletion restrains several elements of the response to injury, including CD45 positive leukocyte accumulation, VSMC phenotype transition, TNC expression and macrophage polarization and induction of VSMC migration. FLAP deletion protects against the intimal hyperplastic response to injury predominantly by disrupting LT synthesis in myeloid cells and thereby depressing local inflammation and VSMC migration and proliferation.
Supplementary Material
Novelty and Significance.
What Is Known?
5-lipoxygenase activating protein (FLAP) is required for enzymatic metabolism of arachidonic acid to leukotriene (LT B4) by lipoxygenase.
LTB4 promotes cellular migration and proliferation in vitro, whereas downstream products, sulfidopeptide leukotrienes, modulate vascular tone.
Elements of the 5-lipoxygenase (5-LOX) pathway are expressed in human atherosclerotic plaque.
What New Information Does This Article Contribute?
Deletion of FLAP attenuates vascular smooth muscle cell phenotypic modulation, intimal migration and proliferation in response to vascular injury.
This phenotype reflects depletion of FLAP-dependent LTB4 formation by myeloid cells.
Local delivery of macrophage-targeted FLAP inhibitors may attenuate restenosis after angioplasty.
Although leukotrienes (LTs) have been clearly implicated in asthma, their importance as drug targets in cardiovascular disease is unclear. FLAP is a necessary partner in the activation of 5-LOX and a pharmaceutical FLAP inhibitor is under development for asthma. Here, deletion of FLAP suppressed LTB4 and pro-inflammatory cytokine formation by macrophages, which, in turn, promote vascular smooth muscle cell (VSMC) migration and proliferation in vitro. In vivo, global or myeloid-specific deletion of FLAP limits the proliferative response to vascular injury in mice, a phenotype rescued by FLAP-replete myeloid cells. Previously, pharmacological disruption of LT pathways has afforded conflicting evidence for the cardiovascular importance of this pathway in model systems, although variance in LT pathway genes has co-segregated with cardiovascular phenotypes in humans. Our studies suggest a dominant role for the macrophage 5-LOX pathway as a modulator of the VSMC response to vascular injury. Although it is difficult to extrapolate directly from such models to the human condition, our data help frame a hypothesis that local delivery of a FLAP inhibitor from a stent and/or directed inhibition of FLAP in macrophages might improve outcomes from procedures, such as percutaneous coronary interventions, by limiting restenosis.
Acknowledgments
We gratefully acknowledge the advice and technical support from Jennifer Bruce, Claire Catherine Morgan, John Lawson, Wenxuan Li, Yanming Xiong, Weili Yan, Jueli Zhen, Helen Zou and the statistical support provided from Gregory Grant.
SOURCES OF FUNDING
These studies were supported by AHA Pre-doctoral Fellowship (0815509D) and NIH grants (HL083799 and HL062250). Dr. FitzGerald is the McNeill Professor of Translational Medicine and Therapeutics.
Non-standard Abbreviations
- 5-HETE
5-Hydroxyeicosatetraenoic acid
- 5-LO
5-lipoxygenase
- AA
arachidonic acid
- BLT1
LTB4 receptor 1
- BrdU
bromodeoxyuridine
- ECM
extracellular matrix
- EdU
5-ethynyl-2′-deoxyuridine
- FLAP
5-LO activating protein
- FLAP KO
FLAP knockout
- I/M
intima to media
- LPS
lipopolysaccharide
- LT
leukotriene
- MS
mass spectrometry
- TNC
tenascin C
- VCAM
vascular cell adhesion molecule
- VSMC
vascular smooth muscle cells
- VWF
Von Willebrand factor
- WT
wild type
- α-SMC
α-smooth muscle cell actin
Footnotes
DISCLOSURES
None.
References
- 1.Funk CD. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 2.Byrum RS, Goulet JL, Griffiths RJ, Koller BH. Role of the 5-lipoxygenase-activating protein (flap) in murine acute inflammatory responses. J Exp Med. 1997;185:1065–1076. doi: 10.1084/jem.185.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spanbroek R, Grabner R, Lotzer K, Hildner M, Urbach A, Ruhling K, Moos MPW, Kaiser B, Cohnert TU, Wahlers T, Zieske A, Plenz G, Robenek H, Salbach P, Kuhn H, Radmark O, Samuelsson B, Habenicht AJR. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1238–1243. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotzer K, Funk CD, Habenicht AJR. The 5-lipoxygenase pathway in arterial wall biology and atherosclerosis. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids. 2005;1736:30–37. doi: 10.1016/j.bbalip.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Knoerzer DL, Rapp SR, Goetz BD, Hanselman J, Butteiger D, Zhang LL, Garner PA, Turk JR, Broschat KO. Inhibition of 5-lipoxygenase pathway targets alone and in combination with statin therapy results in significant cholesterol lowering in atherosclerotic animal models. Arteriosclerosis Thrombosis and Vascular Biology. 2009;29:E25–E25. [Google Scholar]
- 6.Schoenberger SP. Blt for speed. Nat Immunol. 2003;4:937–939. doi: 10.1038/ni1003-937. [DOI] [PubMed] [Google Scholar]
- 7.Bäck M, Bu D-x, Bränström R, Sheikine Y, Yan Z-Q, Hansson GK. Leukotriene b4 signaling through nf-κb-dependent blt1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17501–17506. doi: 10.1073/pnas.0505845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Provost P, Borgeat P, Merhi Y. Platelets, neutrophils, and vasoconstriction after arterial injury by angioplasty in pigs: Effects of mk-886, a leukotriene biosynthesis inhibitor. British Journal of Pharmacology. 1998;123:251–258. doi: 10.1038/sj.bjp.0701611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakonarson H, Thorvaldsson S, Helgadottir A, Gudbjartsson D, Zink F, Andresdottir M, Manolescu A, Arnar DO, Andersen K, Sigurdsson A, Thorgeirsson G, Jonsson A, Agnarsson U, Bjornsdottir H, Gottskalksson G, Einarsson A, Gudmundsdottir H, Adalsteinsdottir AE, Gudmundsson K, Kristjansson K, Hardarson T, Kristinsson A, Topol EJ, Gulcher J, Kong A, Stefansson K. Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction - a randomized trial. Jama-Journal of the American Medical Association. 2005;293:2245–2256. doi: 10.1001/jama.293.18.2245. [DOI] [PubMed] [Google Scholar]
- 10.Helgadottir A, Gretarsdottir S, Clair DS, Manolescu A, Cheung J, Thorleifsson G, Pasdar A, Grant SFA, Whalley LJ, Hakonarson H, Thorsteinsdottir U, Kong A, Gulcher J, Stefansson K, MacLeod MJ. Association between the gene encoding 5-lipoxygenase-activating protein and stroke replicated in a scottish population. American journal of human genetics. 2005;76:505–509. doi: 10.1086/428066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SFA, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 12.Shah SH, Hauser ER, Crosslin D, Wang L, Haynes C, Connelly J, Nelson S, Johnson J, Gadson S, Nelson CL, Seo D, Gregory S, Kraus WE, Granger CB, Goldschmidt-Clermont P, Newby LK. Alox5ap variants are associated with in-stent restenosis after percutaneous coronary intervention. Atherosclerosis. 2008;201:148–154. doi: 10.1016/j.atherosclerosis.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. Br J Surg. 1994;81:1254–1269. doi: 10.1002/bjs.1800810904. [DOI] [PubMed] [Google Scholar]
- 14.Willis AI, Pierre-Paul D, Sumpio BE, Gahtan V. Vascular smooth muscle cell migration: Current research and clinical implications. Vasc Endovascular Surg. 2004;38:11–23. doi: 10.1177/153857440403800102. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz SM, Campbell GR, Campbell JH. Replication of smooth muscle cells in vascular disease. Circ Res. 1986;58:427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- 16.Horwitz AR, Parsons JT. Cell biology:Cell migration--movin’ on. Science. 1999;286:1102–1103. doi: 10.1126/science.286.5442.1102. [DOI] [PubMed] [Google Scholar]
- 17.Jeremy JY, Jackson CL, Bryan AJ, Angelini GD. Eicosanoids, fatty acids and restenosis following coronary artery bypass graft surgery and balloon angioplasty. Prostaglandins Leukot Essent Fatty Acids. 1996;54:385–402. doi: 10.1016/s0952-3278(96)90022-8. [DOI] [PubMed] [Google Scholar]
- 18.Sata M, Maejima Y, Adachi F, Fukino K, Saiura A, Sugiura S, Aoyagi T, Imai Y, Kurihara H, Kimura K, Omata M, Makuuchi M, Hirata Y, Nagai R. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. Journal of Molecular and Cellular Cardiology. 2000;32:2097–2104. doi: 10.1006/jmcc.2000.1238. [DOI] [PubMed] [Google Scholar]
- 19.Gallo R, Padurean A, Toschi V, Bichler J, Fallon JT, Chesebro JH, Fuster V, Badimon JJ. Prolonged thrombin inhibition reduces restenosis after balloon angioplasty in porcine coronary arteries. Circulation. 1998;97:581–588. doi: 10.1161/01.cir.97.6.581. [DOI] [PubMed] [Google Scholar]
- 20.Hui Y, Ricciotti E, Crichton I, Yu Z, Wang D, Stubbe J, Wang M, Pure E, FitzGerald GA. Targeted deletions of cyclooxygenase-2 and atherogenesis in mice. Circulation. 2010;121(24):2654–60. doi: 10.1161/CIRCULATIONAHA.109.910687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuff CA, Kothapalli D, Azonobi I, Chun S, Zhang YM, Belkin R, Yeh C, Secreto A, Assoian RK, Rader DJ, Pure E. The adhesion receptor cd44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation. Journal of Clinical Investigation. 2001;108:1031–1040. doi: 10.1172/JCI12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miwa T, Maldonado MA, Zhou L, Yamada K, Gilkeson GS, Eisenberg RA, Song W-C. Decay-accelerating factor ameliorates systemic autoimmune disease in mrl/lpr mice via both complement-dependent and -independent mechanisms. Am J Pathol. 2007;170:1258–1266. doi: 10.2353/ajpath.2007.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funk CD. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nature Reviews Drug Discovery. 2005;4:664–672. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- 24.Hallstrand TS, Henderson WR. Leukotriene modifiers. Med Clin N Am. 2002;86:1009–1033. doi: 10.1016/s0025-7125(02)00091-3. [DOI] [PubMed] [Google Scholar]
- 25.Hlawaty H, Jacob M-P, Louedec L, Letourneur D, Brink C, Michel J-B, Feldman L, Back M. Leukotriene receptor antagonism and the prevention of extracellular matrix degradation during atherosclerosis and in-stent stenosis. Arterioscler Thromb Vasc Biol. 2009;29:518–524. doi: 10.1161/ATVBAHA.108.181750. [DOI] [PubMed] [Google Scholar]
- 26.Poeckel D, Berry KAZ, Murphy RC, Funk CD. Dual 12/15-and 5-lipoxygenase deficiency in macrophages alters arachidonic acid metabolism and attenuates peritonitis and atherosclerosis in apoe knock-out mice. J Biol Chem. 2009;284:21077–21089. doi: 10.1074/jbc.M109.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L, Moos MPW, Grabner R, Pedrono F, Fan J, Kaiser B, John N, Schmidt S, Spanbroek R, Lotzer K, Huang L, Cui J, Rader DJ, Evans JF, Habenicht AJR, Funk CD. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004;10:966–973. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]
- 28.Mehrabian M, Allayee H, Wong J, Shih W, Wang X-P, Shaposhnik Z, Funk CD, Lusis AJ. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res. 2002;91:120–12629. doi: 10.1161/01.res.0000028008.99774.7f. [DOI] [PubMed] [Google Scholar]
- 29.Yu Z, Crichton I, Tang SY, Hui Y, Ricciotti E, Levin MD, Lawson JA, Pure E, FitzGerald GA. Disruption of the 5-lipoxygenase pathway attenuates atherogenesis consequent to COX-2 deletion in mice. Proc Natl Acad Sci USA. 2012;109(17):6727–32. doi: 10.1073/pnas.1115313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis C, Fischer J, Ley K, Sarembock IJ. The role of inflammation in vascular injury and repair. Journal of Thrombosis and Haemostasis. 2003;1:1699–1709. doi: 10.1046/j.1538-7836.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- 31.Angelini GD, Bryan AJ, Williams HMJ, Morgan R, Newby AC. Distension promotes platelet and leukocyte adhesion and reduces short-term patency in pig arteriovenous bypass grafts. J Thorac Cardiovasc Surg. 1990;99:433–439. [PubMed] [Google Scholar]
- 32.Tanaka H, Sukhova G, Swanson S, Clinton S, Ganz P, Cybulsky M, Libby P. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation. 1993;88:1788–1803. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- 33.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 34.Rensen SSM, Doevendans P, van Eys G. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Netherlands Heart Journal. 2007;15:100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braun M, Pietsch P, Schror K, Baumann G, Felix SB. Cellular adhesion molecules on vascular smooth muscle cells. Cardiovascular Research. 1999;41:395–401. doi: 10.1016/s0008-6363(98)00302-2. [DOI] [PubMed] [Google Scholar]
- 36.Batchelor WB, Robinson R, Strauss BH. The extracellular matrix in balloon arterial injury: A novel target for restenosis prevention. Progress in Cardiovascular Diseases. 1998;41:35–49. doi: 10.1016/s0033-0620(98)80021-2. [DOI] [PubMed] [Google Scholar]
- 37.Hedin U, Holm J, Hansson GK. Induction of tenascin in rat arterial injury. Relationship to altered smooth muscle cell phenotype. American Journal of Pathology. 1991;139:649–656. [PMC free article] [PubMed] [Google Scholar]
- 38.Wang M, Ihida-Stansbury K, Kothapalli D, Tamby MC, Yu Z, Chen L, Grant G, Cheng Y, Lawson JA, Assoian RK, Jones PL, FitzGerald GA. Microsomal prostaglandin e2 synthase-1 modulates the response to vascular injury/clinical perspective. Circulation. 2011;123:631–639. doi: 10.1161/CIRCULATIONAHA.110.973685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones PL, Rabinovitch M. Tenascin-c is induced with progressive pulmonary vascular disease in rats and is functionally related to increased smooth muscle cell proliferation. Circulation Research. 1996;79:1131–1142. doi: 10.1161/01.res.79.6.1131. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto K, Onoda K, Sawada Y, Fujinaga K, Imanaka-Yoshida K, Shimpo H, Yoshida T, Yada I. Tenascin-c is an essential factor for neointimal hyperplasia after aortotomy in mice. Cardiovascular Research. 2005;65:737–742. doi: 10.1016/j.cardiores.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 41.Walker JL, Loscalzo J, Zhang YY. 5-lipoxygenase and human pulmonary artery endothelial cell proliferation. American Journal of Physiology-Heart and Circulatory Physiology. 2002;282:H585–H593. doi: 10.1152/ajpheart.00003.2001. [DOI] [PubMed] [Google Scholar]
- 42.Qiu H, Straat K, Rahbar A, Wan M, Soederberg-Naucler C, Haeggstroem JZ. Human cmv infection induces 5-lipoxygenase expression and leukotriene b-4 production in vascular smooth muscle cells. Journal of Experimental Medicine. 2008;205:19–24. doi: 10.1084/jem.20070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seo KW, Lee SJ, Kim CE, Yun MR, Park HM, Yun JW, Bae SS, Kim CD. Participation of 5-lipoxygenase-derived ltb4 in 4-hydroxynonenal-enhanced mmp-2 production in vascular smooth muscle cells. Atherosclerosis. 2010;208:56–61. doi: 10.1016/j.atherosclerosis.2009.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.