Abstract
Purpose
HIV/AIDS is a worldwide epidemic. Limited evidence suggests that men infected with HIV/AIDS are at increased risk for lower urinary tract symptoms. We determined whether HIV/AIDS status is an independent risk factor for self-reported bothersome lower urinary tract symptoms in a large contemporary cohort.
Materials and Methods
We performed a cross-sectional, Internet based survey of urinary quality of life outcomes in adult HIV infected and HIV uninfected men who have sex with men. The main outcome measure was International Prostate Symptom Score.
Results
Of respondents with complete data 1,507 were HIV uninfected (median age 42 years, mean 43) and 323 HIV infected (median age 45 years, mean 45.1). Of the HIV infected respondents 148 were nonAIDS defining HIV infected and 175 were AIDS defining HIV infected. After adjusting for age and other comorbid conditions, nonAIDS defining HIV infected and AIDS defining HIV infected status increased the odds of severe lower urinary tract symptoms by 2.07 (95% CI 1.04–3.79) and 2.49 (95% CI 1.43–4.33), respectively. HIV infected men had a worse total International Prostate Symptom Score for all domains including quality of life compared to HIV uninfected men. Within the population of men with HIV, those with AIDS had worse mean total International Prostate Symptom Score and all individual International Prostate Symptom Score components relative to nonAIDS defining HIV infected men.
Conclusions
HIV status is an independent risk factor for bothersome lower urinary tract symptoms. The odds of severe lower urinary tract symptoms are greater in HIV infected men with a history of AIDS.
Keywords: urinary tract, signs and symptoms, HIV
Human immunodeficiency virus and AIDS are major public health concerns worldwide. In the United States recent estimates indicate the prevalence of HIV infection is at more than 1.1 million cases with 55,400 new infections annually.1,2 It is predicted that the prevalence of HIV/AIDS will continue to increase as new cases continue to be diagnosed and patients with HIV live progressively longer.2
With the development of HAART, HIV infection has evolved from a terminal diagnosis to a chronic disease in patients with access to treatment.3 As HIV infected individuals live longer lives, age associated illnesses are becoming more common sources of morbidity.3 Indeed there is evidence that HIV infected individuals are at greater risk for nonAIDS related complications such as heart, renal and bone disease compared with age matched HIV uninfected subjects.4–6 These data indicate that age related conditions tend to appear earlier in people infected with HIV.
Urinary problems may pose a major detriment to quality of life and have been associated with significant patient morbidity.7 Urinary problems are common in aging men and are also likely to affect aging men with HIV/AIDS. However, it is unclear whether HIV/AIDS imposes an additional risk of urinary conditions beyond the normal risk associated with age. Evidence exists that HIV infection is associated with LUTS and abnormalities of bladder function as demonstrated by urodynamics.8,9 However, existing case series are hampered by small numbers of subjects, lack of an HIV uninfected control group and potential lack of applicability to patients in the modern era of HAART.
There is a clear need for data on the prevalence of bothersome urinary tract symptoms in HIV positive individuals in the modern era. In a contemporary cohort of MSM we determined whether HIV infected status is associated with greater odds of self-reported LUTS. We hypothesized that HIV infection is an independent risk factor for the presence and severity of LUTS.
METHODS
Study Design and Cohort Description
Institutional review board approval was obtained before initiating the study. We performed a cross-sectional, Internet based survey of urinary quality of life outcomes in MSM. The cohort was restricted to literate, Internet using MSM 30 years old or older. International sampling was achieved by distribution of a survey invitation to local, national and international Lesbian, Gay, Bisexual and Transgender community centers, organizations catering to MSM and advertisements on Facebook (www.facebook.com, Palo Alto, California) aimed at self-identified MSM. Potential subjects were given the option of clicking on a link to the survey which was posted on an Internet based survey site SurveyMonkey® (www.surveymonkey.com). Respondents were informed that they would be asked questions regarding their sexual and urinary wellness, and given the option to decline participation or stop the survey at any time. To maintain privacy no personally identifying information was collected. Responses were collected from January 19 to May 19, 2010.
Description of Variables
Outcome variables
The main outcome variable was the I-PSS, an internationally validated metric of bothersome LUTS.10,11 The I-PSS is graded on a scale of 0 to 35, and based on 7 questions pertaining to the urinary symptoms of frequency, urgency, nocturia, intermittency, weak stream, straining and incomplete emptying. Validated categorical severity scales exist which divide LUTS into none/mild (I-PSS 0 to 7), moderate (8 to 19) or severe (20 to 35). An additional eighth I-PSS question regarding quality of life is scored separately (0 to 5). Each component of the I-PSS was examined and compared by self-reported HIV/AIDS status. In addition, components of the I-PSS were grouped to summarize voiding symptoms (intermittency, weak stream, straining, incomplete emptying) and storage symptoms (frequency, urgency, nocturia).12,13 For multiple logistic regression I-PSS was made binary as (none/mild—0 to 7 vs moderate/severe—8 to 35) or (none/mild/moderate—0 to 19 vs severe—20 to 35). Respondents with missing data were excluded from all subsequent analyses.11
HIV specific questions
Respondents were asked if they were HIV infected (yes/no/uncertain), if they ever had a CD4 count less than 200 cells per μl (yes/no/uncertain) and if they ever had an AIDS defining illness (yes/no/uncertain).14 HIV infected individuals who responded yes to having had an AIDS defining illness or a CD4 count less than 200 cells per μl met AIDS defining criteria.14 Individuals who were uncertain regarding their HIV disease specific characteristics were not included in subsequent analyses.
Exposure variables
Respondents reported their age, geographic location, size of city and race/ethnicity (African-American, Asian-American, Caucasian, Latin American, Native American, other). Respondents were asked if they had ever been diagnosed or treated for the medical conditions diabetes (yes/no), coronary artery disease (yes/no), hyperlipidemia (yes/no), high blood pressure (yes/no) and depression (yes/no). We determined the history of previous UTI (yes/no), previous prostatitis (yes/no) and sexual transmitted infection status (chlamydia [yes/no], gonorrhea [yes/no]).
Statistical Analysis
Descriptive statistics
Descriptive statistics were used to characterize the study population. Mean I-PSS was compared among HIV uninfected, nonAIDS defining HIV infected and AIDS defining HIV infected groups using the Cuzick nonparametric test for trend across ordered groups.15 This test is essentially an extension of the Wilcoxon rank sum/Mann-Whitney test to the ordered category case. Groups were stratified by HIV/AIDS status. The distribution of none/mild, moderate and severe I-PSS scores were compared across HIV/AIDS status using a chi-square test of independence.
Statistical modeling
Odds ratios with 95% confidence intervals were reported to estimate the association between HIV/AIDS status and demographic factors with I-PSS as a binary generated threshold version of the continuous I-PSS. Multiple logistic regression models were fitted with a priori selected predictor variables based on variables known to influence the prevalence/severity of LUTS. These variables included age in 10-year increments, the presence of comorbidities (diabetes, coronary artery disease, hyperlipidemia, depression), sexually transmitted infection status (gonorrhea, chlamydia), previous UTI and history of prostatitis. Only variables associated with p ≤0.20 in the initial model were included in the final multiple logistic regression models. We performed a Hosmer-Lemeshow goodness-of-fit test and a le Cessie - van Houwelingen - Copas - Hosmer unweighted sum of squares test for global goodness of fit on the final models which did not violate goodness of fit. Statistical significance was set at p <0.05 and all tests were 2-sided. STATA® 11 was used for all analysis.
RESULTS
Demographics
The survey website was accessed by 2,783 men, of whom 2,368 (85.1%) completed the instrument. Men who reported an uncertain HIV serostatus (121) were excluded from study as were those younger than 30 years old (417), leaving 1,830 men for the analysis. Of this population 1,507 were HIV uninfected and 323 were HIV infected. Of those with HIV, nonAIDS defining and AIDS defining status were present in 148 and 175, respectively. Of the men who were certain of their HIV infection status all were certain of their CD4 count and AIDS defining illness history.
Selected demographic information is presented in table 1. The HIV infected cohort was older than the HIV uninfected cohort (45 vs 43 years old, respectively). The population was from 6 continents with most indicating they lived in North America, Europe or Australia. The majority of respondents were of Caucasian race. Respondents who were HIV infected were more likely to have coronary artery disease, hyperlipidemia, high blood pressure and depression than HIV uninfected men. HIV infected men were more likely to have a history of chlamydia, gonorrhea and UTI. The respondents defined their orientation as homosexual (93%), bisexual (5%), “queer” (2%), heterosexual (less than 0.1%) or asexual (less than 0.1%).
Table 1.
Demographic information
| No. HIV + (%) | No. HIV − (%) | |
|---|---|---|
| Geographic location: | ||
| Western US | 77 (23.8) | 265 (17.7) |
| Midwest US | 46 (14.2) | 223 (14.9) |
| Northeast US | 53 (16.3) | 262 (17.5) |
| Southern US | 59 (18.2) | 220 (14.7) |
| Southwest US | 20 (6.2) | 103 (6.8) |
| Northwest US | 17 (5.3) | 49 (3.3) |
| Canada | 13 (4) | 116 (7.8) |
| Europe | 22 (6.8) | 161 (10.8) |
| Australia | 13 (4) | 87 (5.8) |
| Other | 3 (1.2) | 11 (0.7) |
| City population: | ||
| Less than 100,000 | 82 (25.4) | 485 (32.4) |
| 100,000–1,000,000 | 136 (42.1) | 529 (35.3) |
| Greater than 1,000,000 | 105 (32.5) | 484 (32.3) |
| Race/ethnicity: | ||
| African-American | 11 (3.4) | 44 (2.9) |
| Asian-American | 7 (2.2) | 31 (2.1) |
| Caucasian | 270 (83.1) | 1,277 (84.9) |
| Latin American | 27 (8.3) | 85 (5.6) |
| Native American | 1 (0.3) | 24 (1.6) |
| Other* | 7 (2.7) | 44 (2.9) |
| Comorbid medical conditions: | ||
| Diabetes | 21 (6.5) | 130 (8.6) |
| Coronary artery disease | 28 (8.6) | 81 (5.4) |
| Hyperlipidemia | 92 (28.3) | 319 (21.2) |
| High blood pressure | 91 (28) | 409 (27.2) |
| Depression | 170 (52.3) | 589 (39.1) |
| Lifetime history of infectious conditions: | ||
| Chlamydia | 82 (25.2) | 170 (11.3) |
| Gonorrhea | 145 (44.6) | 253 (16.8) |
| Urinary tract infection | 104 (32.2) | 361 (24.1) |
| Prostatitis | 27 (8.4) | 133 (8.9) |
Categories may not add up because of missing values.
Other international (Asia, Africa, South America, Central America).
International Prostate Symptom Score
Overall HIV infected men had more severe LUTS (11.4% vs 4.2%) and moderate LUTS (33.2% vs 29.2%) compared to HIV uninfected men (fig. 1). HIV infected men had higher (ie worse) mean I-PSS for all domains including the quality of life question compared to HIV uninfected men (fig. 2). Within the population of men with HIV, those with AIDS had higher total I-PSS scores and individual domain scores on all I-PSS components relative to nonAIDS defining HIV infected men.
Figure 1.
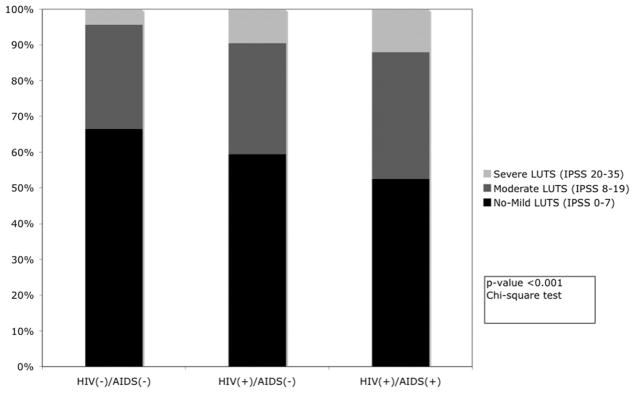
Distribution of categorized I-PSS by HIV/AIDS status
Figure 2.
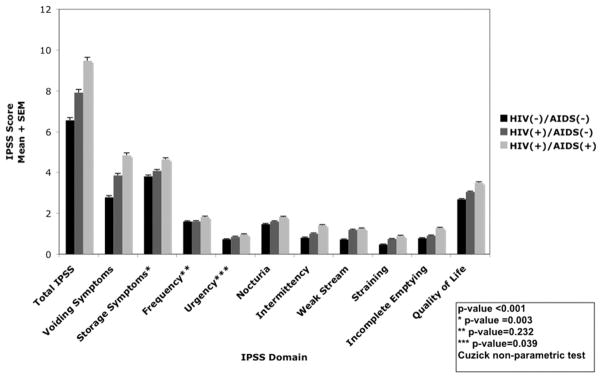
Mean I-PSS by HIV/AIDS status
Multiple Logistic Regression Models
The first multiple logistic regression model (in 1,813 cases) assessing the odds of reporting moderate/severe LUTS (I-PSS total greater than 8) relative to men with no or mild LUTS is presented in table 2. Men with HIV infection were more likely to report moderate/severe LUTS relative to HIV uninfected men in the unadjusted model (OR 1.6, 95% CI 1.26–2.05). HIV infected men without AIDS were less likely than HIV infected men with AIDS to experience moderate/severe LUTS (OR 1.35 95% CI 0.96–1.91 vs OR 1.79 95% CI 1.31–2.45, respectively). After adjusting for age, diabetes, hyperlipidemia, depression, gonorrhea, chlamydia, UTI and prostatitis history, men who were AIDS defining HIV infected were still more likely to report moderate/severe LUTS (OR 1.52, 95% CI 1.09–2.12). Interestingly nonAIDS defining HIV infected status was no longer a significant independent risk factor for moderate/severe LUTS (OR 1.07, 95% CI 0.0.74–1.5) but the estimate is likely indicative of a slightly increased risk. Increasing age, depression, diabetes, a history of gonorrhea, UTI or prostatitis were also significant risk factors for moderate to severe LUTS in the adjusted multiple logistic regression model.
Table 2.
Multiple logistic regression of risk factors for moderate or severe LUTS
| Odds Ratio | 95% CI | p Value | |
|---|---|---|---|
| Unadjusted: | |||
| HIV pos | 1.6 | 1.26–2.05 | <0.001 |
| Unadjusted: | |||
| HIV pos + no AIDS defining illness | 1.35 | 0.96–1.91 | 0.086 |
| HIV pos + AIDS defining illness | 1.79 | 1.31–2.45 | <0.001 |
| Adjusted* | |||
| HIV pos + no AIDS defining illness | 1.07 | 0.74–1.5 | 0.71 |
| HIV pos + AIDS defining illness | 1.52 | 1.09–2.12 | 0.015 |
| Age (10-yr interval) | 1.25 | 1.12–1.39 | <0.001 |
| Hyperlipidemia | 1.25 | 0.98–1.6 | 0.074 |
| Depression | 1.62 | 1.32–1.99 | <0.001 |
| Diabetes | 1.54 | 1.08–2.21 | 0.018 |
| Gonorrhea | 1.39 | 1.09–1.79 | 0.009 |
| Urinary tract infection | 1.29 | 1.02–1.62 | 0.034 |
| Prostatitis | 1.64 | 1.15–2.33 | 0.006 |
History of coronary artery disease and chlamydia did not achieve the p value threshold of 0.2 and were not included in the final adjusted model.
A second multiple logistic regression model (in 1,830 cases) assessing the odds of severe LUTS only (I-PSS total greater than 20) is presented in table 3. The odds of severe LUTS were much greater in the HIV infected population vs the HIV uninfected population (OR 2.94, 95% CI 1.92–4.5). After adjusting for age, diabetes, hyperlipidemia, depression, gonorrhea, chlamydia, UTI and prostatitis history, nonAIDS defining HIV infected and AIDS defining HIV infected status were still associated with increased odds of reporting severe LUTS by 2.07 (95% CI 1.04–3.79) and 2.49 (95% CI 1.43–4.33), respectively, compared to HIV uninfected men. Age, hyperlipidemia and depression were also statistically significant independent risk factors for severe LUTS. Interestingly having a history of an infectious urinary tract condition was not an independent risk factor for reporting severe LUTS.
Table 3.
Multiple logistic regression of risk factors for severe LUTS
| Odds Ratio | 95% CI | p Value | |
|---|---|---|---|
| Unadjusted: | |||
| HIV pos | 2.94 | 1.92–4.5 | <0.001 |
| Unadjusted: | |||
| HIV pos + no AIDS defining illness | 2.32 | 1.27–4.2 | 0.006 |
| HIV pos + AIDS defining illness | 3.03 | 1.8–5.08 | <0.001 |
| Adjusted* | |||
| HIV pos + no AIDS defining illness | 2.07 | 1.04–3.79 | 0.038 |
| HIV pos + AIDS defining illness | 2.49 | 1.43–4.33 | 0.001 |
| Age (10-yr interval) | 1.69 | 1.37–2.08 | <0.001 |
| Hyperlipidemia | 1.65 | 1.07–2.57 | 0.025 |
| Depression | 1.92 | 1.26–2.93 | 0.004 |
History of diabetes, coronary heart disease, gonorrhea, chlamydia, urinary tract infection and prostatitis did not achieve the p value threshold of 0.2 and were not included in the final adjusted model.
Summary
A cross-sectional, Internet based survey was delivered to a large international sampling of MSM. HIV positivity was an independent risk factor for severe LUTS after adjustment for age and comorbid health conditions. HIV disease severity was also related to LUTS. Men with a history of an AIDS defining illness or CD4 count less than 200 cells per μl had greater odds of moderate/severe and severe LUTS relative to nonAIDS defining HIV infected men.
DISCUSSION
To our knowledge this is the first large scale epidemiologic study to investigate the association between HIV and LUTS. We also provide insight into voiding function in MSM, a minority group that has been frequently ignored in urological research. Older case series have highlighted the effect of HIV/AIDS on voiding based on urodynamic findings.8,9 Kane et al studied 18 HIV infected patients who presented for urodynamic testing.9 Detrusor hyper-reflexia, detrusor-sphincter dyssynergia and detrusor areflexia were present in 5, 5 and 1 patient, respectively. Hermieu et al studied 39 HIV infected patients with voiding symptoms during a 3-year period and 87% had urodynamic abnormalities.8 A neurological cause was identified in 61.5% of these cases with the most common disorders arising from AIDS defining illnesses (ie cerebral toxoplasmosis and HIV encephalitis). While the urodynamic specifics of these cases is of interest for understanding the pathophysiology of LUTS in HIV infected patients, it is not clear from these studies that HIV in and of itself confers a greater risk of LUTS.
It has been demonstrated that HIV infection alone produces an increased risk of nonHIV related chronic medical conditions such as cardiovascular, liver, renal and bone disease.4–6 These conditions may be the result of a weakened immune system, chronic inflammation, toxicity of HAART and/or direct effects of HIV on cells.16 Interestingly cohort studies examining the appropriate time to initiate antiretroviral therapy have shown that renal, hepatic and cardiovascular function may be adversely affected by sustained HIV replication and/or immunodeficiency.3
Theoretical risk factors for LUTS in HIV infected persons include chronic urinary tract inflammation, toxicity of HAART, sequelae of opportunistic infections, and direct effects of the virus on the central and peripheral nervous systems.16,17 In our analysis UTI, prostatitis, and gonorrhea were independent risk factors for moderate (but not severe) LUTS. It is implied that infection is unlikely to be the sole underlying cause of bothersome urinary symptoms in HIV infected men. Therefore, it is likely that a direct toxic effect of the virus and/or HAART may be contributing to the burden of LUTS in HIV infected men.
Several important limitations warrant mention. The cross-sectional nature of the data set prohibits statements regarding causality. However, that does not decrease the usefulness of these results in drawing attention to HIV infected individuals as a population at increased risk for LUTS. The cohort is limited to self-identified MSM. Given that sexual practices may have influenced the self-report of LUTS in the study population, our findings may not necessarily be generalizable to HIV infected men who have sex exclusively with women. The use of a web based survey may have introduced selection bias. There could be undercoverage of people who do not read or use computers. In addition, the survey was available only in English. Nonresponse bias and volunteer bias may also diminish the generalizability of our results to the general HIV infected population. It is also important to note that we did find positive associations where we expected them (eg age and depression) in keeping with established risk factors of LUTS. Unfortunately we did not obtain information regarding the use of HAART and the role it may have in the development of LUTS.
Despite these limitations, the current report suggests an important area of directed clinical inquiry and further research for the HIV infected population. Providers should be aware that HIV infected men are at increased risk for reporting LUTS. These men should be screened accordingly and treated appropriately. Future studies should confirm these findings in population based samples and focus on the pathophysiology that underlies our observations so as to better tailor treatments. Future studies should also investigate the role of HAART in LUTS in HIV infected people.
CONCLUSIONS
After adjustment for age and other comorbidities, HIV infection is an independent risk factor for bothersome LUTS. The odds of reporting severe LUTS increase in men with a history of AIDS defining HIV infection.
Acknowledgments
Supported by a grant from the Sexual Medicine Society of North America and by NIH/NCRR UCSF-CTSI Grant UL1 RR024131. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the Sexual Medicine Society of North America.
John Kornak, Department of Epidemiology and Biostatistics, University of California, San Francisco provided statistical evaluation and support.
Abbreviations and Acronyms
- HAART
highly active antiretroviral therapy
- I-PSS
International Prostate Symptom Score
- LUTS
lower urinary tract symptoms
- MSM
men who have sex with men
- UTI
urinary tract infection
References
- 1.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall HI, Green TA, Wolitski RJ, et al. Estimated future HIV prevalence, incidence, and potential infections averted in the United States: a multiple scenario analysis. J Acquir Immune Defic Syndr. 2010;55:271. doi: 10.1097/QAI.0b013e3181e8f90c. [DOI] [PubMed] [Google Scholar]
- 3.Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376:49. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- 4.Grund B, Peng G, Gibert CL, et al. Continuous antiretroviral therapy decreases bone mineral density. Aids. 2009;23:1519. doi: 10.1097/QAD.0b013e32832c1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marin B, Thiebaut R, Bucher HC, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. Aids. 2009;23:1743. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferry T, Raffi F, Collin-Filleul F, et al. Uncontrolled viral replication as a risk factor for non-AIDS severe clinical events in HIV-infected patients on long-term antiretroviral therapy: APROCO/COPILOTE (ANRS CO8) cohort study. J Acquir Immune Defic Syndr. 2009;51:407. doi: 10.1097/QAI.0b013e3181acb65f. [DOI] [PubMed] [Google Scholar]
- 7.Roberts RO, Rhodes T, Panser LA, et al. Natural history of prostatism: worry and embarrassment from urinary symptoms and health care-seeking behavior. Urology. 1994;43:621. doi: 10.1016/0090-4295(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 8.Hermieu JF, Delmas V, Boccon-Gibod L. Micturition disturbances and human immunodeficiency virus infection. J Urol. 1996;156:157. [PubMed] [Google Scholar]
- 9.Kane CJ, Bolton DM, Connolly JA, et al. Voiding dysfunction in human immunodeficiency virus infections. J Urol. 1996;155:523. [PubMed] [Google Scholar]
- 10.Cockett AT, Aso Y, Denis L, et al. World Health Organization Consensus Committee recommendations concerning the diagnosis of BPH. Prog Urol. 1991;1:957. [PubMed] [Google Scholar]
- 11.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 12.Welch G, Kawachi I, Barry MJ, et al. Distinction between symptoms of voiding and filling in benign prostatic hyperplasia: findings from the Health Professionals Follow-up Study. Urology. 1998;51:422. doi: 10.1016/s0090-4295(97)00626-2. [DOI] [PubMed] [Google Scholar]
- 13.Barry MJ, Williford WO, Fowler FJ, Jr, et al. Filling and voiding symptoms in the American Urological Association symptom index: the value of their distinction in a Veterans Affairs randomized trial of medical therapy in men with a clinical diagnosis of benign prostatic hyperplasia. J Urol. 2000;164:1559. [PubMed] [Google Scholar]
- 14.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1. [PubMed] [Google Scholar]
- 15.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 16.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Pinho AM, Lopes GS, Ramos-Filho CF, et al. Urinary tract infection in men with AIDS. Genitourin Med. 1994;70:30. doi: 10.1136/sti.70.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]


