Abstract
Background
The American Cancer Society (ACS), the Centers for Disease Control and Prevention (CDC), the National Cancer Institute (NCI), and the North American Association of Central Cancer Registries (NAACCR) collaborate annually to provide updates on cancer incidence and death rates and trends in these outcomes for the United States. This year’s report includes incidence trends for human papillomavirus (HPV)–associated cancers and HPV vaccination (recommended for adolescents aged 11–12 years).
Methods
Data on cancer incidence were obtained from the CDC, NCI, and NAACCR, and data on mortality were obtained from the CDC. Long- (1975/1992–2009) and short-term (2000–2009) trends in age-standardized incidence and death rates for all cancers combined and for the leading cancers among men and among women were examined by joinpoint analysis. Prevalence of HPV vaccination coverage during 2008 and 2010 and of Papanicolaou (Pap) testing during 2010 were obtained from national surveys.
Results
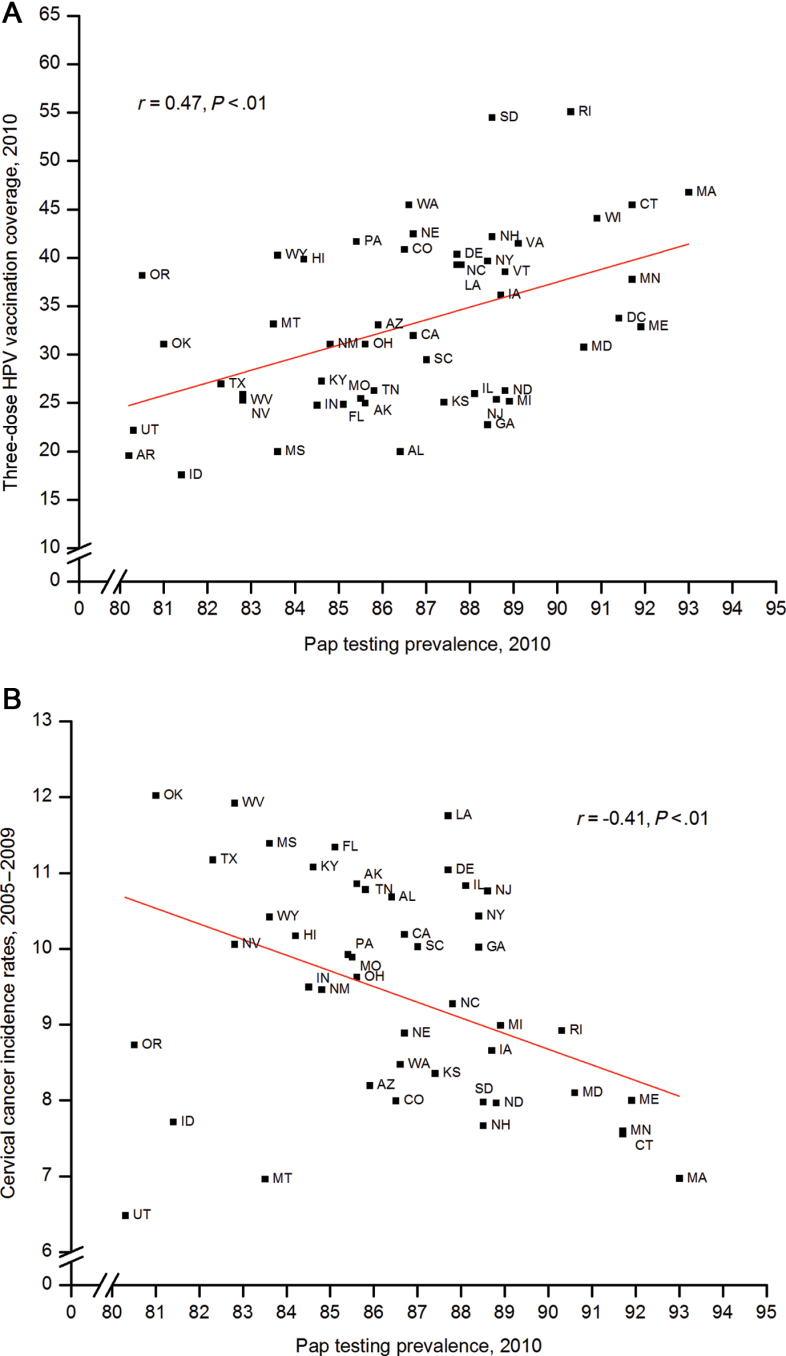
Death rates continued to decline for all cancers combined for men and women of all major racial and ethnic groups and for most major cancer sites; rates for both sexes combined decreased by 1.5% per year from 2000 to 2009. Overall incidence rates decreased in men but stabilized in women. Incidence rates increased for two HPV-associated cancers (oropharynx, anus) and some cancers not associated with HPV (eg, liver, kidney, thyroid). Nationally, 32.0% (95% confidence interval [CI] = 30.3% to 33.6%) of girls aged 13 to 17 years in 2010 had received three doses of the HPV vaccine, and coverage was statistically significantly lower among the uninsured (14.1%, 95% CI = 9.4% to 20.6%) and in some Southern states (eg, 20.0% in Alabama [95% CI = 13.9% to 27.9%] and Mississippi [95% CI = 13.8% to 28.2%]), where cervical cancer rates were highest and recent Pap testing prevalence was the lowest.
Conclusions
The overall trends in declining cancer death rates continue. However, increases in incidence rates for some HPV-associated cancers and low vaccination coverage among adolescents underscore the need for additional prevention efforts for HPV-associated cancers, including efforts to increase vaccination coverage.
The American Cancer Society (ACS), the Centers for Disease Control and Prevention (CDC), the National Cancer Institute (NCI), and the North American Association of Central Cancer Registries (NAACCR) collaborate annually to provide updated cancer incidence and mortality data for the United States. The initial report documented the first steady decline in cancer death rates, beginning in the early 1990s, since national record keeping on vital statistics began in 1930 (1). In addition to providing updates on incidence and mortality patterns, each report features a topic of special interest (2–14). This report features the burden and trends in human papillomavirus (HPV)–associated cancers among persons aged 15 years or older and HPV vaccination coverage levels among adolescents aged 13 to 17 years.
Exposure to HPV is common through sexual contact, and most infections resolve over time. However, persistent infection with oncogenic HPV types is etiologically linked to cervical cancer (15), as well as cancers of the oropharynx (16), anus (17), vagina and vulva (18), and penis (19,20). Virtually all cervical cancers are due to HPV infection, along with 90% of anal cancers, more than 60% of certain subsites of oropharyngeal cancers, and 40% of vagina, vulva, and penile cancers (20). Although there are approximately a dozen oncogenic HPV types, HPV 16 and 18 are the most common HPV types and are found in approximately 70% of cervical cancers. Human papillomavirus 16 is found in approximately 90% of the noncervical cancers often associated with HPV infection (20). Human papillomavirus types 6 and 11 are associated with the development of 90% of anogenital warts (21). Two vaccines (bivalent and quadrivalent) are available to protect against HPV types 16 and 18. Data from clinical trials have shown that both vaccines prevent vaccine type–related cervical precancers (22,23); the quadrivalent vaccine has been shown to also prevent vaginal, vulvar, and anal precancers (24,25). Although data show the vaccines prevent various outcomes, no data are available on the efficacy for prevention of HPV-associated cancers or lesions of the oropharynx. Because HPV 16 is responsible for the majority of HPV-associated cancers (20), the vaccines likely protect against these outcomes. The quadrivalent vaccine also protects against HPV 6 and 11, and clinical trials show the vaccine prevents vaccine type–related genital warts (26). The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination of girls aged 11 or 12 years with three doses of either vaccine and routine vaccination of boys aged 11 or 12 years with three doses of quadrivalent vaccine (27–29). Vaccination is also recommended for women aged 13 through 26 years and men aged 13 through 21 years who were not vaccinated previously. Men aged 22 through 26 years may also receive the vaccine. The goals of the current vaccination recommendations for adolescents are to prevent persistent HPV infections and the occurrence of anogenital warts beginning in young adulthood and cervical, vaginal, vulvar, and anal cancers that occur later in life. The occurrence of cervical cancer can also be prevented through screening (eg, Papanicolaou [Pap] and HPV testing) (30–32), and Pap testing has contributed to the substantial declines in cervical cancer rates in the United States and other developed countries over the past several decades (33).
Subjects and Methods
New Cancer Cases and Deaths
Population-based data on cancer incidence were based on the CDC’s National Program of Cancer Registries (NPCR) and/or the NCI’s Surveillance, Epidemiology, and End Results (SEER) program that met NAACCR’s data quality criteria (34). Site and histology for incident invasive cancers were coded according to the International Classification of Diseases for Oncology edition in use at the time of diagnosis, converted to the Third Edition coding (35), and categorized according to SEER site groups (36). Cancers of the oral cavity and pharynx mentioned in the main sections of the annual report include all cancers arising at these sites. Incidence rates were calculated for all sites combined, childhood cancers (ages 0–14 and 0–19 years), and the 17 most common cancers among men and 18 most common cancers among women to accommodate the 15 most common cancers for all races and ethnicities combined and for each of the five major racial and ethnic groups (white, black, Asian and Pacific Islander [API], American Indian/Alaska Native [AI/AN], and Hispanic) by sex. Hispanic ethnicity includes men and women from all races identified as Hispanic. Rates for AI/ANs were based on cases and deaths occurring in counties covered by the Indian Health Service’s Contract Health Service Delivery Area because of the high-quality ascertainment of race/ethnicity in these areas (10,37).
Incidence data were not available uniformly for every calendar year, geographic area, and racial and ethnic group in the United States. Therefore, long-term (1992–2009) incidence trends for all racial and ethnic groups combined were estimated using data from the 13 SEER registries covering approximately 14% of the US population (38). Five-year (2005–2009) average annual incidence rates and short-term (2000–2009) incidence trends for all racial and ethnic groups combined and for each of the five major racial and ethnic populations were calculated using combined data from NPCR and SEER registries, covering 93% (for the rates) and 87% (for the trends) of the US population.
Cause of death was based on death certificate information reported to state vital statistics offices and compiled into a national file through the CDC National Center for Health Statistics’ National Vital Statistics System (39). To maximize comparability among International Classification of Diseases and International Classification of Diseases for Oncology versions, cause of death was categorized according to SEER site groups (36). The underlying causes of death were selected according to the version of the International Classification of Diseases codes and selection rules in use at the time of death (International Classification of Diseases 6 to International Classification of Diseases 10). Death rates were calculated for all sites combined, childhood cancers, and the 17 most common cancers among men and 18 most common cancers in women in order to include the 15 most common cancers for all races and ethnicities combined and for each of the five major racial and ethnic groups by sex. We examined long-term (1975–2009) mortality trends for all racial and ethnic groups combined, and 5-year (2005–2009) average annual death rates and short-term (2000–2009) mortality trends for all racial and ethnic groups combined and for each of the five major racial and ethnic groups.
For classifying HPV-associated cancers, we used the same framework as in a prior study (40), by selecting invasive, microscopically confirmed squamous cell carcinomas (histology codes 8050–8084 and 8120–8131) of certain subsites of the oropharynx (including the base of the tongue, tonsils, and other oropharynx), anus (including rectum), vagina, vulva, and penis (35,36) in which HPV DNA is frequently found. For cervical cancers, all epithelial carcinomas were selected using histology codes 8010 to 8671 and 8940 to 8941 because all are considered to be HPV associated (15). However, it is noteworthy that information about the HPV DNA status of the specific cancers included in these analyses was not available and not all of these tumors were necessarily HPV positive. In contrast to all ages combined for the other sites, analyses of HPV-associated cancers were restricted to men and women aged 15 years or older. We present the burden as the total number of HPV-associated cancers for the most recent year of diagnosis (2009) by sex, and we present average annual incidence rates (per 100 000 population) for 2005–2009 by sex, race and ethnicity, and area-level socioeconomic status (41). In addition, we examined temporal trends in the annual incidence rates for HPV-associated cancers from 2000 to 2009 by sex and race and ethnicity.
HPV Vaccination Coverage
Data on HPV vaccination coverage for receipt of one or more and three (some received more than three) doses of HPV vaccine among girls aged 13 to 17 years for 2008 and 2010 were obtained from the National Immunization Survey-Teen (NIS-Teen), a random-digit-dialed landline telephone survey of US households (42,43). The parents and guardians of eligible adolescents are asked during the telephone interview for verbal consent to contact the adolescents’ vaccination provider(s). The NIS-Teen uses the list-assisted method of random-digit-dialed survey, and the sampling frame of telephone numbers is updated each quarter to reflect new telephone exchanges and area codes (42,43). The NIS-Teen is the only national data source to assess provider-verified vaccination coverage among adolescents. Estimates are reported among adolescents aged 13 to 17 years. Measuring at ages 13 through 17 years allows sufficient time for those who initiated the series at age 11 or 12 years to complete it, and it captures information on those vaccinated at older ages. Data from this national survey are weighted based on the sampling design. National estimates are used to monitor coverage as vaccination histories are obtained from medical records and sociodemographic information is obtained from parents (44). Three-dose series completion rates were determined among girls who received at least one dose of the HPV vaccine 24 weeks or more before the NIS-Teen interview date because administration of the third dose is recommended 24 weeks after receipt of the first dose. Vaccination coverage estimates are presented by demographic characteristics, including by insurance status and Vaccines for Children (VFC) program eligibility. The VFC program provides free vaccine to children and adolescents through 18 years of age who are uninsured, eligible for the Medicaid program, American Indian or Alaska Native, or underinsured. Underinsured children (whose insurance does not cover vaccine) are eligible to receive VFC vaccine only through a federally qualified health center or rural health clinic (45).
Prevalence of Pap Testing
Data from the 2010 Behavioral Risk Factor Surveillance System (BRFSS), a state-based, random-digit-dialed telephone survey, were used to estimate the state-level (and national average) prevalence of recent Pap testing (during the previous 3 years) for women aged 21 to 65 years with an intact uterus, overall, and by usual source of medical care (46). Verbal consent is obtained during the interview from survey participants.
Population Estimates
Population estimates from the Census Bureau’s Vintage 2009 National Tables were used in the SEER*Stat software (http://seer.cancer.gov/seerstat) to produce mortality and incidence rates by age, sex, race, and ethnicity at the county level (38,47). Because the Census, beginning in 2000, allowed for selection of multiple races, mortality and incidence data by race (ie, the numerators for death and incidence rates) are not wholly compatible with the population data collected in the Census. Therefore, bridged single-race estimates were produced by the Census Bureau in collaboration with the National Center for Health Statistics (48). For most states, population estimates as of July 1 of each year were used to calculate annual incidence rates because these estimates are presumed to reflect the average population of a defined geographic area for a calendar year. However, certain county population estimates were adjusted to account for populations displaced along the Gulf Coast of Louisiana, Alabama, Mississippi, and Texas during 2005 by Hurricanes Katrina and Rita (38). National total population estimates were not affected by these adjustments. Other specific modifications included using additional local information to estimate the native Hawaiian population accurately and to derive population estimates for a newly created county in Colorado (38). These modified county-level population estimates, summed to the state and national level, were used as denominators in rate calculations. Population estimates were grouped into three categories according to the percent of the population in the county living below the federally defined poverty threshold: less than 10%, 10.0% to 19.99%, and 20% or greater, with the last group considered a severely disadvantaged area (41).
Statistical Methods
Incidence and Death Rates and Trends.
Average annual cancer incidence and death rates per 100 000 persons were age standardized to the 2000 US standard population by the direct method (49). Corresponding 95% confidence intervals (CIs) were calculated as modified gamma intervals (50). For stability and reliability, rates were not reported if the numerator included less than 16 observations.
Trends in age-standardized cancer incidence and death rates were analyzed using joinpoint regression, which involves fitting a series of joined straight lines on a logarithmic scale to the trends in the annual age-standardized rates (http://www.srab.cancer.gov/joinpoint) (51) with at least three data points between changes in joinpoints. Up to three joinpoints were allowed in models for the period 1992 to 2009, up to five joinpoints were allowed in models for the period 1975 to 2009, and up to two joinpoints were allowed in models for the period 2000 to 2009. The number of joinpoints is constrained by the number of intervals available to identify the years in which there was a statistically significant change in the trends. The resulting trends of varying time periods were described by the slope of the line segment or annual percentage change (APC). The average annual percentage change (AAPC) was estimated as a geometric weighted average of the APCs, with the weights equal to the length of each line segment during the prespecified fixed interval (eg, 2000–2009) (http://srab.cancer.gov/joinpoint/aapc.html) (52). Long-term incidence trends were calculated using both observed and delay-adjusted SEER 13 data; however, descriptions of these trends were based on the delay-adjusted data, except when noted. Delay adjustment is a statistical method to correct for unreported (delayed) or updated cases and mostly affects cancers diagnosed in recent years and cancers diagnosed in nonhospital settings (eg, melanoma or leukemia) (53). Delay-adjusted rates include two sources of variability. The first is the usual variability of the rates themselves, and the second is the variability from the delay model, which includes the uncertainty of the delay adjustments. The delay-adjustment method is not available for NPCR areas. Therefore, short-term trends (2000–2009) by race and ethnicity were based on observed NPCR and SEER combined data. We used the t test and the Z test, respectively, to assess whether the APC and the AAPC were statistically different from zero; all statistical tests were two-sided. In describing trends, the terms “increase” or “decrease” were used when the slope (APC or AAPC) of the trend was statistically significant (P < .05). For non-statistically significant trends, terms such as “stable,” “nonsignificant increase,” and “nonsignificant decrease” were used. Incidence rates of HPV-associated cancers by area-level socioeconomic status were considered to be statistically significantly different if the 95% confidence intervals for the groups being compared did not overlap.
HPV Vaccination Coverage Levels.
Sample-weighted national vaccination coverage estimates, as well as the percentage point changes in estimates from 2008 to 2010, for selected sociodemographic characteristics and by state were calculated using Statistical Analysis Software (SAS, version 9.3, SAS Institute, Cary, NC)–callable- SUDAAN (release 10.0, Research Triangle Institute, Research Triangle Park, NC) (54). The two-sided t test was used to determine whether overall differences in vaccination estimate percentage point changes from 2008 to 2010 were statistically significant (P < .05). Differences in vaccination coverage estimates by sociodemographic characteristics were considered to be statistically different if the 95% confidence intervals for the groups being compared did not overlap. State-level coverage estimates are displayed by their overall quartile distribution. State-level vaccination coverage estimates were considered to be statistically significantly different from the national estimate if their 95% confidence intervals did not overlap.
Prevalence of Pap Testing.
SAS-callable-SUDAAN (54) was used to calculate national and state-level weighted prevalence estimates of recent Pap testing and corresponding 95% confidence intervals, taking into account the complex survey design of the BRFSS. The relationships between state-level HPV vaccination coverage and Pap testing prevalence and between cervical cancer incidence rates and Pap testing prevalence were assessed with the Pearson correlation coefficient (r, weighted by the inverse of the variance of Pap testing prevalence). The correlation coefficients were calculated in SAS, and a two-sided t test was used to assess statistical significance (P < .05).
Results
Long-Term (1992–2009) Cancer Incidence Trends for All Racial and Ethnic Groups Combined
Trend analysis based on SEER 13 data showed that overall delay-adjusted cancer incidence rates in all racial and ethnic groups and sexes combined were stable from 2000 to 2009 (Table 1). Among men, overall cancer incidence decreased on average by 0.6% annually from 1994 to 2009. Overall cancer incidence rates among women decreased 0.5% annually from 1998 to 2006, but rates were stable from 2006 to 2009. Overall cancer incidence rates increased by 0.6% per year among children aged 0 to 14 years and by 0.7% per year among children aged 0 to 19 years from 2000 to 2009, continuing trends from 1992.
Table 1.
Surveillance, Epidemiology, and End Results (SEER) cancer incidence rate trends with joinpoint analyses from 1992 to 2009 for the most common cancers, by sex, for all racial and ethnic groups combined*
| Joinpoint analyses (1992–2009)† | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trend 1 | Trend 2 | Trend 3 | Trend 4 | AAPC‡ | ||||||
| Cancer site or type by sex | Years | APC§ | Years | APC§ | Years | APC§ | Years | APC§ | 2000–2009 | 2005–2009 |
| All sites|| | ||||||||||
| Both sexes | 1992–1994 | −3.1¶ | 1994–1999 | 0.3 | 1999–2009 | −0.6¶ | −0.6# | −0.6# | ||
| Delay adjusted | 1992–1994 | −3.1¶ | 1994–1999 | 0.4 | 1999–2005 | −0.7¶ | 2005–2009 | 0.1 | −0.4 | 0.1 |
| Men | 1992–1995 | −4.5¶ | 1995–2000 | 0.2 | 2000–2009 | −1.0¶ | −1.0# | −1.0# | ||
| Delay adjusted | 1992–1994 | −5.6¶ | 1994–2009 | −0.6¶ | −0.6# | −0.6# | ||||
| Women | 1992–1994 | −0.4 | 1994–1998 | 1.2 | 1998–2004 | −0.8¶ | 2004–2009 | 0.2 | −0.2 | 0.2 |
| Delay adjusted | 1992–1998 | 0.8¶ | 1998–2006 | −0.5¶ | 2006–2009 | 1.0 | 0.0 | 0.6 | ||
| Children (ages 0–14) | 1992–2009 | 0.5¶ | 0.5# | 0.5# | ||||||
| Delay adjusted | 1992–2009 | 0.6¶ | 0.6# | 0.6# | ||||||
| Children (ages 0–19) | 1992–2009 | 0.6¶ | 0.6# | 0.6# | ||||||
| Delay adjusted | 1992–2009 | 0.7¶ | 0.7# | 0.7# | ||||||
| 17 most common cancers for men** | ||||||||||
| Prostate | 1992–1995 | −11.2¶ | 1995–2000 | 2.1 | 2000–2009 | −2.1¶ | −2.1# | −2.1# | ||
| Delay adjusted | 1992–1995 | −11.1¶ | 1995–2000 | 2.0 | 2000–2009 | −1.9¶ | −1.9# | −1.9# | ||
| Lung and bronchus | 1992–2009 | −2.0¶ | −2.0# | −2.0# | ||||||
| Delay adjusted | 1992–2009 | −1.9¶ | −1.9# | −1.9# | ||||||
| Colon and rectum | 1992–1995 | −2.6¶ | 1995–1998 | 1.6 | 1998–2009 | −2.7¶ | −2.7# | −2.7# | ||
| Delay adjusted | 1992–1995 | −2.6¶ | 1995–1998 | 1.5 | 1998–2009 | −2.6¶ | −2.6# | −2.6# | ||
| Urinary bladder | 1992–2007 | 0.1 | 2007–2009 | −3.3 | −0.7 | −1.6 | ||||
| Delay adjusted | 1992–2007 | 0.1 | 2007–2009 | −2.5 | −0.5 | −1.2 | ||||
| Melanoma of the skin | 1992–2009 | 2.4¶ | 2.4# | 2.4# | ||||||
| Delay adjusted | 1992–2009 | 2.5¶ | 2.5# | 2.5# | ||||||
| Non-Hodgkin lymphoma | 1992–2009 | 0.1 | 0.1 | 0.1 | ||||||
| Delay adjusted | 1992–2009 | 0.2 | 0.2 | 0.2 | ||||||
| Kidney and renal pelvis | 1992–2009 | 2.3¶ | 2.3# | 2.3# | ||||||
| Delay adjusted | 1992–1999 | 1.3¶ | 1999–2009 | 2.9¶ | 2.9# | 2.9# | ||||
| Oral cavity and pharynx | 1992–2005 | −1.4¶ | 2005–2009 | 1.5 | −0.1 | 1.5 | ||||
| Delay adjusted | 1992–2005 | −1.4¶ | 2005–2009 | 1.8 | 0.0 | 1.8 | ||||
| Leukemia | 1992–2009 | −0.3¶ | −0.3# | −0.3# | ||||||
| Delay adjusted | 1992–2009 | 0.2 | 0.2 | 0.2 | ||||||
| Pancreas | 1992–2002 | 0.0 | 2002–2009 | 1.4¶ | 1.1# | 1.4# | ||||
| Delay adjusted | 1992–2002 | 0.0 | 2002–2009 | 1.7¶ | 1.3# | 1.7# | ||||
| Liver and intrahepatic bile duct | 1992–2009 | 3.5¶ | 3.5# | 3.5# | ||||||
| Delay adjusted | 1992–2009 | 3.7¶ | 3.7# | 3.7# | ||||||
| Stomach | 1992–2009 | −1.7¶ | −1.7# | −1.7# | ||||||
| Delay adjusted | 1992–2009 | −1.7¶ | −1.7# | −1.7# | ||||||
| Esophagus | 1992–2009 | 0.0 | 0.0 | 0.0 | ||||||
| Delay adjusted | 1992–2009 | 0.0 | 0.0 | 0.0 | ||||||
| Brain and other nervous system | 1992–2009 | −0.3¶ | −0.3# | −0.3# | ||||||
| Delay adjusted | 1992–2009 | −0.2 | −0.2 | −0.2 | ||||||
| Myeloma | 1992–2009 | 0.2 | 0.2 | 0.2 | ||||||
| Delay adjusted | 1992–2009 | 0.5¶ | 0.5# | 0.5# | ||||||
| Larynx | 1992–2009 | −2.8¶ | −2.8# | −2.8# | ||||||
| Delay adjusted | 1992–2009 | −2.8¶ | −2.8# | −2.8# | ||||||
| Thyroid | 1992–1996 | −1.4 | 1996–2009 | 5.5¶ | 5.5# | 5.5# | ||||
| Delay adjusted | 1992–1996 | −1.4 | 1996–2009 | 5.6¶ | 5.6# | 5.6# | ||||
| 18 most common cancers for women** | ||||||||||
| Breast | 1992–1999 | 1.3¶ | 1999–2005 | −2.0¶ | 2005–2009 | 0.9 | −0.7 | 0.9 | ||
| Delay adjusted | 1992–1999 | 1.3¶ | 1999–2005 | −2.0¶ | 2005–2009 | 1.1 | −0.6 | 1.1 | ||
| Lung and bronchus | 1992–1998 | 0.8¶ | 1998–2001 | −1.3 | 2001–2005 | 0.5 | 2005–2009 | −1.2¶ | −0.5 | −1.2# |
| Delay adjusted | 1992–1997 | 0.7 | 1997–2009 | −0.3¶ | −0.3# | −0.3# | ||||
| Colon and rectum | 1992–1995 | −1.9¶ | 1995–1998 | 1.9 | 1998–2009 | −2.1¶ | −2.1# | −2.1# | ||
| Delay adjusted | 1992–1995 | −1.8¶ | 1995–1998 | 1.9 | 1998–2009 | −2.1¶ | −2.1# | −2.1# | ||
| Corpus and uterus, NOS | 1992–2006 | −0.2 | 2006–2009 | 3.1¶ | 0.9# | 2.3# | ||||
| Delay adjusted | 1992–2007 | −0.1 | 2007–2009 | 5.2¶ | 1.0# | 2.5# | ||||
| Thyroid | 1992–1999 | 4.1¶ | 1999–2009 | 6.9¶ | 6.9# | 6.9# | ||||
| Delay adjusted | 1992–1999 | 4.1¶ | 1999–2009 | 7.0¶ | 7.0# | 7.0# | ||||
| Non-Hodgkin lymphoma | 1992–2003 | 1.3¶ | 2003–2009 | −0.5 | 0.1 | −0.5 | ||||
| Delay adjusted | 1992–2003 | 1.4¶ | 2003–2009 | −0.1 | 0.4 | −0.1 | ||||
| Melanoma of the skin | 1992–1997 | 4.0¶ | 1997–2009 | 1.6¶ | 1.6# | 1.6# | ||||
| Delay adjusted | 1992–1997 | 3.9¶ | 1997–2009 | 1.7¶ | 1.7# | 1.7# | ||||
| Ovary|| | 1992–2001 | −0.6¶ | 2001–2009 | −1.4¶ | −1.3# | −1.4# | ||||
| Delay adjusted|| | 1992–2009 | −0.9¶ | −0.9# | −0.9# | ||||||
| Kidney and renal pelvis | 1992–2009 | 2.5¶ | 2.5# | 2.5# | ||||||
| Delay adjusted | 1992–1998 | 1.3 | 1998–2009 | 3.1¶ | 3.1# | 3.1# | ||||
| Pancreas | 1992–2009 | 0.6¶ | 0.6# | 0.6# | ||||||
| Delay adjusted | 1992–2000 | −0.1 | 2000–2009 | 1.4¶ | 1.4# | 1.4# | ||||
| Leukemia | 1992–2009 | 0.0 | 0.0 | 0.0 | ||||||
| Delay adjusted | 1992–2009 | 0.5¶ | 0.5# | 0.5# | ||||||
| Urinary bladder | 1992–2004 | −0.2 | 2004–2009 | −1.7¶ | −1.0# | −1.7# | ||||
| Delay adjusted | 1992–2004 | −0.1 | 2004–2009 | −1.3¶ | −0.8# | −1.3# | ||||
| Cervix uteri | 1992–2009 | −2.6¶ | −2.6# | −2.6# | ||||||
| Delay adjusted | 1992–2009 | −2.5¶ | −2.5# | −2.5# | ||||||
| Oral cavity and pharynx | 1992–2009 | −1.0¶ | −1.0# | −1.0# | ||||||
| Delay adjusted | 1992–2009 | −0.9¶ | −0.9# | −0.9# | ||||||
| Brain and other nervous system | 1992–2009 | −0.2 | −0.2 | −0.2 | ||||||
| Delay adjusted | 1992–2009 | 0.0 | 0.0 | 0.0 | ||||||
| Myeloma | 1992–2009 | −0.1 | −0.1 | −0.1 | ||||||
| Delay adjusted | 1992–2009 | 0.3 | 0.3 | 0.3 | ||||||
| Stomach | 1992–2009 | −0.8¶ | −0.8# | −0.8# | ||||||
| Delay adjusted | 1992–2009 | −0.8¶ | −0.8# | −0.8# | ||||||
| Liver and intrahepatic bile duct | 1992–2009 | 2.8¶ | 2.8# | 2.8# | ||||||
| Delay adjusted | 1992–2009 | 3.0¶ | 3.0# | 3.0# | ||||||
* Source: Surveillance, Epidemiology, and End Results (SEER) 13 areas covering about 14% of the US population (Connecticut, Hawaii, Iowa, Utah, and New Mexico, the Alaska Native Tumor Registry, rural Georgia, and the metropolitan areas of San Francisco, Los Angeles, San Jose-Monterey, Detroit, Atlanta, and Seattle-Puget Sound). AAPC = average annual percent change; APC = annual percent change; NOS = not otherwise specified.
† Joinpoint analyses with up to three joinpoints yielding up to four trend segments (Trends 1–4) were based on rates per 100 000 persons and were age adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, … , 80–84 years, ≥85 years; Census publication p25-1130; US Bureau of the Census, Current Population Reports. Washington, DC: US Government Printing Office, 2000). For joinpoint analysis, the Joinpoint Regression Program was used (version 3.5.1, July 2011; Surveillance Research Program, National Cancer Institute, Bethesda, MD).
‡ The AAPC is a weighted average of the APCs that is calculated by joinpoint regression.
§ The APC is based on age-adjusted rates (see above).
|| All sites excludes myelodysplastic syndromes and borderline tumors; ovary excludes borderline tumors.
¶ The APC is statistically significantly different from zero (2-sided t test; P < .05).
# The AAPC is statistically significantly different from zero (2-sided Z test; P < .05).
** Cancers are listed in descending rank order of sex-specific, age-adjusted incidence rates for 2005 through 2009 for all racial and ethnic groups combined (using data from the National Program of Cancer Registries [NPCR] and SEER Program areas reported by the North American Association of Central Cancer Registries [NAACCR] as meeting high-quality incidence data standards for 2005–2009). To include the 15 most common cancers in each racial and ethnic group, more than 15 cancers are given for men and women in total.
Among men, incidence rates from 2000 to 2009 decreased for five of the 17 most common cancers: prostate, lung and bronchus (lung), colon and rectum (colorectal), stomach, and larynx. In contrast, rates among men during the same time interval increased for six cancers: kidney and renal pelvis (kidney), pancreas, liver and intrahepatic bile duct (liver), thyroid, melanoma of the skin (melanoma), and myeloma. Among women, incidence rates decreased from 2000 to 2009 for seven of the 18 most common cancers: lung, colorectal, urinary bladder (bladder), cervix uteri (cervix), oral cavity and pharynx (all tumors regardless of their potential association with HPV infection), ovary, and stomach. Incidence rates among women increased from 2000 to 2009 for seven cancers: thyroid, melanoma, kidney, pancreas, leukemia, liver, and corpus and uterus (uterus). Incidence rates were stable for all other cancers during the period from 2000 to 2009, including female breast cancer and non-Hodgkin lymphoma in men and women.
Long-Term (1975–2009) Cancer Mortality Trends for All Racial and Ethnic Groups Combined
Overall cancer death rates have been declining since the early 1990s, with rates decreasing by about 1.8% per year in men and by 1.4% per year in women from 2000 to 2009 (Table 2). Among children, rates have continued to decrease since 1975, although the decrease was briefly interrupted from 1998 to 2003. During the period from 2000 to 2009 and the period from 2005 to 2009, death rates among men decreased for 10 of the 17 most common cancers (lung, prostate, colorectal, leukemia, non-Hodgkin’s lymphoma, kidney, stomach, myeloma, oral cavity and pharynx, and larynx), whereas rates increased for cancers of the pancreas, liver, and melanoma of the skin. During the same time periods, death rates among women decreased for 15 of the 18 most common cancers (lung, breast, colorectal, ovary, leukemia, non-Hodgkin lymphoma, brain and central nervous system, myeloma, kidney, stomach, cervix, bladder, esophagus, oral cavity and pharynx, and gallbladder), whereas they increased for cancers of the pancreas, liver, and uterus.
Table 2.
US cancer death rate trends with joinpoint analyses from 1975 to 2009 for the most common cancers, by sex, for all racial and ethnic groups combined*
| Joinpoint analyses (1975–2009)† | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trend 1 | Trend 2 | Trend 3 | Trend 4 | Trend 5 | Trend 6 | AAPC‡ | ||||||||
| Cancer site or type by sex | Years | APC§ | Years | APC§ | Years | APC§ | Years | APC§ | Years | APC§ | Years | APC§ | 2000–2009 | 2005–2009 |
| All sites | ||||||||||||||
| Both sexes | 1975–1984 | 0.5|| | 1984–1991 | 0.3|| | 1991–1994 | −0.5 | 1994–1998 | −1.3|| | 1998–2001 | −0.8 | 2001–2009 | −1.6|| | −1.5¶ | −1.6¶ |
| Men | 1975–1979 | 1.0|| | 1979–1990 | 0.3|| | 1990–1993 | −0.5 | 1993–2001 | −1.5|| | 2001–2009 | −1.8|| | −1.8¶ | −1.8¶ | ||
| Women | 1975–1990 | 0.6|| | 1990–1994 | −0.2 | 1994–2002 | −0.8|| | 2002–2009 | −1.5|| | −1.4¶ | −1.5¶ | ||||
| Children (aged 0–14 y) | 1975–1998 | −2.9|| | 1998–2003 | 0.2 | 2003–2009 | −2.8|| | −1.8¶ | −2.8¶ | ||||||
| Children (aged 0–19 y) | 1975–1998 | −2.7|| | 1998–2003 | 0.0 | 2003–2009 | −2.7|| | −1.8¶ | −2.7¶ | ||||||
| 17 most common cancers for men# | ||||||||||||||
| Lung and bronchus | 1975–1978 | 2.5|| | 1978–1984 | 1.2|| | 1984–1990 | 0.4|| | 1990–1993 | −1.1 | 1993–2005 | −1.9|| | 2005–2009 | −2.8|| | −2.3¶ | −2.8¶ |
| Prostate | 1975–1987 | 0.9|| | 1987–1991 | 3.1|| | 1991–1994 | −0.7 | 1994–2004 | −3.9|| | 2004–2009 | −3.2|| | −3.5¶ | −3.2¶ | ||
| Colon and rectum | 1975–1978 | 0.8 | 1978–1984 | −0.4 | 1984–1990 | −1.3|| | 1990–2002 | −2.0|| | 2002–2005 | −3.9|| | 2005–2009 | −2.5|| | −2.9¶ | −2.5¶ |
| Pancreas | 1975–1986 | −0.8|| | 1986–2001 | −0.3|| | 2001–2009 | 0.5|| | 0.4¶ | 0.5¶ | ||||||
| Leukemia | 1975–1995 | −0.2|| | 1995–2009 | −0.8|| | −0.8¶ | −0.8¶ | ||||||||
| Non-Hodgkin lymphoma | 1975–1991 | 2.7|| | 1991–1997 | 1.5|| | 1997–2009 | −2.7|| | −2.7¶ | −2.7¶ | ||||||
| Liver and intrahepatic bile duct | 1975–1985 | 1.5|| | 1985–1996 | 3.8|| | 1996–1999 | 0.5 | 1999–2009 | 2.6|| | 2.6¶ | 2.6¶ | ||||
| Esophagus | 1975–1985 | 0.7|| | 1985–1994 | 1.2|| | 1994–2005 | 0.5|| | 2005–2009 | −1.2|| | −0.3 | −1.2¶ | ||||
| Urinary bladder | 1975–1983 | −1.4|| | 1983–1987 | −2.8|| | 1987–1993 | 0.2 | 1993–1997 | −1.1 | 1997–2009 | 0.0 | 0.0 | 0.0 | ||
| Kidney and renal pelvis | 1975–1992 | 1.1|| | 1992–2009 | −0.4|| | −0.4¶ | −0.4¶ | ||||||||
| Brain and other nervous system | 1975–1977 | 4.4 | 1977–1982 | −0.4 | 1982–1991 | 1.3|| | 1991–2007 | −1.0|| | 2007–2009 | 1.5 | −0.4 | 0.3 | ||
| Stomach | 1975–1987 | −2.3|| | 1987–1991 | −1.0 | 1991–2009 | −3.4|| | −3.4¶ | −3.4¶ | ||||||
| Myeloma | 1975–1994 | 1.5|| | 1994–2009 | −1.1|| | −1.1¶ | −1.1¶ | ||||||||
| Melanoma of the skin | 1975–1990 | 2.2|| | 1990–2002 | 0.0 | 2002–2009 | 1.0|| | 0.8¶ | 1.0¶ | ||||||
| Oral cavity and pharynx | 1975–1993 | −1.9|| | 1993–1999 | −3.1|| | 1999–2009 | −1.3|| | −1.3¶ | −1.3¶ | ||||||
| Larynx | 1975–1994 | −0.8|| | 1994–2009 | −2.5|| | −2.5¶ | −2.5¶ | ||||||||
| Soft tissue including heart | 1975–1980 | 7.6|| | 1980–1997 | 1.2|| | 1997–2002 | −3.5|| | 2002–2009 | 1.2|| | 0.2 | 1.2¶ | ||||
| 18 most common cancers for women# | ||||||||||||||
| Lung and bronchus | 1975–1982 | 6.0|| | 1982–1990 | 4.2|| | 1990–1995 | 1.7|| | 1995–2003 | 0.3|| | 2003–2009 | −1.1|| | −0.6¶ | −1.1¶ | ||
| Breast | 1975–1990 | 0.4|| | 1990–1995 | −1.8|| | 1995–1998 | −3.2|| | 1998–2009 | −1.9|| | −1.9¶ | −1.9¶ | ||||
| Colon and rectum | 1975–1984 | −1.0|| | 1984–2001 | −1.8|| | 2001–2009 | −3.1|| | −2.9¶ | −3.1¶ | ||||||
| Pancreas | 1975–1984 | 0.8|| | 1984–2002 | 0.1 | 2002–2009 | 0.4|| | 0.4¶ | 0.4¶ | ||||||
| Ovary | 1975–1982 | −1.2|| | 1982–1992 | 0.3|| | 1992–1998 | −1.2|| | 1998–2002 | 1.0 | 2002–2009 | −2.0|| | −1.3¶ | −2.0¶ | ||
| Leukemia | 1975–1980 | 0.8 | 1980–2000 | −0.4|| | 2000–2009 | −1.5|| | −1.5¶ | −1.5¶ | ||||||
| Non-Hodgkin lymphoma | 1975–1994 | 2.2|| | 1994–1997 | 1.0 | 1997–2009 | −3.4|| | −3.4¶ | −3.4¶ | ||||||
| Corpus and uterus, NOS | 1975–1989 | −1.6|| | 1989–1997 | −0.7|| | 1997–2009 | 0.3|| | 0.3¶ | 0.3¶ | ||||||
| Brain and other nervous system | 1975–1992 | 0.9|| | 1992–2009 | −0.9|| | −0.9¶ | −0.9¶ | ||||||||
| Liver and intrahepatic bile duct | 1975–1987 | 0.8|| | 1987–1995 | 3.8|| | 1995–2000 | 0.4 | 2000–2009 | 1.5|| | 1.5¶ | 1.5¶ | ||||
| Myeloma | 1975–1993 | 1.5|| | 1993–2002 | −0.5|| | 2002–2009 | −2.9|| | −2.3¶ | −2.9¶ | ||||||
| Kidney and renal pelvis | 1975–1994 | 1.1|| | 1994–2009 | −0.9|| | −0.9¶ | −0.9¶ | ||||||||
| Stomach | 1975–1987 | −2.8|| | 1987–1990 | −0.2 | 1990–2009 | −2.7|| | −2.7¶ | −2.7¶ | ||||||
| Cervix uteri | 1975–1982 | −4.3|| | 1982–1996 | −1.6|| | 1996–2003 | −3.8|| | 2003–2009 | −0.9|| | −1.9¶ | −0.9¶ | ||||
| Urinary bladder | 1975–1986 | −1.6|| | 1986–2009 | −0.4|| | −0.4¶ | −0.4¶ | ||||||||
| Esophagus | 1975–2001 | 0.0 | 2001–2009 | −2.0|| | −1.7¶ | −2.0¶ | ||||||||
| Oral cavity and pharynx | 1975–1990 | −1.0|| | 1990–2009 | −2.3|| | −2.3¶ | −2.3¶ | ||||||||
| Gallbladder | 1975–1990 | −3.0|| | 1990–2009 | −2.3|| | −2.3¶ | −2.3¶ | ||||||||
* Source: National Center for Health Statistics public-use data file for the total US, 1975 through 2009. AAPC = average annual percent change; APC = annual percent change; NOS = not otherwise specified.
† Joinpoint analyses with up to five joinpoints yielding up to six trend segments (Trends 1–6) were based on rates per 100 000 persons and were age adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, … , 80–84 years, ≥85 years; Census publication p25-1130; US Bureau of the Census, Current Population Reports. Washington, DC: US Government Printing Office, 2000). For joinpoint analysis, the Joinpoint Regression Program was used (version 3.5.1, July 2011; Surveillance Research Program, National Cancer Institute, Bethesda, MD).
‡ The AAPC is a weighted average of the APCs calculated by joinpoint regression.
§ The APC is based on age-adjusted rates (see above).
|| The APC is statistically significantly different from zero (two-sided t test; P < .05).
¶ The AAPC is statistically significantly different from zero (two-sided Z test; P < .05).
# Cancers are listed in descending rank order of sex-specific, age-adjusted incidence rates for 2005 through 2009 for all racial and ethnic groups combined. To include the 15 most common cancers in each racial and ethnic group, more than 15 cancers are given for men and women in total.
Cancer Incidence Rates (2005–2009) and Short-Term (2000–2009) Trends by Race and Ethnicity
Five-year incidence rates (2005–2009) and short-term trends (2000–2009), which were not delay adjusted, are presented for men and women by race and ethnicity in Table 3. For all cancer sites combined and all racial and ethnic groups, cancer incidence rates for the period from 2005 to 2009 were higher among men than women. Black men had the highest overall cancer incidence rate of any racial and ethnic group. Among men, the highest incidence rates were observed for prostate cancer, followed by lung and colorectal cancer in each racial and ethnic group, except for Hispanics, in whom colorectal cancer ranked second. Among women, the highest overall incidence rates during the period from 2005 to 2009 were in whites followed by blacks. Generally, breast cancer had the highest incidence rate, followed by lung and colorectal cancers, except among API and Hispanic women, in whom colorectal cancer was more common than lung cancer. Uterine cancer ranked fourth among women of each racial and ethnic group except API women, in whom thyroid cancer was the fourth most common cancer. Beyond the three most commonly diagnosed cancers for men and four most commonly diagnosed cancers for women, cancer ranking varied by race and ethnicity.
Table 3.
Incidence rates for the period from 2005 to 2009 and fixed-interval trends for the period from 2000 to 2009 for the most common cancers by sex, race, and ethnicity, for areas in the United States with high-quality incidence data*
| All races/ethnicities | White‡ | Black‡ | API‡ | AI/AN (CHSDA)‡ | Hispanic‡ | Non-Hispanic‡ | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer site or type by sex† | Rank | Rate§ | 2000–2009 AAPC|| | 2005–2009 AAPC|| | Rank | Rate§ | 2000–2009 AAPC|| | Rank | Rate§ | 2000–2009 AAPC|| | Rank | Rate§ | 2000–2009 AAPC|| | Rank | Rate§ | 2000–2009 AAPC|| | Rank | Rate§ | 2000–2009 AAPC|| | Rank | Rate§ | 2000–2009 AAPC|| |
| All sites¶ | ||||||||||||||||||||||
| Both sexes | 473.4 | −0.6# | −0.6# | 472.8 | −0.7# | 484.7 | −0.7# | 301.2 | −0.7# | 385.4 | −0.3 | 366.1 | −0.9# | 483.7 | −0.5# | |||||||
| Men | 550.7 | −1.1# | −1.1# | 543.1 | −1.2# | 619.7 | −1.4# | 327.5 | −1.8# | 423.2 | −0.7 | 418.7 | −1.7# | 563.0 | −1.0# | |||||||
| Women | 419.3 | −0.3# | −0.3# | 424.0 | −0.3# | 396.8 | 0.0 | 286.2 | 0.2 | 360.3 | 0.0 | 333.2 | −0.3# | 427.6 | −0.2# | |||||||
| Children (aged 0–14 y) | 15.6 | 0.3 | 0.3 | 16.0 | −0.1 | 12.3 | 1.4# | 12.7 | 0.5 | 12.1 | −0.6 | 15.5 | 0.2 | 15.6 | 0.3 | |||||||
| Children (aged 0–19 y) | 17.2 | 0.4 | 0.4 | 17.8 | 0.1 | 13.1 | 1.4# | 14.0 | 1.4 | 13.8 | −0.2 | 17.1 | 0.6# | 17.3 | 0.4 | |||||||
| 17 most common cancers for men | ||||||||||||||||||||||
| Prostate | 1 | 151.4 | −1.9# | −1.9# | 1 | 141.0 | −2.3# | 1 | 228.7 | −2.0# | 1 | 77.2 | −3.1# | 1 | 98.8 | −2.1# | 1 | 124.9 | −2.4# | 1 | 153.8 | −1.9# |
| Lung and bronchus | 2 | 82.7 | −2.0# | −2.6# | 2 | 82.3 | −1.9# | 2 | 99.3 | −2.4# | 2 | 49.4 | −1.6# | 2 | 67.4 | −2.1# | 3 | 45.4 | −2.9# | 2 | 85.9 | −1.8# |
| Colon and rectum | 3 | 54.0 | −3.2# | −3.2# | 3 | 52.8 | −3.5# | 3 | 65.1 | −1.9# | 3 | 41.4 | −2.9# | 3 | 50.7 | −0.8 | 2 | 46.9 | −2.3# | 3 | 54.6 | −3.2# |
| Urinary bladder | 4 | 37.5 | −0.8# | −1.8# | 4 | 39.5 | −0.9# | 5 | 19.5 | 0.4 | 6 | 15.3 | −1.1 | 5 | 18.0 | 0.1 | 4 | 20.1 | −2.0# | 4 | 38.9 | −0.7# |
| Melanoma of the skin | 5 | 24.6 | 1.9# | 1.9# | 5 | 27.2 | 1.9# | 25 | 1.1 | −0.1 | 20 | 1.6 | −0.8 | 13 | 6.4 | −1.6 | 16 | 4.6 | −0.9 | 5 | 26.7 | 2.2# |
| Non-Hodgkin lymphoma | 6 | 23.3 | 0.1 | −0.7# | 6 | 23.9 | 0.0 | 6 | 17.0 | 0.0 | 7 | 14.8 | −0.5 | 7 | 16.0 | −1.2 | 6 | 19.3 | −0.7 | 6 | 23.7 | 0.2 |
| Kidney and renal pelvis | 7 | 21.2 | 2.0# | 0.9 | 7 | 21.2 | 2.0# | 4 | 23.3 | 2.9# | 9 | 10.1 | 2.7# | 4 | 29.0 | 3.6# | 5 | 19.8 | 1.6# | 7 | 21.4 | 2.1# |
| Oral cavity and pharynx | 8 | 16.4 | 0.2 | 0.2 | 8 | 16.5 | 0.5# | 9 | 15.7 | −2.7# | 8 | 10.7 | −0.6 | 9 | 12.7 | 0.2 | 11 | 10.4 | −1.4# | 8 | 17.1 | 0.5# |
| Leukemia | 9 | 16.1 | −1.1# | −1.1# | 9 | 16.5 | −1.3# | 12 | 12.3 | −1.0# | 11 | 8.5 | −1.6# | 10 | 11.8 | 1.0 | 9 | 12.0 | −1.3# | 9 | 16.3 | −1.0# |
| Pancreas | 10 | 13.6 | 0.8# | 0.8# | 10 | 13.4 | 0.9# | 7 | 16.9 | 0.9# | 10 | 9.6 | 0.1 | 11 | 10.8 | 1.7 | 10 | 11.5 | −0.4 | 10 | 13.8 | 1.0# |
| Liver and intrahepatic bile duct | 11 | 10.3 | 4.0# | 4.0# | 11 | 9.1 | 4.0# | 10 | 15.0 | 5.2# | 4 | 21.6 | −0.1 | 6 | 16.4 | 4.4# | 7 | 17.5 | 2.6# | 11 | 9.7 | 4.0# |
| Stomach | 12 | 9.4 | −1.8# | −1.8# | 13 | 8.4 | −2.0# | 8 | 16.3 | −1.4# | 5 | 16.1 | −3.2# | 8 | 13.0 | −3.8 | 8 | 13.5 | −2.5# | 12 | 9.0 | −1.9# |
| Esophagus | 13 | 8.7 | 0.0 | 0.0 | 12 | 8.8 | 0.6# | 14 | 9.2 | −4.7# | 15 | 3.8 | −1.1 | 12 | 7.3 | −2.4 | 15 | 5.5 | −1.1 | 13 | 9.0 | 0.2 |
| Brain and other nervous system | 14 | 7.9 | −0.4# | −0.4# | 14 | 8.4 | −0.4# | 15 | 4.7 | −0.4 | 13 | 4.3 | 0.4 | 16 | 5.3 | −0.1 | 13 | 5.9 | −1.3# | 14 | 8.1 | −0.3 |
| Myeloma | 15 | 7.2 | 0.1 | −0.7# | 15 | 6.6 | −0.2 | 11 | 13.6 | 0.4 | 14 | 4.1 | 1.2# | 15 | 6.0 | −4.5# | 12 | 6.6 | −0.8 | 15 | 7.2 | 0.1 |
| Larynx | 16 | 6.8 | −2.4# | −2.4# | 16 | 6.6 | −2.5# | 13 | 10.3 | −3.1# | 18 | 2.2 | −4.8# | 14 | 6.2 | −0.4 | 14 | 5.5 | −3.3# | 16 | 6.9 | −2.2# |
| Thyroid | 17 | 6.0 | 6.5# | 6.5# | 17 | 6.3 | 6.6# | 18 | 3.2 | 5.6# | 12 | 5.3 | 6.0# | 19 | 3.3 | — | 17 | 4.6 | 4.4# | 17 | 6.3 | 6.8# |
| 18 most common cancers for women | ||||||||||||||||||||||
| Breast | 1 | 122.3 | −0.9# | 0.5 | 1 | 123.3 | −1.0# | 1 | 118.0 | 0.7# | 1 | 85.9 | 0.7# | 1 | 89.1 | −0.4 | 1 | 93.0 | −0.2 | 1 | 125.1 | −0.8# |
| Lung and bronchus | 2 | 55.9 | −0.2 | −1.1# | 2 | 57.5 | −0.1 | 2 | 51.3 | 0.2 | 3 | 28.1 | 0.1 | 2 | 49.5 | −0.7 | 3 | 26.6 | −0.6 | 2 | 58.4 | 0.0 |
| Colon and rectum | 3 | 40.3 | −2.8# | −3.4# | 3 | 39.2 | −3.0# | 3 | 48.0 | −2.1# | 2 | 32.1 | −1.6# | 3 | 41.1 | −0.3 | 2 | 33.3 | −2.4# | 3 | 40.9 | −2.8# |
| Corpus and uterus, NOS | 4 | 24.5 | 0.6# | 0.6# | 4 | 24.9 | 0.4 | 4 | 22.5 | 2.2# | 5 | 16.9 | 2.7# | 4 | 22.1 | 2.3 | 4 | 20.1 | 1.2# | 4 | 24.8 | 0.6# |
| Thyroid | 5 | 17.7 | 7.0# | 7.0# | 5 | 18.5 | 7.0# | 8 | 10.8 | 6.1# | 4 | 18.4 | 6.5# | 8 | 11.6 | 5.4# | 5 | 17.2 | 6.7# | 5 | 17.9 | 7.0# |
| Non-Hodgkin lymphoma | 6 | 16.2 | −0.2 | −1.0# | 7 | 16.7 | −0.3 | 7 | 11.6 | 0.1 | 6 | 10.5 | −0.6 | 6 | 13.5 | −0.7 | 6 | 15.3 | 0.5 | 7 | 16.3 | −0.3 |
| Melanoma of the skin | 7 | 15.8 | 1.9# | 1.9# | 6 | 17.9 | 2.0# | 29 | 1.0 | −0.1 | 21 | 1.2 | −0.8 | 14 | 5.5 | 1.9 | 18 | 4.1 | −0.7 | 6 | 17.2 | 2.3# |
| Ovary | 8 | 12.5 | −1.9# | −1.9# | 8 | 12.9 | −2.0# | 11 | 9.5 | −1.0# | 8 | 9.2 | −1.3# | 7 | 11.8 | −2.2 | 9 | 11.1 | −1.5# | 8 | 12.6 | −1.9# |
| Kidney and renal pelvis | 9 | 11.1 | 2.5# | 1.2# | 9 | 11.2 | 2.5# | 6 | 12.1 | 3.6# | 13 | 5.1 | 3.0# | 5 | 16.6 | 2.9# | 8 | 11.4 | 2.6# | 9 | 11.1 | 2.5# |
| Pancreas | 10 | 10.5 | 0.9# | 0.9# | 10 | 10.2 | 0.9# | 5 | 13.9 | 0.4 | 9 | 8.1 | 0.1 | 10 | 9.1 | −1.6 | 10 | 9.9 | 0.1 | 10 | 10.6 | 0.9# |
| Leukemia | 11 | 9.7 | −0.8# | −0.8# | 11 | 10.0 | −0.9# | 13 | 7.7 | −1.3# | 12 | 5.9 | 0.4 | 12 | 7.5 | −0.7 | 11 | 8.5 | −0.5 | 11 | 9.7 | −0.8# |
| Urinary bladder | 12 | 9.3 | −1.0# | −1.0# | 12 | 9.7 | −1.1# | 14 | 6.6 | −0.7 | 15 | 3.8 | −1.6 | 18 | 4.3 | −2.8 | 14 | 5.3 | −2.3# | 12 | 9.6 | −0.9# |
| Cervix uteri | 13 | 8.1 | −2.0# | −0.6 | 13 | 7.8 | −1.9# | 9 | 10.4 | −3.0# | 11 | 7.2 | −3.0# | 9 | 10.1 | 0.4 | 7 | 11.8 | −3.6# | 13 | 7.7 | −1.8# |
| Oral cavity and pharynx | 14 | 6.2 | 0.0 | 0.0 | 14 | 6.2 | 0.1 | 15 | 5.3 | −0.7 | 14 | 5.0 | −2.5# | 15 | 5.0 | −2.1 | 17 | 4.2 | −1.4 | 14 | 6.4 | 0.1 |
| Brain and other nervous system | 15 | 5.7 | −0.2 | −0.2 | 15 | 6.1 | −0.3 | 17 | 3.7 | 0.5 | 16 | 3.0 | −0.2 | 17 | 4.5 | −3.9 | 16 | 4.7 | −0.9 | 15 | 5.9 | −0.1 |
| Myeloma | 16 | 4.7 | −0.5# | −0.5# | 16 | 4.1 | −0.7# | 10 | 9.8 | −0.2 | 17 | 2.8 | −1.8# | 16 | 4.5 | −3.9 | 15 | 4.8 | −1.3# | 16 | 4.7 | −0.4# |
| Stomach | 17 | 4.7 | −1.2# | −1.9# | 17 | 4.0 | −1.5# | 12 | 8.2 | −1.8# | 7 | 9.3 | −2.9# | 13 | 6.4 | −4.4# | 12 | 8.1 | −2.2# | 17 | 4.3 | −1.5# |
| Liver and intrahepatic bile duct | 18 | 3.4 | 2.9# | 2.9# | 18 | 3.1 | 2.8# | 16 | 4.2 | 3.5# | 10 | 8.1 | −0.5 | 11 | 7.6 | 4.1 | 13 | 6.6 | 2.0# | 18 | 3.2 | 2.8# |
* Source: National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) areas reported by the North American Association of Central Cancer Registries (NAACCR) as meeting high-quality incidence data standards for the specified time periods. The rates for all races/ethnicities for the period from 2005 to 2009 are from 47 states: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Washington, West Virginia, Wyoming. The AAPCs for all races/ethnicities for the period from 2000 to 2009 are from 42 states: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Texas, Utah, Vermont, Washington, West Virginia, Wyoming. AAPC = average annual percent change; APC = annual percent change; API = Asian/Pacific Islander; AI/AN = American Indian/Alaska Native; CHSDA = Indian Health Services Contract Health Services Delivery Area; NOS = not otherwise specified; — = statistic could not be calculated because the AAPC is based on less than 10 cases for at least 1 year within the time interval.
† Cancers are sorted in descending order according to sex-specific rates for all races/ethnicities. To include the 15 most common cancers in each racial and ethnic group, more than 15 cancers are given for men and women in total.
‡ White, black, API, and AI/AN (CHSDA counties) include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive. AI/AN (CHSDA) statistics exclude data from Kansas and Minnesota.
§ Rates are per 100 000 persons and were age adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, … , 80–84 years, and ≥85 years; Census publication p25-1130; US Bureau of the Census, Current Population Reports. Washington, DC: US Government Printing Office, 2000).
|| AAPC is the average annual percent change and is a weighted average of the APCs calculated by Joinpoint over the time period from 2000 to 2009 unless otherwise noted. Joinpoint analyses with up to two joinpoints are based on age-adjusted rates (see above). Joinpoint Regression Program, Version 3.5.1 July 2011, Surveillance Research Program, National Cancer Institute.
¶ For all sites, myelodysplastic syndromes are included for the rate calculations but not for the APC calculations; they are excluded from cancer-specific analysis. Ovary excludes borderline tumors.
# AAPC is statistically significantly different from zero (two-sided Z test; P < .05).
During the period from 2000 to 2009, incidence rates for all cancers combined declined among men of each racial and ethnic group, although the decrease was not statistically significant for AI/AN men. In contrast, rates of all cancers combined among women decreased only in whites and Hispanics. Childhood cancer incidence rates among those aged 0 to 19 years increased for black and Hispanic children but were stable for children of all other racial and ethnic groups; however, blacks had the lowest rates of any racial and ethnic group. Prostate cancer incidence rates declined among men of all racial and ethnic groups. Breast cancer incidence rates declined during the period from 2000 to 2009 among white women but increased among black and API women and were stable among AI/AN and among Hispanic women; however, in the most recent 5-year period (2005–2009), rates were stable among women of all racial and ethnic groups. Lung cancer incidence rates from 2000 to 2009 declined in men and were stable among women of all racial and ethnic groups, although rates decreased among all women from 2005 to 2009. Colorectal cancer incidence rates from 2000 to 2009 decreased among both men and women of every racial and ethnic group, although this decrease was not statistically significant for AI/AN men or women. Liver cancer incidence rates increased among white, black, AI/AN, and Hispanic men and among white, black, and Hispanic women. Pancreas cancer incidence rates increased only among white men and women and black men. Kidney and thyroid cancer incidence rates increased among men and women of every racial and ethnic group, except the thyroid cancer incidence rate did not increase among AI/AN men. Uterine cancer incidence rates increased among women of all racial and ethnic groups, although increases were not statistically significant among white and AI/AN women.
Current Cancer Death Rates (2005–2009) and Short-Term (2000–2009) Trends by Race and Ethnicity
For individuals of all racial and ethnic groups, overall cancer death rates declined during the most recent 10-year time period (2000–2009) among both sexes combined (1.5% per year) and among children aged 0 to 19 years (2.0% per year), although the decreases were not statistically significant among AI/AN persons (Table 4). Similarly, death rates for the most common cancers (lung, colorectal, and prostate) among men decreased in all racial and ethnic groups, except among AI/AN men, in whom the decreases for lung and colorectal cancers were not statistically significant. Among women, death rates for lung, breast and colorectal cancers decreased in all racial and ethnic groups, except among AI/AN women for all three cancers and among API women for lung cancer. Death rates increased for liver cancer in white, black, and Hispanic men and among white and Hispanic women, whereas rates decreased among API men and women. Pancreatic cancer death rates increased among white men and women and API men, whereas they were stable among the other population subgroups. Melanoma death rates increased only among white men.
Table 4.
US cancer death rates for the period from 2005 to 2009 and fixed-interval trends from 2000 to 2009 for the most common cancers by sex, race, and ethnicity*
| All racial and ethnic groups combined | White† | Black† | API† | AI/AN (CHSDA)† | Hispanic†,‡ | Non-Hispanic†,‡ | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Site or Type by sex§ | Rank | Rate|| | 2000– 2009 AAPC¶ | 2005– 2009 AAPC¶ | Rank | Rate|| | 2000– 2009 AAPC¶ | Rank | Rate|| | 2000– 2009 AAPC¶ | Rank | Rate|| | 2000– 2009 AAPC¶ | Rank | Rate|| | 2000– 2009 AAPC¶ | Rank | Rate|| | 2000– 2009 AAPC¶ | Rank | Rate|| | 2000– 2009 AAPC¶ |
| All sites | ||||||||||||||||||||||
| Both sexes | 178.7 | −1.5# | −1.5# | 177.6 | −1.5# | 216.4 | −2.0# | 109.5 | −1.3# | 156.2 | −0.7 | 119.4 | −1.8# | 183.2 | −1.4# | |||||||
| Men | 219.4 | −1.8# | −1.8# | 216.7 | −1.7# | 288.3 | −2.4# | 132.6 | −1.5# | 184.9 | −0.8 | 146.4 | −2.3# | 224.8 | −1.7# | |||||||
| Women | 151.1 | −1.4# | −1.4# | 150.8 | −1.4# | 174.6 | −1.5# | 93.2 | −1.1# | 135.9 | −0.8 | 100.6 | −1.4# | 155.1 | −1.4# | |||||||
| Children (aged 0–14 y) | 2.2 | −2.0# | −2.0# | 2.3 | −2.0# | 2.2 | −1.5 | 1.8 | −3.8# | 1.5 | ** | 2.3 | −2.2# | 2.2 | −1.8# | |||||||
| Children (aged 0–19 y) | 2.5 | −2.0# | −2.0# | 2.5 | −2.0# | 2.5 | −1.9# | 2.1 | −2.2# | 2.0 | −1.8 | 2.7 | −1.9# | 2.4 | −2.1# | |||||||
| 17 most common cancers for men§ | ||||||||||||||||||||||
| Lung and bronchus | 1 | 65.7 | −2.3# | −2.8# | 1 | 65.3 | −2.2# | 1 | 82.6 | −3.0# | 1 | 35.9 | −1.5# | 1 | 48.3 | −0.4 | 1 | 30.8 | −3.3# | 1 | 68.4 | −2.1# |
| Prostate | 2 | 23.6 | −3.5# | −3.5# | 2 | 21.7 | −3.4# | 2 | 53.1 | −3.7# | 4 | 10.0 | −2.8# | 2 | 19.7 | −2.9# | 2 | 17.8 | −3.8# | 2 | 23.8 | −3.4# |
| Colon and rectum | 3 | 20.2 | −3.1# | −3.1# | 3 | 19.5 | −3.0# | 3 | 29.8 | −2.3# | 3 | 13.1 | −2.8# | 3 | 18.8 | −2.3 | 3 | 15.3 | −1.9# | 3 | 20.5 | −3.0# |
| Pancreas | 4 | 12.5 | 0.4# | 0.4# | 4 | 12.4 | 0.5# | 4 | 15.5 | −0.1 | 6 | 8.4 | 1.0# | 5 | 10.1 | 2.2 | 5 | 9.2 | 0.2 | 4 | 12.8 | 0.5# |
| Leukemia | 5 | 9.6 | −0.9# | −0.9# | 5 | 9.9 | −0.9# | 7 | 8.5 | −0.8 | 8 | 4.9 | −0.9 | 9 | 6.3 | 1.9 | 8 | 5.9 | −2.0# | 5 | 9.8 | −0.8# |
| Non-Hodgkin lymphoma | 6 | 8.4 | −2.6# | −1.9# | 6 | 8.7 | −2.7# | 10 | 6.1 | −1.8# | 7 | 5.2 | −2.2# | 10 | 5.0 | −4.0 | 7 | 6.3 | −2.6# | 6 | 8.5 | −2.6# |
| Liver and intrahepatic bile duct | 7 | 8.1 | 2.6# | 2.6# | 9 | 7.4 | 2.6# | 5 | 11.9 | 3.4# | 2 | 14.5 | −1.2# | 4 | 11.9 | 1.6 | 4 | 11.8 | 1.5# | 9 | 7.8 | 2.6# |
| Esophagus | 8 | 7.7 | −0.4 | −1.2# | 8 | 7.9 | 0.3 | 8 | 8.2 | −4.5# | 9 | 3.0 | −0.2 | 8 | 6.4 | −1.0 | 10 | 4.2 | −0.7 | 7 | 8.0 | −0.2 |
| Urinary bladder | 9 | 7.7 | 0.1 | 0.1 | 7 | 8.0 | 0.2 | 13 | 5.6 | −0.1 | 12 | 2.7 | −0.7 | 12 | 3.6 | — | 11 | 3.8 | −1.5 | 8 | 7.9 | 0.4# |
| Kidney and renal pelvis | 10 | 5.8 | −0.9# | −0.9# | 10 | 5.9 | −0.9# | 11 | 6.0 | −0.7 | 11 | 2.9 | 2.7 | 6 | 8.8 | −1.1 | 9 | 5.0 | −1.5 | 10 | 5.9 | −0.8# |
| Brain and other nervous system | 11 | 5.2 | −0.5 | 0.4 | 11 | 5.6 | −0.4 | 15 | 3.1 | −0.8 | 13 | 2.3 | −1.4 | 14 | 2.9 | 2.7 | 13 | 3.3 | −1.0 | 11 | 5.4 | −0.3 |
| Stomach | 12 | 5.0 | −3.3# | −3.3# | 13 | 4.3 | −3.5# | 6 | 10.3 | −2.9# | 5 | 9.0 | −3.6# | 7 | 8.3 | −3.7 | 6 | 7.4 | −4.0# | 12 | 4.8 | −3.4# |
| Myeloma | 13 | 4.4 | −1.2# | −1.2# | 14 | 4.1 | −1.2# | 9 | 8.0 | −1.2# | 14 | 2.1 | 2.5# | 11 | 3.8 | −1.7 | 12 | 3.3 | −2.5# | 13 | 4.4 | −1.0# |
| Melanoma of the skin | 14 | 4.1 | 1.0# | 1.0# | 12 | 4.6 | 1.1# | 21 | 0.5 | 1.0 | 20 | 0.5 | — | 16 | 1.7 | — | 17 | 1.0 | 0.5 | 14 | 4.4 | 1.2# |
| Oral cavity and pharynx | 15 | 3.8 | −1.3# | −1.3# | 15 | 3.6 | −0.8# | 12 | 5.7 | −3.6# | 10 | 3.0 | −2.8# | 13 | 3.5 | −3.1 | 14 | 2.4 | −2.9# | 15 | 3.9 | −1.0# |
| Larynx | 16 | 2.1 | −2.7# | −2.7# | 16 | 2.0 | −2.5# | 14 | 4.2 | −3.4# | 16 | 0.8 | −2.2 | 15 | 2.0 | — | 15 | 1.6 | −3.2# | 16 | 2.1 | −2.6# |
| Soft tissue including heart | 17 | 1.5 | 0.1 | 1.2# | 18 | 1.5 | 0.0 | 16 | 1.4 | 0.1 | 15 | 1.0 | 1.5 | 17 | 1.2 | — | 16 | 1.0 | −0.3 | 18 | 1.5 | 0.1 |
| 18 most common cancers for women§ | ||||||||||||||||||||||
| Lung and bronchus | 1 | 39.6 | −0.7# | −1.3# | 1 | 40.8 | −0.6# | 1 | 38.0 | −1.0# | 1 | 18.5 | −0.3 | 1 | 33.2 | 0.0 | 2 | 14.1 | −1.0# | 1 | 41.8 | −0.6# |
| Breast | 2 | 23.0 | −2.1# | −2.1# | 2 | 22.4 | −2.1# | 2 | 31.6 | −1.4# | 2 | 11.9 | −1.3# | 2 | 16.6 | 0.4 | 1 | 14.9 | −1.6# | 2 | 23.7 | −2.0# |
| Colon and rectum | 3 | 14.1 | −3.1# | −3.1# | 3 | 13.6 | −3.1# | 3 | 19.8 | −3.0# | 3 | 9.6 | −1.6# | 3 | 14.6 | 1.1 | 3 | 10.2 | −2.0# | 3 | 14.4 | −3.0# |
| Pancreas | 4 | 9.5 | 0.4# | 0.4# | 4 | 9.3 | 0.5# | 4 | 12.6 | −0.3 | 4 | 6.9 | −0.2 | 4 | 7.9 | 0.1 | 4 | 7.5 | −0.3 | 4 | 9.6 | 0.5# |
| Ovary | 5 | 8.2 | −1.5# | −2.5# | 5 | 8.6 | −1.5# | 6 | 6.8 | −0.8 | 7 | 5.0 | −0.1 | 5 | 6.8 | −1.5 | 5 | 5.9 | −1.1# | 5 | 8.4 | −1.5# |
| Leukemia | 6 | 5.3 | −1.4# | −1.4# | 6 | 5.5 | −1.4# | 8 | 4.8 | −1.6# | 9 | 3.1 | 0.5 | 11 | 3.3 | −3.8 | 9 | 3.9 | −0.8 | 6 | 5.4 | −1.5# |
| Non-Hodgkin lymphoma | 7 | 5.2 | −3.5# | −3.1# | 7 | 5.4 | −3.5# | 12 | 3.6 | −2.9# | 8 | 3.4 | −2.7# | 7 | 4.5 | −3.3 | 7 | 4.3 | −2.3# | 7 | 5.3 | −3.4# |
| Corpus and uterus, NOS | 8 | 4.2 | 0.2 | 0.2 | 8 | 3.9 | 0.1 | 5 | 7.3 | 0.5 | 10 | 2.6 | 1.5# | 12 | 3.0 | — | 10 | 3.3 | 0.6 | 8 | 4.2 | 0.2 |
| Brain and other nervous system | 9 | 3.5 | −0.5# | −0.5# | 9 | 3.8 | −0.4 | 16 | 2.1 | −0.5 | 12 | 1.5 | −0.2 | 15 | 2.0 | — | 12 | 2.4 | −0.1 | 9 | 3.6 | −0.5# |
| Liver and intrahepatic bile duct | 10 | 3.3 | 1.5# | 1.5# | 10 | 3.1 | 1.7# | 11 | 4.0 | 1.0 | 5 | 6.1 | −2.1# | 6 | 5.9 | −0.5 | 6 | 5.3 | 1.0# | 10 | 3.1 | 1.3# |
| Myeloma | 11 | 2.7 | −2.6# | −2.6# | 12 | 2.5 | −2.5# | 7 | 5.4 | −2.9# | 13 | 1.4 | −2.3 | 13 | 2.5 | −5.1 | 13 | 2.3 | −2.9# | 11 | 2.8 | −2.5# |
| Kidney and renal pelvis | 12 | 2.6 | −1.3# | −2.1# | 11 | 2.7 | −1.3# | 13 | 2.6 | −0.8 | 14 | 1.3 | 0.4 | 8 | 4.1 | 0.1 | 14 | 2.3 | −0.7 | 12 | 2.6 | −1.2# |
| Stomach | 13 | 2.6 | −3.1# | −3.1# | 13 | 2.2 | −3.2# | 9 | 4.8 | −3.8# | 6 | 5.3 | −3.9# | 9 | 3.8 | −6.4# | 8 | 4.3 | −3.0# | 13 | 2.4 | −3.3# |
| Cervix uteri | 14 | 2.4 | −2.0# | −1.2 | 15 | 2.2 | −1.9# | 10 | 4.3 | −2.6# | 11 | 2.0 | −4.4# | 10 | 3.5 | −0.6 | 11 | 3.0 | −3.2# | 14 | 2.3 | −2.0# |
| Urinary bladder | 15 | 2.2 | −0.7# | −0.7# | 14 | 2.2 | −0.5 | 14 | 2.6 | −1.4 | 16 | 0.9 | −1.7 | 18 | 1.0 | — | 16 | 1.2 | −1.7 | 15 | 2.3 | −0.5 |
| Esophagus | 17 | 1.6 | −1.9# | −1.9# | 17 | 1.6 | −1.2# | 15 | 2.2 | −5.3# | 17 | 0.9 | 0.3 | 16 | 1.5 | — | 18 | 0.8 | −3.8# | 17 | 1.7 | −1.7# |
| Oral cavity and pharynx | 18 | 1.4 | −1.9# | −1.9# | 18 | 1.4 | −1.7# | 17 | 1.4 | −3.2# | 15 | 1.3 | −2.8 | 17 | 1.3 | — | 19 | 0.7 | −1.6 | 18 | 1.4 | −1.8# |
| Gallbladder | 20 | 0.8 | −1.7# | −1.7# | 20 | 0.7 | −1.9# | 19 | 1.0 | −1.1 | 20 | 0.8 | −0.7 | 14 | 2.1 | −3.1 | 15 | 1.3 | −2.1 | 20 | 0.7 | −1.9# |
* Source: National Center for Health Statistics public-use data file for the total US, 1975−2009. AAPC = average annual percent change; API = Asian/Pacific Islander; AI/AN = American Indian/Alaska Native; CHSDA = Indian Health Services Contract Health Services Delivery Area; NOS = not otherwise specified; — = statistic could not be calculated because the AAPC is based on less than 10 cases for at least 1 year within the time interval.
† White, black, API, and AI/AN (CHSDA counties) populations include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive.
‡ Data for Hispanic and non-Hispanic exclude the District of Columbia, Minnesota, New Hampshire, North Dakota, and South Carolina.
§ Cancers are listed in descending rank order of sex-specific, age-adjusted incidence rates for the period from 2005 to 2009 for all racial and ethnic groups combined. To include the 15 most common cancers in each racial and ethnic group, more than 15 cancers are given for men and women in total.
|| Rates are per 100 000 persons and are age adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, . . . , 80–84 years, >85 years; Census publication; US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office, 2000).
¶ The AAPC is a weighted average of the annual percent change and is calculated by joinpoint analyses with up to two joinpoints yielding up to three trend segments based on age adjusted rates (see above). For joinpoint analysis, the Joinpoint Regression Program was used (version 3.5.1, July 2011; Surveillance Research Program, National Cancer Institute, Bethesda, MD).
# The AAPC is statistically significantly different from zero (two-sided Z test; P < .05).
HPV-Associated Cancer Incidence Rates (2005–2009) and Short-Term (2000–2009) Trends
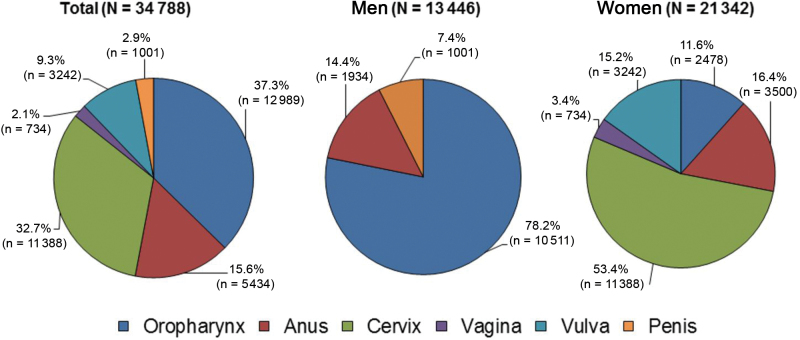
HPV-associated cancers accounted for 3.3% (21 342 of 646 684) of all cancer cases among women and 2.0% (13 446 of 676 672) of the total cancer cases among men diagnosed in 2009 in the combined SEER and NPCR databases (Figure 1). Cervical cancer alone represents 53.4% of the total number of HPV-associated cancers among women and 32.7% of all HPV-associated cancers. Oropharyngeal cancer accounts for 78.2% of HPV-associated cancers among men, 11.6% of HPV-associated cancers among women, and 37.3% of HPV-associated cancers among men and women combined.
Figure 1.
Number of new human papillomavirus (HPV)–associated cancers overall, and by sex, in the United States, 2009. Source: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results areas reported by the North American Association of Central Cancer Registries as meeting high-quality incidence data standards for the specified time period. Note that the number of cancer cases underestimates the actual number of cases occurring because of incomplete coverage of population-based registries in 2009 (93%). HPV-associated cancers are defined as cancers at specific anatomic sites and with specific cellular types in which HPV DNA frequently is found. Some of these cancers may not necessarily be HPV-positive because no testing was conducted. Virtually all cervical cancers are due to HPV infection, along with 90% of anal cancers, more than 60% of certain subsites of oropharyngeal cancers, and approximately 40% of vagina, vulva, and penile cancers.
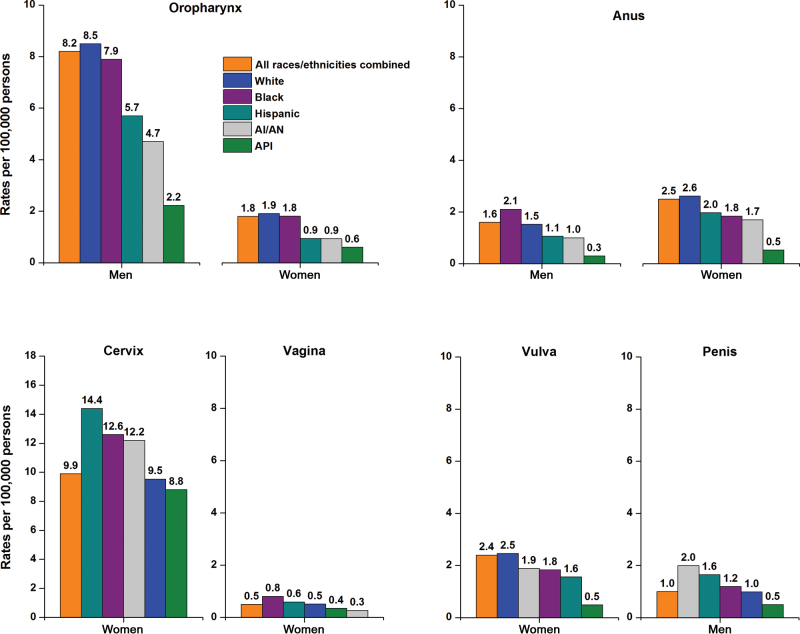
Incidence rates for HPV-associated oropharyngeal cancers (2005–2009) were highest among white and black men (Figure 2). Among women, the highest incidence rates occurred among blacks and whites for oropharyngeal cancers, among whites for anal and vulvar cancers, among blacks for vaginal cancer, and among Hispanics for cervical cancer. Anal cancer incidence rates were highest among black men. API men and women demonstrated the lowest incidence rates for each of the HPV-associated cancers, except for vaginal cancer, which was lowest among AI/AN women. Cervical cancer rates were markedly elevated among most women living in low vs high socioeconomic status areas (Table 5), and similar disparities were noted among men for HPV-associated cancers of the anus and penis. No striking socioeconomic status disparities were apparent for the other HPV-associated cancers among men or women.
Figure 2.
Age-adjusted incidence rates for human papillomavirus (HPV)–associated cancers in the United States by sex and race and ethnicity, 2005 to 2009. The scale of the y axis differs for cervical cancer. The rates for the period from 2005 to 2009 for the five major racial and ethnic groups are from 47 states: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Washington, West Virginia, Wyoming. Source: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results areas reported by the North American Association of Central Cancer Registries as meeting high-quality incidence data standards for the specified time period. HPV-associated cancers are defined as cancers at specific anatomic sites and with specific cellular types in which HPV DNA frequently is found. Some of these cancers may not necessarily be HPV-positive because no testing was conducted. White, black, Asian/Pacific Islander (API), and American Indian/Alaska Native (AI/AN) (IHS Contract Health Services Delivery Area counties) include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive.
Table 5.
Incidence of human papillomavirus (HPV)–associated cancers in the United States, by sex, race and ethnicity, and area-level socioeconomic status (SES) during the period from 2005 to 2009, for areas with high-quality incidence data*
| All racial and ethnic groups combined | White‡ | Black‡ | API‡ | AI/AN (CHSDA)‡ | Hispanic | |
|---|---|---|---|---|---|---|
| Cancer site by sex and area-level SES† | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) |
| Men | ||||||
| Oropharynx | ||||||
| <10% | 8.0 (7.8 to 8.1) | 8.3 (8.2 to 8.4) | 6.5 (6.0 to 7.1) | 2.3 (2.0 to 2.06) | 3.5 (1.9 to 6.3) | 5.0 (4.5 to 5.5) |
| 10.0%–19.9% | 8.5 (8.4 to 8.6) | 8.8 (8.7 to 8.9) | 8.2 (7.9 to 8.5) | 2.2 (1.9 to 2.5) | 6.5 (5.2 to 8.0) | 4.5 (4.3 to 4.8) |
| >20% | 7.8 (7.6 to 8.1) | 7.9 (7.6 to 8.2) | 8.7 (8.1 to 9.3) | 2.0 (1.3 to 2.9) | 4.9 (3.4 to 6.9) | 5.4 (4.8 to 5.9) |
| Anus | ||||||
| <10% | 1.3 (1.2 to 1.3) | 1.3 (1.2 to 1.3) | 1.6 (1.4 to 1.9) | 0.2 (0.1 to 0.4) | — | 0.8 (0.6 to 1.0) |
| 10.0%–19.9% | 1.7 (1.7 to 1.8) | 1.7 (1.6 to 1.8) | 2.1 (2.0 to 2.3) | 0.3 (0.2 to 0.4) | 1.3 (0.8 to 2.0) | 1.1 (1.0 to 1.2) |
| >20% | 2.0 (1.9 to 2.1) | 1.8 (1.7 to 2.0) | 2.7 (2.3 to 3.0) | 0.5 (0.2 to 1.1) | — | 1.3 (1.0 to 1.6) |
| Penis | ||||||
| <10% | 0.9 (0.8 to 0.9) | 0.9 (0.8 to 0.9) | 1.2 (0.9 to 1.5) | 0.4 (0.3 to 0.6) | — | 1.7 (1.4 to 2.0) |
| 10.0%–19.9% | 1.0 (1.0 to 1.0) | 1.0 (1.0 to 1.0) | 1.1 (1.0 to 1.3) | 0.5 (0.4 to 0.7) | 1.6 (0.9 to 2.6) | 1.4 (1.3 to 1.6) |
| >20% | 1.5 (1.3 to 1.6) | 1.4 (1.3 to 1.6) | 1.4 (1.2 to 1.7) | 0.9 (0.5 to 1.7) | 2.8 (1.5 to 4.7) | 2.4 (2.1 to 2.8) |
| Women | ||||||
| Oropharynx | ||||||
| <10% | 1.8 (1.7 to 1.8) | 1.8 (1.8 to 1.9) | 1.3 (1.2 to 1.6) | 0.7 (0.5 to 0.9) | — | 1.0 (0.8 to 1.3) |
| 10.0%–19.9% | 1.9 (1.9 to 1.9) | 2.0 (1.9 to 2.0) | 1.9 (1.8 to 2.0) | 0.6 (0.5 to 0.7) | 1.2 (0.7 to 1.9) | 0.9 (0.8 to 1.0) |
| >20% | 1.7 (1.6 to 1.9) | 1.7 (1.6 to 1.9) | 2.0 (1.7 to 2.2) | 0.6 (0.3 to 1.1) | — | 1.0 (0.8 to 1.3) |
| Anus | ||||||
| <10% | 2.4 (2.3 to 2.4) | 2.5 (2.4 to 2.6) | 1.6 (1.4 to 1.8) | 0.5 (0.4 to 0.7) | — | 2.0 (1.7 to 2.3) |
| 10.0%–19.9% | 2.5 (2.5 to 2.6) | 2.7 (2.7 to 2.8) | 1.9 (1.7 to 2.0) | 0.5 (0.4 to 0.7) | 1.9 (1.3 to 2.8) | 2.0 (1.8 to 2.1) |
| >20% | 2.4 (2.3 to 2.5) | 2.6 (2.4 to 2.8) | 2.0 (1.8 to 2.3) | 0.5 (0.2 to 1.1) | 1.4 (0.7 to 2.4) | 2.0 (1.7 to 2.3) |
| Cervix | ||||||
| <10% | 8.4 (8.3 to 8.6) | 8.2 (8.1 to 8.4) | 10.4 (9.9 to 11.0) | 7.5 (7.0 to 8.0) | 16.0 (12.2 to 20.8) | 13.5 (12.8 to 14.2) |
| 10.0%–19.9% | 10.5 (10.4 to 10.6) | 10.2 (10.0 to 10.3) | 12.7 (12.4 to 13.1) | 9.4 (8.9 to 9.9) | 11.6 (10.1 to 13.4) | 14.6 (14.2 to 15.0) |
| >20% | 12.4 (12.1 to 12.8) | 11.4 (11.0 to 11.8) | 14.9 (14.2 to 15.6) | 12.0 (10.3 to 13.9) | 11.7 (9.4 to 14.3) | 14.4 (13.6 to 15.2) |
| Vagina | ||||||
| <10% | 0.5 (0.4 to 0.5) | 0.4 (0.4 to 0.5) | 0.8 (0.6 to 0.9) | 0.4 (0.3 to 0.6) | — | 0.5 (0.3 to 0.7) |
| 10.0%–19.9% | 0.6 (0.5 to 0.6) | 0.5 (0.5 to 0.6) | 0.8 (0.8 to 0.9) | 0.3 (0.2 to 0.4) | — | 0.6 (0.5 to 0.7) |
| >20% | 0.7 (0.6 to 0.7) | 0.6 (0.5 to 0.7) | 0.8 (0.6 to 1.0) | 0.4 (0.2 to 0.8) | — | 0.6 (0.5 to 0.8) |
| Vulva | ||||||
| <10% | 2.4 (2.3 to 2.4) | 2.5 (2.4 to 2.5) | 1.6 (1.4 to 1.8) | 0.6 (0.4 to 0.8) | 2.3 (1.0 to 4.7) | 1.7 (1.4 to 2.0) |
| 10.0%–19.9% | 2.4 (2.4 to 2.5) | 2.5 (2.5 to 2.6) | 1.9 (1.7 to 2.0) | 0.4 (0.3 to 0.5) | 1.8 (1.2 to 2.6) | 1.5 (1.3 to 1.6) |
| >20% | 2.3 (2.2 to 2.5) | 2.5 (2.3 to 2.7) | 1.9 (1.7 to 2.2) | — | 1.8 (0.9 to 3.0) | 1.7 (1.4 to 2.0) |
* Source: National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) areas reported by the North American Association of Central Cancer Registries (NAACCR) as meeting high-quality incidence data standards for the specified time periods. HPV-associated cancers are defined as cancers at specific anatomic sites and with specific cellular types in which HPV DNA frequently is found. Some of these cancers may not necessarily be HPV positive because no testing was conducted. The rates for all races and ethnicities for the period from 2005 to 2009 are from 47 states: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Washington, West Virginia, Wyoming. AI/AN = American Indian/Alaska Native; API = Asian/Pacific Islander; CHSDA = Indian Health Service Contract Health Services Delivery Area; CI = confidence interval; — = statistic not given because of less than six cases.
† Area-level SES was defined using criteria discussed in the Methods section and presented as the percent of the population in the county of the cancer case’s diagnosis living beneath the poverty threshold. Areas with 20% or more of the population beneath the poverty threshold represent severely disadvantaged areas. Rates are per 100 000 persons and were age adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, . . . , 80–84 years, and >85 years; Census publication p25-1130, US Bureau of the Census, Current Population Reports, Washington, DC: US Government Printing Office, 2000). Analyses restricted to persons aged 15 years or older.
‡ White, black, API, and AI/AN (CHSDA counties) include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive.
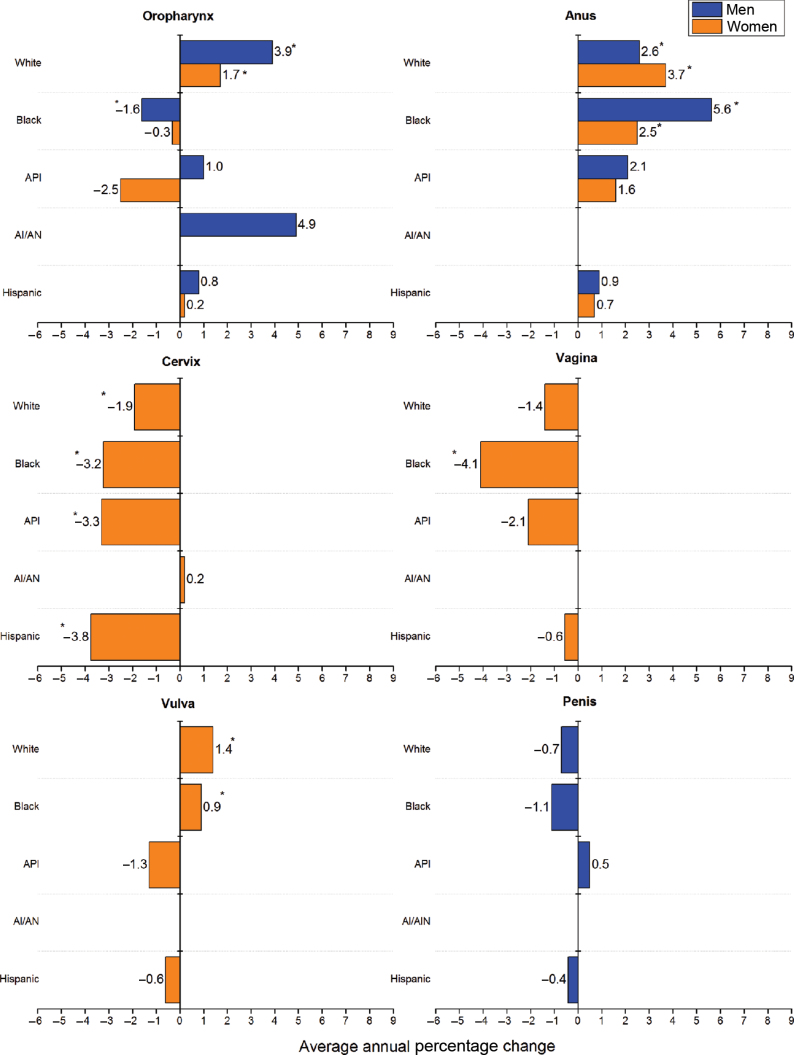
From 2000 to 2009, incidence rates increased for HPV-associated cancer of the oropharynx among white men and women, for anal cancer among white and black men and women, and for cancer of the vulva among white and black women (Figure 3). By age, the increases in incidence rates for these cancers were generally larger among persons aged 55 to 64 years than among younger or older individuals (data not shown). In contrast, cervical cancer incidence rates decreased for women in all racial and ethnic groups, except AI/AN women. Vaginal cancer incidence decreased among black women. Rates remained unchanged for penile cancer among men in all racial and ethnic groups.
Figure 3.
Trends in age-adjusted human papillomavirus (HPV)–associated cancer incidence rates by sex and race and ethnicity in the United States, 2000 to 2009. An asterisk indicates average annual percentage change was statistically significantly different from zero at P less than .05. Trends could not be determined for American Indians/Alaska Natives (AI/ANs) for cancers of anus, vagina, vulva, and penis because of sparse data. The rates for the period from 2005 to 2009 for the five major racial and ethnic groups are from 42 states: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Texas, Utah, Vermont, Washington, West Virginia, Wyoming. Source: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results areas reported by the North American Association of Central Cancer Registries as meeting high-quality incidence data standards for the specified time period. HPV-associated cancers are defined as cancers at specific anatomic sites and with specific cellular types in which HPV DNA frequently is found. Some of these cancers may not necessarily be HPV-positive because no testing was conducted. White, black, Asian/Pacific Islander (API), and AI/AN (IHS Contract Health Services Delivery Area counties) include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive.
HPV Vaccination Coverage Levels and Prevalence of Pap Testing by State, Race and Ethnicity, and Other Sociodemographic Factors
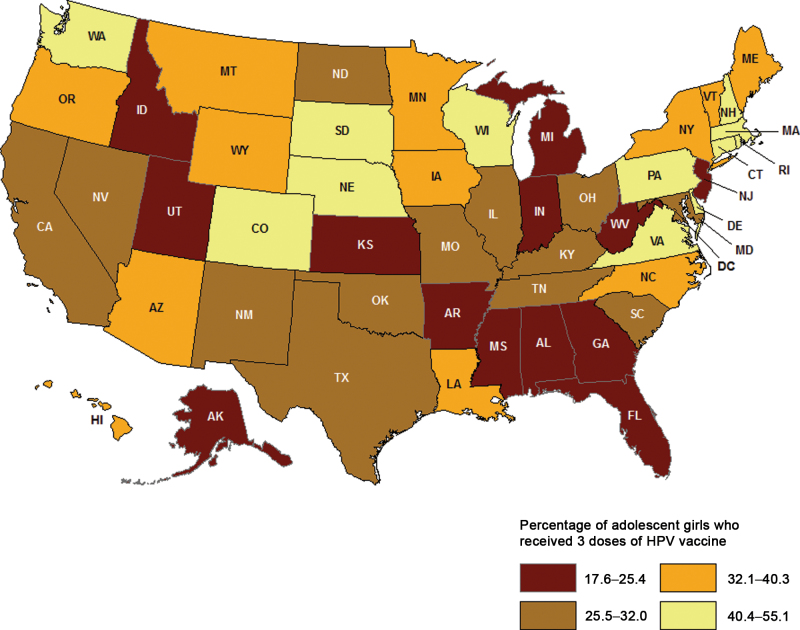
Nationally, less than half (48.7%, 95% CI = 46.9% to 50.5%) of adolescent girls aged 13 to 17 years in 2010 had received or more doses, and 32.0% (95% CI = 30.3% to 33.6%) had received three doses of the HPV vaccine (Table 6; Figure 4). Of the girls who initiated the series (≥1 dose) and had at least 24 weeks between their first dose and their NIS-Teen interview date, more than two-thirds (69.6%, 95% CI = 66.8% to 72.2%) completed the three-dose series. State-level HPV vaccination coverage levels varied widely in 2010, ranging from 28.8% (95% CI = 21.6% to 37.3%) in Idaho to 73.0% (95% CI = 64.6% to 80.0%) in Rhode Island for one or more doses, from 17.6% (95% CI = 11.8% to 25.4%) in Idaho to 55.1% (95% CI = 46.0% to 63.9%) in Rhode Island for three doses, and from 47.4% (95% CI = 34.3% to 60.9%) in Alabama to 87.1% (95% CI = 77.1 to 93.1%) in New Hampshire for three-dose series completion, with coverage in many states being statistically significantly lower than the national estimate (Supplementary Table 2, available online). In 2010, three-dose coverage was statistically significantly lower in some Southern states (eg, 20.0% in both Alabama [95% CI = 13.9% to 27.9%] and Mississippi [95% CI = 13.8% to 28.2%]) compared with the national coverage level of 32.0% (Supplementary Table 2, available online). Between 2008 and 2010, overall initiation of the HPV vaccination series statistically significantly increased by 11.5 (95% CI = 8.7 to 14.3) percentage points, and receipt of three doses increased by 14.1 (95% CI = 11.8 to 16.4) percentage points nationally (Supplementary Table 1, available online). Vaccination coverage rates for one or more doses and three doses increased in most states between 2008 and 2010.
Table 6.
Human papillomavirus (HPV) vaccination coverage among adolescent girls, aged 13 to 17 years, by select sociodemographic characteristics, National Immunization Survey-Teen, United States, 2008 and 2010*
| ≥1 dose† | 3 dose | 3-dose series completion‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2008 | 2010 | Difference | 2008 | 2010 | Difference | 2008 | 2010 | Difference | |
| % (95% CI)§ | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |
| Overall | 37.2 (35.1 to 39.3) | 48.7 (46.9 to 50.5) | 11.5 (8.7 to 14.3)|| | 17.9 (16.3 to 19.6) | 32.0 (30.3 to 33.6) | 14.1 (11.8 to 16.4)|| | 59.6 (55.5 to 63.5) | 69.6 (66.8 to 72.2) | 10.0 (5.2 to 14.8)|| |
| Age at interview, y | |||||||||
| 13 | 35.2 (31.1 to 39.6) | 38.9 (34.9 to 43.1) | 3.7 ( to 2.2 to 9.6) | 14.5 (11.9 to 17.5) | 23.2 (20.1 to 26.6) | 8.7 (4.4 to 13.0)|| | 53.1 (44.3 to 61.7) | 64.1 (55.9 to 71.5) | 11.0 (−0.8 to 22.8) |
| 14 | 33.8 (29.5 to 38.3) | 48.5 (44.5 to 52.6) | 14.7 (8.7 to 20.7)|| | 16.6 (13.5 to 20.2) | 30.5 (26.9 to 34.3) | 13.9 (8.9 to 18.9)|| | 62.9 (53.9 to 71.2) | 68.2 (61.7 to 74.0) | 5.3 (−5.4 to 16.0) |
| 15 | 42.2 (37.5 to 47.2) | 51.1 (47.0 to 55.3) | 8.9 (2.5 to 15.3)|| | 18.5 (15.5 to 21.8) | 31.9 (28.3 to 35.6) | 13.4 (8.6 to 18.2)|| | 54.1 (45.7 to 62.3) | 65.6 (59.4 to 71.3) | 11.5 (1.2 to 21.8)|| |
| 16 | 35.7 (31.7 to 39.9) | 51.7 (47.8 to 55.7) | 16.0 (10.3 to 21.7)|| | 18.8 (15.6 to 22.4) | 36.9 (33.2 to 40.8) | 18.1 (13.0 to 23.2)|| | 65.2 (57.8 to 71.9) | 74.3 (69.4 to 78.7) | 9.1 (0.6 to 17.6)|| |
| 17 | 38.5 (33.3 to 43.9) | 53.1 (49.1 to 57.1) | 14.6 (8.0 to 21.2)|| | 20.9 (16.3 to 26.3) | 37.5 (33.7 to 41.5) | 16.6 (10.3 to 22.9)|| | 63.1 (52.9 to 72.3) | 74.6 (68.8 to 79.6) | 11.5 (0.3 to 22.7)|| |
| Race/ethnicity¶ | |||||||||
| White, non-Hispanic | 35.0 (32.9 to 37.2) | 45.8 (43.8 to 47.9) | 10.8 (7.9 to 13.7)|| | 19.5 (17.8 to 21.4) | 32.4 (30.6 to 34.2) | 12.9 (10.3 to 15.5)|| | 67.9 (63.9 to 71.6) | 74.7 (71.6 to 77.5) | 6.8 (1.9 to 11.7)|| |
| Black, non-Hispanic | 35.7 (29.6 to 42.4) | 48.9 (43.8 to 54.1) | 13.2 (4.9 to 21.5)|| | 14.9 (9.7 to 22.3) | 30.2 (25.5 to 35.4) | 15.3 (7.3 to 23.3)|| | 48.5 (34.9 to 62.3) | 65.4 (57.5 to 72.5) | 16.9 (1.0 to 32.8)|| |
| Hispanic | 44.4 (38.0 to 50.9) | 56.2 (50.6 to 61.6) | 11.8 (3.3 to 20.3)|| | 14.7 (11.4 to 18.8) | 29.5 (25.0 to 34.4) | 14.8 (8.8 to 20.8)|| | 45.5 (34.9 to 56.4) | 56.1 (48.5 to 63.5) | 10.6 (−2.6 to 23.8) |
| American Indian/Alaska Native, non-Hispanic | 52.8 (35.4 to 69.6) | 64.8 (46.6 to 79.5) | 12.0 (−12.6 to 36.6) | — | 40.5 (26.7 to 56.0) | — | — | 64.0 (45.6 to 79.1) | — |
| Asian, non-Hispanic | 40.6 (27.6 to 55.2) | 50.1 (38.2 to 61.9) | 9.5 (−9.1 to 28.1) | — | 39.8 (28.3 to 52.5) | — | — | 86.0 (75.4 to 92.5) | — |
| Other | 40.1 (28.2 to 53.2) | 52.3 (44.0 to 60.5) | 12.2 (−3.0 to 27.4) | 20.2 (12.2 to 31.6) | 37.3 (29.9 to 45.3) | 17.1 (4.7 to 29.5)|| | 64.4 (46.1 to 79.4) | 75.4 (62.7 to 84.8) | 11.0 (−9.5 to 31.5) |
| Household poverty level# | |||||||||
| Above the poverty level | 35.8 (33.7 to 37.9) | 47.7 (45.7 to 49.6) | 11.9 (9.0 to 14.8)|| | 18.6 (17.0 to 20.3) | 32.9 (31.1 to 34.7) | 14.3 (11.9 to 16.7)|| | 63.7 (59.7 to 67.5) | 73.2 (70.3 to 76.0) | 9.5 (4.7 to 14.3)|| |
| Below the poverty level | 46.4 (39.8 to 53.1) | 51.8 (46.8 to 56.8) | 5.4 (−3.0 to 13.8) | 14.8 (9.6 to 22.2) | 28.2 (24.2 to 32.4) | 13.4 (6.0 to 20.8)|| | 41.1 (28.3 to 55.3) | 57.3 (50.1 to 64.2) | 16.2 (0.6 to 31.8)|| |
| Unknown | 31.1 (23.5 to 39.9) | 52.1 (44.2 to 59.8) | 21.0 (9.6 to 32.4)|| | 16.3 (11.4 to 22.8) | 33.0 (25.8 to 41.0) | 16.7 (7.2 to 26.2)|| | 66.9 (51.5 to 79.3) | 68.1 (56.2 to 78.1) | 1.2 (−16.8 to 19.2) |
| Maternal education level | |||||||||
| Less than 12 years | 39.4 (32.6 to 46.5) | 56.6 (50.3 to 62.7) | 17.2 (7.9 to 26.5)|| | 11.5 (8.1 to 16.1) | 28.0 (23.2 to 33.2) | 16.5 (10.1 to 22.9)|| | 38.1 (26.6 to 51.1) | 51.0 (42.8 to 59.1) | 12.9 (−2.0 to 27.8) |
| 12 years | 35.4 (30.9 to 40.0) | 47.4 (43.7 to 51.1) | 12.0 (6.1 to 17.9)|| | 16.2 (12.7 to 20.5) | 28.7 (25.4 to 32.2) | 12.5 (7.4 to 17.6)|| | 57.9 (48.8 to 66.4) | 65.8 (60.0 to 71.2) | 7.9 (−2.6 to 18.4) |
| More than 12 years, non-college graduate | 36.1 (32.6 to 39.6) | 46.7 (43.5 to 50.0) | 10.6 (5.8 to 15.4)|| | 17.6 (15.0 to 20.6) | 32.4 (29.3 to 35.6) | 14.8 (10.6 to 19.0)|| | 61.2 (54.1 to 67.8) | 73.7 (69.0 to 77.9) | 12.5 (4.3 to 20.7)|| |
| College graduate | 38.8 (35.8 to 41.9) | 48.0 (45.2 to 50.8) | 9.2 (5.1 to 13.3)|| | 22.3 (19.9 to 24.9) | 35.8 (33.2 to 38.4) | 13.5 (9.9 to 17.1)|| | 68.1 (62.7 to 73.1) | 78.4 (74.4 to 82.0) | 10.3 (3.9 to 16.7)|| |
| Health insurance | |||||||||
| VFC eligible, uninsured only | 19.1 (13.4 to 26.6) | 34.9 (26.7 to 44.2) | 15.8 (4.8 to 26.8)|| | — | 14.1 (9.4 to 20.6) | — | 54.8 (35.4 to 72.8) | 41.5 (27.9 to 56.6) | −13.3 (−37.9 to 11.3) |
| VFC eligible, all others | 42.7 (37.0 to 48.6) | 55.7 (51.6 to 59.8) | 13.0 (5.8 to 20.2)|| | 15.9 (11.4 to 21.7) | 31.6 (28.0 to 35.4) | 15.7 (9.4 to 22.0)|| | 46.8 (35.6 to 58.2) | 60.2 (54.1 to 66.1) | 13.4 (0.4 to 26.4)|| |
| Private/other insurance | 37.3 (35.1 to 39.5) | 47.7 (45.7 to 49.8) | 10.4 (7.4 to 13.4)|| | 19.2 (17.6 to 20.9) | 33.8 (32.0 to 35.7) | 14.6 (12.1 to 17.1)|| | 63.6 (59.3 to 67.7) | 75.2 (72.4 to 77.9) | 11.6 (6.6 to 16.6)|| |
*Adolescent girls (N = 17 827) in the 2008 and 2010 National Immunization Survey-Teen were born during the period from January 1990 to February 1996 and the period from January 1992 to February 1998, respectively. Source: National Immunization Survey-Teen 2008, 2010; National Center for Health Statistics, Centers for Disease Control and Prevention, 2010, 2011. CI = confidence interval; VFC = Vaccines for Children program; — = estimate not reported because unweighted sample size for the denominator was less than 30 or the confidence interval half-width/estimate was greater than 0.588.
† Either quadrivalent or bivalent human papillomavirus vaccine.
‡ Percentage of girls who received three doses among those who had at least one HPV dose and had at least 24 weeks between the first dose and the interview date.
§ Estimates with confidence interval widths greater than 20 might not be reliable.
|| The percentage point difference in vaccination coverage levels from 2008 to 2010 is statistically significantly different from zero (two-sided t test, P < .05).
¶ Sample adolescents who were reported by the adult respondent as Hispanic were of any race. Sample respondents who were reported as white, black, Asian, or American Indian/Alaska Native were all considered
non-Hispanic. Native Hawaiian, other Pacific Islanders, and persons of multiple races were categorized as other.
# Adolescents were classified as below poverty level if their total family income was less than the federal poverty level specified for the applicable family size and number of children aged less than 18 years. All others were classified
as at or above the poverty level. Additional information available at http://www.census.gov/hhes/www/poverty.html.
Figure 4.
Three-dose human pappilomavirus (HPV) vaccination coverage among girls (aged 13 to 17 years), by state, in the United States, 2010. Source: National Immunization Survey-Teen (NIS-Teen) 2010, National Center for Health Statistics, Centers for Disease Control and Prevention, 2011. Girls in the 2010 NIS-Teen were born during the period from January 1992 to February 1998 and received either quadrivalent or bivalent human papillomavirus vaccine (some girls received more than three doses).
Virtually all sociodemographic groups showed statistically significant increases in one or more–dose and three-dose coverage and series completion rates for HPV vaccination from 2008 to 2010 (P < .05), although not all the increases were statistically significant (Table 6). In 2010, HPV vaccination coverage with one or more doses and three doses, as well as series completion, increased with age (Table 6). Hispanics (56.2%, 95% CI = 50.6% to 61.6%) were statistically significantly more likely than non-Hispanic whites (45.8%, 95% CI = 43.8% to 47.9%) to have received one or more HPV vaccine doses by 2010. Girls who were VFC-eligible and insured (55.7%, 95% CI = 51.6% to 59.8%) were statistically significantly more likely than privately insured (47.7%, 95% CI = 45.7% to 49.8%) and VFC-eligible and uninsured girls (34.9%, 95% CI = 26.7% to 44.2%) to have received one or more HPV vaccine doses by 2010. Girls who were VFC-eligible and uninsured (14.1%, 95% CI = 9.4% to 20.6%) were also significantly less likely than privately insured (33.8%, 95% CI = 32.0% to 35.7%) girls to have received 3 doses of HPV vaccine by 2010. Among girls who initiated the series, Hispanics (56.1%, 95% CI = 48.5% to 63.5%) were statistically significantly less likely than non-Hispanic whites (74.7%, 95% CI = 71.6% to 77.5%), those living below the poverty level (57.3%, 95% CI = 50.1% to 64.2%) were statistically significantly less likely than those living above the poverty level (73.2%, 95% CI = 70.3% to 76.0%), and the privately insured (75.2, 95% CI = 72.4% to 77.9%) were statistically significantly more likely than VFC-eligible and insured girls (60.2%, 95% CI = 54.1% to 66.1%) and VFC-eligible and uninsured girls (41.5%, 95% CI = 27.9% to 56.6%) to complete the three-dose series by 2010.
Nationally, 86.7% (95% CI = 86.3% to 87.0%) of women aged 21 to 65 years had a recent Pap test (during the previous 3 years) in 2010 (Supplementary Table 2, available online). The prevalence of Pap testing varied by state, ranging from 80.2% (95% CI = 76.0% to 83.8%) in Arkansas to 93.0% (95% CI = 91.8% to 94.1%) in Massachusetts (where cervical cancer rates were low) (Figure 5). The prevalence of Pap testing was positively correlated with vaccination coverage levels (r = 0.47, P < .01) (Figure 5) but negatively correlated with cervical cancer incidence rates (r = −0.41, P < .01). State-specific Pap test prevalence was generally low among women with no usual source of medical care or health insurance (Supplementary Table 2, available online).
Figure 5.
Scatter plots of human pappilomavirus (HPV) vaccination coverage levels (A) and cervical cancer incidence rates (B) by Papanicolao (Pap) testing prevalence by state. P values were obtained by a two-sided t test. r, Pearson correlation coefficient. Three-dose HPV vaccination coverage levels are reported for adolescent girls in the 2010 National Immunization Survey-Teen (NIS-Teen) who were born during the period from January 1992 to February 1998. Girls may have received either quadrivalent or bivalent HPV vaccine. Source: National Immunization Survey-Teen 2008, 2010, National Center for Health Statistics, Centers for Disease Control and Prevention, 2010, 2011. Five-year cervical cancer incidence rates during the period from 2005 to 2009 for women aged 15 years or older are per 100,000 population and standardized to the 2000 US standard population. States with missing rates did not meet North American Association of Central Cancer Registries (NAACCR) quality standards for the specified years and are not included in the reporting of incidence. Source: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program reported by NAACCR as meeting high-quality incidence data standards for the specified time periods. Percentage of women aged 21 to 65 years with intact uteri who received a Pap test in the previous 3 years in 2010. Source: Behavioral Risk Factor Surveillance System Public Use Data Tape 2010, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, 2011.
Discussion
Overall cancer death rates continue to decrease in the United States, and this favorable trend involved men and women, all major racial and ethnic groups, and all four major sites, including lung, colorectal, female breast, and prostate cancers. However, death rates continued to increase for cancers of the liver, pancreas, melanoma (men only), and uterus. Similarly, incidence rates continued to increase for these cancers and other cancers, including some associated with HPV infection (ie, oropharynx and anus). Notably, HPV vaccination coverage among girls in 2010 remained low, underscoring the need for broader interventions to increase vaccination uptake.
Factors that contribute to the favorable trends for lung, colorectal, female breast, and prostate cancer death rates have been discussed in previous annual reports and include reductions in important risk factors (eg, smoking for lung cancer) and improved early detection and treatment (eg, screening as well as adjuvant chemotherapy for breast and colorectal cancers) (2–14). In contrast, reasons for the increasing death rates for pancreatic and liver cancers in men and women, melanoma in men, and uterine cancer in women have not been fully elucidated. However, the trends may, in part, reflect a high prevalence of chronic hepatitis C virus infection due to injection drug use during the period from the 1960s to the 1980s for liver cancer (55), increased obesity prevalence for liver, pancreatic, and uterine cancers (14), and increased harmful ultraviolent radiation exposure for melanoma (56). Corresponding increases in incidence rates have been noted for all of these four cancers (57). Additional cancers with increasing incidence trends include thyroid and kidney cancers. Although some studies suggest that the increase in thyroid cancer rates are largely because of increased detection of small and indolent tumors by imaging (57–59), others suggest that unidentified risk factors may also be important because rates increased for both small and large tumors (60,61). Similarly, the increase in kidney cancer incidence rates is thought to reflect, in part, increased diagnosis because of wider application of imaging techniques (62) as well as the obesity epidemic (14).
Although overall breast cancer incidence rates stabilized during the most recent time period (2005–2009) after sharply decreasing between 2002 and 2003 because of reductions in the use of postmenopausal hormone replacement therapy (63,64), during the period from 2000 to 2009 incidence rates increased among black and API women. In addition to reproductive factors and postmenopausal hormone replacement therapy, obesity after menopause, weight gain throughout life, and alcohol consumption are also known risk factors for breast cancer (65). Mammography also increases breast cancer incidence rates by detecting tumors at an earlier time and detecting indolent cases (66). Whereas the obesity epidemic involved women of all races and ethnicities (67), recent increases in mammography are confined to API women (68). However, the extent to which these factors contributed to the increasing breast cancer incidence rates among black and API women is unclear.
With respect to HPV-associated cancers, rates increased for cancer of the oropharynx in white men and women, for vulvar cancer in white and black women, and for anal cancer in white and black men and women. Based on data from three SEER registries, the presence of HPV DNA detected in oropharyngeal tumors increased from 16.3% during the period from 1984 to 1989 to 71.7% during the period from 2000 to 2004 (69). The increasing trend for HPV-associated oropharyngeal cancer rates is in stark contrast with the overall decreasing trend for tobacco-related oropharyngeal cancers, largely because of declines in cigarette smoking (70). Increases in rates of HPV-associated oropharyngeal cancers have also been reported in Canada and several European countries, including Denmark and Sweden (71–74). However, it is unclear why increases in HPV-associated oropharyngeal cancers in the United States are confined to white men and women. There are no data on the natural history of oral HPV infection or on changes in the prevalence of infection over time among the general population or among oropharyngeal cancer patients by race and ethnicity.
Anal cancer incidence rates in the United States increased by more than twofold from 1975 to 2009 in both men and women (17), and the burden is higher among women than men. Among women, it is associated with an increasing number of sexual partners, young age at first sexual intercourse, and sexually transmitted diseases, but most cases occur among those without a history of anal sex (75). Among men, one study found that men who have sex with men had the highest anal cancer incidence rates in California (76). A more recent US study (covering diagnosis years 1980 to 2005) found that increases in anal cancer rates among men were largely influenced by the HIV epidemic (and driven primarily by increasing rates among HIV-positive men who have sex with men) (77). Increases in anal cancer incidence rates in Australia (78) and several European countries (79–81) were found to be associated with increases in high-risk sexual activities. In the United States, we also documented increases in incidence rates for cancers of the vulva among white and black women, and increases in these cancers (and anal cancer) may be due to increased sexual exposure to HPV (82).
In contrast to HPV-associated oropharyngeal and anal cancers, cervical cancer incidence rates declined substantially for women of most racial and ethnic groups because of wider dissemination and utilization of Pap testing and successful treatment of screen-detected precancerous lesions (30–32), although the change for AI/ANs was not statistically significant. The lack of decrease among AI/ANs and the substantially higher burden of cervical cancer among Hispanics may, in part, reflect issues regarding adherence to screening guidelines and other factors (83–85). According to national estimates from the 2010 NHIS, Asians and Hispanics women aged 21 years and older had a lower prevalence (75.4%, 78.7%, respectively) of having had a Pap test in the past 3 years, compared with non-Hispanic blacks and non-Hispanic whites (85.0%, 83.4% respectively) (86). Racial disparities in cervical cancer rates may persist due to differences in screening, follow-up, and treatment of abnormal lesions (31,87,88). Cervical cancer remains a leading cause of cancer death among women in several economically developing countries because of lack of screening (89–91).
In 2006, the ACIP recommended routine vaccination of girls aged 11 to 12 years with the quadrivalent HPV vaccine and vaccination for unvaccinated girls and women aged 13 to 26 years (27). In 2009, when the bivalent vaccine was licensed in the United States, updated recommendations were issued to state that either vaccine should be used for routine vaccination and vaccination of previously unvaccinated women (28). According to NIS-Teen data, HPV vaccination uptake (one or more–dose, three-dose, and series completion rates) increased for adolescent girls of virtually all sociodemographic groups from 2008 to 2010, although not all increases were statistically significant. In 2010, less than half (48.7%) of girls aged 13 to 17 years had received one or more doses of vaccine, one in three girls (32.0%) received the entire three-dose series, and 69.6% of those who initiated the series completed it (92). Nevertheless, the 2010 national three-dose coverage estimate among girls aged 13 to 17 years (32%) falls well short of the Healthy People 2020 target of 80% for girls aged 13 to 15 years (93) and was much lower than uptake reported in Canada (50%–85%), a region in Mexico (67%) (94), and the United Kingdom and Australia (>70%) (95,96). The low vaccine uptake in the United States overall and among socioeconomic disadvantaged groups is likely because of a combination of factors, including inadequate provider recommendations, issues regarding provider reimbursement, infrequent use of reminder/recall systems that would foster completion of the three-dose series, and factors such as parental hesitancy, health-care access, and general challenges in vaccine delivery to adolescents (92,97–100).
Previous studies have found that health-care provider recommendation is the most important predictor of vaccine acceptance (101–104). A survey of pediatricians and family medicine physicians conducted in 2008 (18 months after the initial licensure of the HPV vaccine) found that 57% strongly recommended vaccination for girls aged 11 to 12 years and 90% strongly recommended vaccination for girls aged 13 to 15 years (102); another survey of primary care physicians in 2009 found only 34.6% “always recommended” vaccination to appropriately aged adolescent girls (105). Barriers to not “strongly or always” recommending vaccination included the need to discuss sexuality before recommending the vaccine; the high cost of the vaccine (approximately $390 for the three-dose series) and reimbursement concerns; parental refusal because of vaccine safety concerns, religious, or philosophical concerns; and lack of understanding of the HPV vaccine (102,106).
Although the vaccine is available free of cost through the VFC program for eligible children and adolescents (45), about 50% of surveyed physicians in 2008 reported inadequate insurance coverage and lack of adequate reimbursement as barriers to vaccination (102,107). Some states use state and/or local funding to purchase vaccine and offer it to children or adolescents not eligible for vaccine provided by the VFC program. With this additional funding, some states are able to provide vaccine to all children or adolescents (ie, universal purchase policy), whereas some may only be able to provide vaccine to underinsured children. As of 2010, six states (New Hampshire, New Mexico, Rhode Island, Vermont, Washington, and Wyoming) had universal purchase policies that provided free HPV vaccine to all girls, including those who are VFC eligible, underinsured, and fully insured (108). Three-dose coverage rates in 2010 were higher than the national average (32%) for five of these six states, even though the national average included the six states with universal access. Other factors could also contribute to higher coverage in these states, including program policies or activities that lead to higher coverage independent of the universal policy.
Patient reminder systems in primary care setting, such as telephone calls, postcards, and letters, have been shown to improve immunization rates (97,98), and have been recommended by the Task Force on Community Preventive Services (109) because many patients do not know or remember recommended immunization schedules. However, a 2008 survey of physicians found almost two-thirds of physicians did not use any reminder/recall system for patients needing a second or third dose of the HPV vaccine (102). Implementing such strategies may improve completion rates of three doses of the HPV vaccine in all girls, especially among VFC-eligible and uninsured girls, those living below the poverty level, and Hispanics, in whom the completion rates are the lowest.
Mandates that require immunization for school entry increase vaccination coverage levels for a number of childhood vaccines (110,111). Although many states mandate childhood (eg, measles, mumps, hepatitis B) and adolescent (eg, meningococcal conjugate, tetanus, diphtheria) immunization for school enrollment (112), only the District of Colombia and Virginia have school mandates for HPV vaccination (112,113). Notably, the 2010 national adolescent vaccination coverage for at least one dose of Tdap vaccine (tetanus, diphtheria, and acellular pertussis, 68.7%) (92), which was mandated by 31 states, was substantially higher than one-dose coverage for HPV vaccine (48.7%) (112,113). The initiation rates of HPV vaccination in the District of Colombia (57.5%) and Virginia (54.0%) are only slightly higher than the national average of 48.7%, likely because of broader opt-out provisions in the mandates for HPV vaccination than for other childhood and adolescent vaccinations.
Routine HPV vaccination of boys was recommended by the ACIP in December 2011 (29). Coverage in boys was only 1.4% in 2010 but will likely increase in the coming years, and this could substantially improve the overall herd immunity against HPV infection in view of the existing low vaccine uptake among adolescent girls (92). Increasing current vaccination coverage levels among boys could eventually curb the growing burden of anal cancers, especially among men who have sex with men (76,114), and possibly the burden of oropharyngeal cancers.
Although HPV vaccination coverage among girls increased between 2008 and 2010 in most states, some Southern states continue to have lower HPV vaccination coverage. Pap testing prevalence is also low in these states, which show the highest cervical cancer burden in the United States. These unfavorable patterns may, at least in part, be because these areas are disproportionately represented by economically disadvantaged groups, including uninsured residents, blacks, and Hispanics (115), suggesting the need for focused cancer prevention and control in the region.
Limitations
High-quality cancer surveillance data in the United States are available for the entire population for mortality and for 93% of the population for incidence (2005–2009); however, certain limitations in data sources, data collection, and analyses may have influenced the findings of this report. First, differences between the numerator (incidence data) and denominator (Census population data) can occur in the designation of characteristics such as age, race, ethnicity, and place of residency. Postcensal population estimates based on numbers updated by birth and death data, administrative information, and emigration/immigration information are more subject to error than estimates based on actual Census counts; errors in these estimates may increase as time passes from the original recording of Census data. Additionally, the NCI modified these Census estimates to account for changes in 2005 county-level populations due to displacement of people after Hurricanes Katrina and Rita in the most-affected counties of Louisiana, Mississippi, Alabama, and Texas.
Second, as noted in previous Annual Reports to the Nation (1–14), the broad racial and ethnic groups categorized for our analyses may mask variations in the cancer burden by country of origin or by other unique characteristics of high- or low-risk populations. Also, cancer rates for racial and ethnic groups may be affected by difficulties in ascertaining race and ethnicity information from medical records, death certificates, and Census reports (116).
Third, analyses of trends should be carefully interpreted for several reasons. Changes in incidence may result from changes in the prevalence of risk factors, the introduction or increased use of screening or diagnostic techniques, or a combination of these. The AAPC was used as a summary measure to average trends over a 5- or 10-year period using joinpoint regression; joinpoint models identify recent changes in the magnitude and direction of trends but may give an impression of a continuous increase or decrease over time when this is not the case. Furthermore, delayed case reporting may affect incidence trends if the most recent joinpoint segments overestimate recent declines or underestimate recent increases; methods to adjust for delayed reporting (53) were used only in the analysis of SEER 13 data. The largest effects of adjusting for delayed reporting are seen in cancers diagnosed in nonhospital settings, such as melanoma and leukemia. This report presents trends based on both data from the SEER 13 registries and combined data from NAACCR, which includes SEER and NPCR registries. Both datasets have strengths and limitations and provide valuable insight into cancer trends in the United States. Longer-term trends can be examined using the SEER 13 registries, and these data have also been delay adjusted. However, the combined data from SEER and NPCR registries covers nearly the entire US population and may better capture geographic and population differences in risk factors and incidence.
Fourth, US Department of Veterans Affairs (VA) hospitals traditionally are a critical source of data for cancers diagnosed among veterans, representing approximately 3% to 8% of cancer diagnoses among men (117). A 2007 policy change regarding the transfer of VA cancer data to central state cancer registries resulted in incomplete reporting of VA hospital cases in some, but not all, state registries, beginning in the third quarter of the 2004 diagnosis year through the current time period. However, with the enactment of special data-sharing agreements with the VA, progress in collecting data from VA hospitals has been made, which resulted in more complete and accurate national cancer incidence estimates. As a result, the cancer incidence rates from 2005 to 2009 among men are underestimated by approximately 1% for all cancers combined, based on an analysis of data from the SEER registries, with slightly higher amounts (2%–4%) for rates among black men.
Fifth, we considered the cancers included in the special section to be HPV-associated based on numerous rigorous etiologic studies and previously established methods (proportions of cancers generally found to be associated with HPV are listed in the Introduction) (20,118). However, specific information about the presence of HPV DNA in tumors was unavailable for this analysis, and the number of HPV-associated cancers attributed to HPV infection should be cautiously interpreted because not all cancers termed “HPV-associated” reflect actual HPV infections.
Finally, both the BRFSS and NIS-Teen are landline telephone surveys and exclude households without landlines. The estimates from these surveys may be under- or overestimated, although they were adjusted for noncoverage of households with no landline telephones and for nonresponse (46,119). Generally, BRFSS respondents tend to overreport behaviors that are considered desirable, such as screening (120). Therefore, the Pap test estimates from BRFSS (a self-reported survey) may have been overestimated. Further, estimates of Pap test and vaccination coverage for particular states and for racial and ethnic populations should be interpreted with caution because they may be unstable due to smaller sample sizes.
Future Directions
Although substantial progress in cancer prevention and control has been made for many cancers, including lung, colorectal, female breast, and prostate cancers (121), incidence and/or death rates continue to increase for some cancer sites (eg, liver, pancreas, kidney, thyroid, and melanoma), underscoring the need for additional etiologic research for the identification of major risk factors and the development of appropriate interventions. Further, programs that increase uptake of proven cancer prevention strategies at the population level should be strengthened, along with broader access to early detection and treatment through increased access to the health-care system.
A greater understanding of the increasing incidence rates for HPV-associated cancers requires continued monitoring of changes in sexual practices that increase HPV exposure as well as of trends in the population-based prevalence of HPV infections at anatomic sites where these cancers arise. Notably, HPV-associated cancers occur in excess among people with HIV and AIDS relative to the general population, warranting additional monitoring and prevention activities in this high-risk population (122–124).
Primary prevention of HPV-associated cervical, vaginal, vulvar, and anal cancers is achieved through childhood vaccination of girls and boys, although vaccine coverage remains low compared with the Healthy People 2020 target of 80% (93), and strategies are needed to increase coverage among adolescents. Educating health-care workers about the importance of provider recommendation as an influential factor in a parent’s decision to allow HPV vaccination of their child may be the single most important way to increase coverage levels (101–104). Also, programs to educate parents about the importance of HPV vaccination as an anticancer vaccine and system changes, such as the implementation of automatic electronic reminders (for series completion) are also likely to be important in increasing coverage (99). Increasing HPV vaccination coverage would also likely result in the added benefit of attaining levels of herd immunity observed in other countries (eg, Australia) and associated with decreased HPV transmission (125–127).
Research on HPV vaccines is ongoing in several areas. There is interest in evaluating the efficacy of less than three doses (128), and further data may be available on these schedules in the future. In addition, second-generation vaccines that target additional HPV types are being developed. Current data suggest good duration of protection afforded by the currently approved bivalent and quadrivalent vaccines, and ongoing studies will provide further data (129,130). Monitoring of vaccine safety is part of routine activities of the US Food and Drug Administration and the CDC, and current data are reassuring. A recent evaluation of quadrivalent HPV vaccine after the administration of more than 600,000 doses in girls and women found no statistically significant increased rate of prespecified adverse events (131). Safety monitoring of HPV vaccines will continue to investigate potential rare outcomes.
Early detection of cervical and noncervical cancers associated with HPV infection also deserves brief comment. For cervical cancers, the ACS and multiple clinical organizations, as well as the US Preventive Services Task Force, have recently issued updated age-specific guidelines for cytologic and HPV testing for the early detection of cervical cancer (30,31). These guidelines addressed critical issues about the harms of unnecessary procedures and treatments associated with transient HPV infections and associated cervical lesions, which regress without medical intervention. In addition, as HPV vaccination coverage levels increase, cervical cancer screening recommendations may need to be modified. The efficacy of anal Pap testing to reduce mortality from HPV-associated anal cancer is unknown, and no national guidelines exist for anal cytology among men or women with or without HIV infection, although one study found it to be both clinically and cost effective to conduct such testing among HIV-positive gay and bisexual men (132,133).
As incidence rates for some HPV-associated cancers continue to rise, these cases will contribute to the overall growing number of cancers associated with population aging and expansion, requiring additional resources for medical research and treatment. Continued monitoring of incidence and mortality trends for all cancers is warranted to inform cancer prevention and control policies and programs.
Funding
This work was supported by the American Cancer Society, the Centers for Disease Control and Prevention, the National Cancer Institute, the National Institutes of Health, and the North American Association of Central Cancer Registries.
Supplementary Material
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
We gratefully acknowledge the contributions of the state and regional cancer registry staffs for their work in collecting the data used in this study. In addition we thank Lynn A. G. Ries, a contractor with the National Cancer Institute, and Andrew Lake, Martin Krapcho, and Rick Firth, of Information Management Services, Inc, for assistance in compiling the data used in this report.
References
- 1. Wingo PA, Ries LA, Rosenberg HM, et al. Cancer incidence and mortality, 1973–1995: a report card for the U.S. Cancer. 1998; 82(6):1197–1207 [DOI] [PubMed] [Google Scholar]
- 2. Wingo PA, Ries LA, Giovino GA, et al. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999; 91(8):675–690 [DOI] [PubMed] [Google Scholar]
- 3. Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer. 2000; 88(10):2398–2424 [DOI] [PubMed] [Google Scholar]
- 4. Howe HL, Wingo PA, Thun MJ, et al. Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. J Natl Cancer Inst. 2001; 93(11):824–842 [DOI] [PubMed] [Google Scholar]
- 5. Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002; 94(10):2766–2792 [DOI] [PubMed] [Google Scholar]
- 6. Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003; 95(17):1276–1299 [DOI] [PubMed] [Google Scholar]
- 7. Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004; 101(1):3–27 [DOI] [PubMed] [Google Scholar]
- 8. Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005; 97(19):1407–1427 [DOI] [PubMed] [Google Scholar]
- 9. Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006; 107(8):1711–1742 [DOI] [PubMed] [Google Scholar]
- 10. Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007; 110(10):2119–2152 [DOI] [PubMed] [Google Scholar]
- 11. Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008; 100(23):1672–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010; 116(3):544–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011; 103(9):714–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eheman C, Henley SJ, Ballard-Barbash R, et al. Annual report to the nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012; 18(9): 2338–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schiffman M, Hildesheim A. Cervical cancer.. In: Schottenfeld D, Fraumeni J, eds. Cancer Epidemiology and Prevention. 3rded. New York: Oxford; 2006; 1044–1067 [Google Scholar]
- 16. Mayne S, Morse D, Winn D. Cancers of the oral cavity and pharynx.. In: Schottenfeld D, Fraumeni J, eds. Cancer Epidemiology and Prevention. 3rded. New York: Oxford; 2006; 674–696 [Google Scholar]
- 17. Frisch M, Melbye M. Anal cancer.. In: Schottenfeld D, Fraumeni J, eds. Cancer Epidemiology and Prevention. 3rded. New York: Oxford; 2006; 830–840 [Google Scholar]
- 18. Madeline M, Daling J. Cancers of the vulva and vagina.. In: Schottenfeld D, Fraumeni J, eds. Cancer Epidemiology and Prevention. 3rded. New York: Oxford; 2006; 1068–1074 [Google Scholar]
- 19. Wideroff L, Schottenfeld D. Penile cancer.. In: Schottenfeld D, Fraumeni J, eds. Cancer Epidemiology and Prevention. 3rded. New York: Oxford; 2006; 1166–1172 [Google Scholar]
- 20. Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008; 113(10 Suppl):3036–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bouvard V, Baan R, Straif K, et al. A review of human carcinogens—part B: biological agents. Lancet Oncol. 2009; 10(4):321–322 [DOI] [PubMed] [Google Scholar]
- 22. The FUTURE II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007; 356(19):1915–1927 [DOI] [PubMed] [Google Scholar]
- 23. Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009; 374(9686): 301–314 [DOI] [PubMed] [Google Scholar]
- 24. Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like- particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007; 369(9574):1693–1702 [DOI] [PubMed] [Google Scholar]
- 25. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011; 364(5):401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dillner J, Kjaer SK, Wheeler CM, et al. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 2010; 341 c3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007; 56(RR-2):1–24 [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010; 59(20):626–629 [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011; 60(50):1705–1708 [PubMed] [Google Scholar]
- 30. Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012; 156(12):880–891 [DOI] [PubMed] [Google Scholar]
- 31. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;;62(3):147–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol. 2009; 114(6):1409–1420 [DOI] [PubMed] [Google Scholar]
- 33. Pierce Campbell CM, Menezes LJ, Paskett ED, et al. Prevention of invasive cervical cancer in the United States: past, present, and future. Cancer Epidemiol Biomarkers Prev. 2012; 21(9):1402–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. North American Association of Central Cancer Registries Data Quality Standards http://www.naaccr.org/Certification/CertificationLevels.aspxAccessed July 27, 2012
- 35. World Health Organization International Classification of Diseases for Oncology. 3rded. Geneva, Switzerland: WHO; 2000; [Google Scholar]
- 36. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). Bethesda, MD: National Cancer Institute; based on November 2011 SEER data submission, posted to the SEER web site, April 2012 [Google Scholar]
- 37. Espey DK, Wiggins CL, Jim MA, et al. Methods for improving cancer surveillance data in American Indian and Alaska Native populations. Cancer. 2008; 113(5 Suppl):1120–1130 [DOI] [PubMed] [Google Scholar]
- 38. National Cancer Institute Surveillance Epidemiology and End Results (SEER) Program. Population Estimates Used in NCI’s SEER*Stat Software. http://seer.cancer.gov/popdata/methods.html Accessed July 27, 2012 [Google Scholar]
- 39. National Center for Health Statistics Deaths: final data for 2009. Natl Vital Stat Rep. 2012; 60(3):1–116 [PubMed] [Google Scholar]
- 40. Watson M, Saraiya M, Ahmed F, et al. Using population-based cancer registry data to assess the burden of human papillomavirus-associated cancers in the United States: overview of methods. Cancer. 2008; 113(10 Suppl):2841–2854 [DOI] [PubMed] [Google Scholar]
- 41. Singh G, Miller B, Hankey B, et al. Area Socioeconomic Variations in U.S. Cancer Incidence, Mortality, Stage, Treatment, and Survival, 1975–1999. Bethesda, MD: National Cancer Institute; 2003. [Google Scholar]
- 42. Centers for Disease Control and Prevention National Immunization Survey-Teen: A User’s Guide for the 2008 Public-Use Data File. ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NIS/NISteenPUF08_DUG.pdf Accessed August 14, 2012 [Google Scholar]
- 43. Centers for Disease Control and Prevention National Immunization Survey-Teen: A User’s Guide for the 2010 Public-Use Data File. ftp://ftp.cdc.gov/pub/health_statistics/nchs/dataset_documentation/nis/nisteenpuf10_dug.pdf Accessed August 14, 2012 [Google Scholar]
- 44. Jain N, Singleton JA, Montgomery M, et al. Determining accurate vaccination coverage rates for adolescents: the National Immunization Survey-Teen 2006. Public Health Rep. 2009; 124(5):642–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Centers for Disease Control and Prevention Vaccines for Children Program (VFC). http://www.cdc.gov/vaccines/programs/vfc/default.htm Accessed July 27, 2012 [Google Scholar]
- 46. Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System Survey Data. U.S. Department of Health and Human Services; http://www.cdc.gov/brfss/technical_infodata/surveydata/2010.htm Accessed July 27, 2012; [Google Scholar]
- 47. US Bureau of the Census Population Estimates. http://www.census.gov/popest/data/historical/2000s/vintage_2009/index.html Accessed July 27, 2012 [Google Scholar]
- 48. National Center for Health Statistics Postcensal Estimates of the Resident Population of the United States for July 1, 2000–July 1, 2009, by Year, County, Single-Year of age (0, 1, 2, .., 85 years and over), Bridged Race, Hispanic Origin, and Sex (Vintage 2009). ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Datasets/NVSS/bridgepop/2009/DocumentationBridgedPostcenV2009.pdf Accessed August 9, 2012;
- 49. Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep. 1998; 47(3): 1–16, 20 [PubMed] [Google Scholar]
- 50. Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006; 15(6):547–569 [DOI] [PubMed] [Google Scholar]
- 51. Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000; 19(3):335–351 [DOI] [PubMed] [Google Scholar]
- 52. Clegg LX, Hankey BF, Tiwari R, et al. Estimating average annual per cent change in trend analysis. Stat Med. 2009; 28(29):3670–3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clegg LX, Feuer EJ, Midthune DN, et al. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002; 94(20):1537–1545 [DOI] [PubMed] [Google Scholar]
- 54. RTI International SUDAAN Language Manual, Release 10.0. Research Triangle Park, NC: Research Triangle Institute; 2008. [Google Scholar]
- 55. Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006; 144(10):705–714 [DOI] [PubMed] [Google Scholar]
- 56. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol. 2011; 65(5 Suppl 1):S17–25 e1–3 [DOI] [PubMed] [Google Scholar]
- 57. Simard EP, Ward EM, Siegel R, et al. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012; 62(2) 118–128 [DOI] [PubMed] [Google Scholar]
- 58. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006; 295(18):2164–2167 [DOI] [PubMed] [Google Scholar]
- 59. Davies L, Ouellette M, Hunter M, et al. The increasing incidence of small thyroid cancers: where are the cases coming from?. Laryngoscope. 2010; 120(12):2446–2451 [DOI] [PubMed] [Google Scholar]
- 60. Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009; 115(16): 3801–3807 [DOI] [PubMed] [Google Scholar]
- 61. Aschebrook-Kilfoy B, Sabra MM, Brenner A, et al. Diabetes and thyroid cancer risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid. 2011; 21(9):957–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998; 51(2):203–205 [DOI] [PubMed] [Google Scholar]
- 63. Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast- cancer incidence in 2003 in the United States. N Engl J Med. 2007; 356(16): 1670–1674 [DOI] [PubMed] [Google Scholar]
- 64. Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res. 2007; 9(3):R28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Colditz G, Baer H, Tamimi R. Breast cancer.. In: Schottenfeld D, Fraumeni J, eds. Cancer Epidemiology and Prevention. New York: Oxford; 2006; 959–974 [Google Scholar]
- 66. Kalager M, Adami HO, Bretthauer M, et al. Overdiagnosis of invasive breast cancer due to mammography screening: results from the Norwegian screening program. Ann Intern Med. 2012; 156(7):491–499 [DOI] [PubMed] [Google Scholar]
- 67. Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010; 303(3):235–241 [DOI] [PubMed] [Google Scholar]
- 68. Breen N, Gentleman JF, Schiller JS. Update on mammography trends: comparisons of rates in 2000, 2005, and 2008. Cancer. 2011; 117(10):2209–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011; 29(32):4294–4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008; 26(4):612–619 [DOI] [PubMed] [Google Scholar]
- 71. Johnson-Obaseki S, McDonald JT, Corsten M, et al. Head and neck cancer in Canada: trends 1992 to 2007. Otolaryngol Head Neck Surg. 2012;;147(1):74–78 [DOI] [PubMed] [Google Scholar]
- 72. Blomberg M, Nielsen A, Munk C, et al. Trends in head and neck cancer incidence in Denmark, 1978–2007: focus on human papillomavirus associated sites. Int J Cancer. 2011; 129(3):733–741 [DOI] [PubMed] [Google Scholar]
- 73. Ramqvist T, Dalianis T. An epidemic of oropharyngeal squamous cell carcinoma (OSCC) due to human papillomavirus (HPV) infection and aspects of treatment and prevention. Anticancer Res. 2011; 31(5):1515–1519 [PubMed] [Google Scholar]
- 74. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009; 45(4–5):309–316 [DOI] [PubMed] [Google Scholar]
- 75. Frisch M, Glimelius B, van den Brule AJ, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med. 1997; 337(19):1350–1358 [DOI] [PubMed] [Google Scholar]
- 76. Cress RD, Holly EA. Incidence of anal cancer in California: increased incidence among men in San Francisco, 1973–1999. Prev Med. 2003; 36(5):555–560 [DOI] [PubMed] [Google Scholar]
- 77. Shiels MS, Pfeiffer RM, Chaturvedi AK, et al. Impact of the HIV epidemic on the incidence rates of anal cancer in the United States. J Natl Cancer Inst. 2012;;104(20):1591–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival: the Surveillance, Epidemiology, and End Results experience, 1973–2000. Cancer. 2004; 101(2):281–288 [DOI] [PubMed] [Google Scholar]
- 79. Jin F, Stein AN, Conway EL, et al. Trends in anal cancer in Australia, 1982–2005. Vaccine. 2011; 29(12):2322–2327 [DOI] [PubMed] [Google Scholar]
- 80. Nielsen A, Munk C, Kjaer SK. Trends in incidence of anal cancer and high-grade anal intraepithelial neoplasia in Denmark, 1978–2008. Int J Cancer. 2012; 130(5):1168–1173 [DOI] [PubMed] [Google Scholar]
- 81. Robinson D, Coupland V, Moller H. An analysis of temporal and generational trends in the incidence of anal and other HPV-related cancers in Southeast England. Br J Cancer. 2009; 100(3):527–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Saraiya M, Watson M, Wu X, et al. Incidence of in situ and invasive vulvar cancer in the US, 1998–2003. Cancer. 2008; 113(10 Suppl):2865–2872 [DOI] [PubMed] [Google Scholar]
- 83. Benard VB, Lawson HW, Eheman CR, et al. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet Gynecol. 2005; 105(6):1323–1328 [DOI] [PubMed] [Google Scholar]
- 84. Becker TM, Espey DK, Lawson HW, et al. Regional differences in cervical cancer incidence among American Indians and Alaska Natives, 1999–2004. Cancer. 2008; 113(5 Suppl):1234–1243 [DOI] [PubMed] [Google Scholar]
- 85. Scarinci IC, Garcia FA, Kobetz E, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010; 116(11):2531–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Centers for Disease Control and Prevention. Cancer screening—United States, 2010 MMWR Morb Mortal Wkly Rep. 2012; 61(3):41–45 [PubMed] [Google Scholar]
- 87. Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000; 132(10):810–819 [DOI] [PubMed] [Google Scholar]
- 88. Wang SS, Sherman ME, Hildesheim A, et al. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer. 2004; 100(5):1035–1044 [DOI] [PubMed] [Google Scholar]
- 89. Arbyn M, Castellsague X, de Sanjose S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011; 22(12):2675–2686 [DOI] [PubMed] [Google Scholar]
- 90. de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012; 13(6):607–615 [DOI] [PubMed] [Google Scholar]
- 91. Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012; 5(1):18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011; 60(33):1117–1123 [PubMed] [Google Scholar]
- 93. US Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People. 2020; http://www.healthypeople.gov/2020/default.aspxAccessed August 14, 2012 [Google Scholar]
- 94.Centers for Disease Control and Prevention. Progress toward implementation of human papillomavirus vaccination—the Americas, 2006–2010. MMWR Morb Mortal Wkly Rep. 2011; 60(40):1382–1384 [PubMed] [Google Scholar]
- 95. Sheridan A, White J. for the Department of Health. Annual HPV Vaccine Coverage in England in 2009/2010. [published online ahead of print January 27, 2011]. http://www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_123795 Accessed November 15, 2012.
- 96. Garland SM, Skinner SR, Brotherton JM. Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Prev Med. 2011; 53(Suppl 1):S29–S35 [DOI] [PubMed] [Google Scholar]
- 97. Szilagyi PG, Bordley C, Vann JC, et al. Effect of patient reminder/recall interventions on immunization rates: a review. JAMA. 2000; 284(14): 1820–1827 [DOI] [PubMed] [Google Scholar]
- 98. Jacobson VJ, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;;(3): CD003941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McCauley MM, Fishbein DB, Santoli JM. Introduction: strengthening the delivery of new vaccines for adolescents. Pediatrics. 2008; 121(Suppl 1):S1–S4 [DOI] [PubMed] [Google Scholar]
- 100. Bugenske E, Stokley S, Kennedy A, et al. Middle school vaccination requirements and adolescent vaccination coverage. Pediatrics. 2012; 129(6): 1056–1063 [DOI] [PubMed] [Google Scholar]
- 101. Dorell CG, Yankey D, Santibanez TA, et al. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics. 2011; 128(5):830–839 [DOI] [PubMed] [Google Scholar]
- 102. Daley MF, Crane LA, Markowitz LE, et al. Human papillomavirus vaccination practices: a survey of US physicians 18 months after licensure. Pediatrics. 2010; 126(3):425–433 [DOI] [PubMed] [Google Scholar]
- 103. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011; 29(5):890–895 [DOI] [PubMed] [Google Scholar]
- 104. Dorell C, Yankey D, Strasser S. Parent-reported reasons for nonreceipt of recommended adolescent vaccinations, national immunization survey: teen, 2009. Clin Pediatr (Phila). 2011; 50(12):1116–1124 [DOI] [PubMed] [Google Scholar]
- 105. Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine. 2011; 29(47):8634–8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Freed GL, Cowan AE, Clark SJ. Primary care physician perspectives on reimbursement for childhood immunizations. Pediatrics. 2009; 124(Suppl 5):S466–S471 [DOI] [PubMed] [Google Scholar]
- 107. Kahn JA, Cooper HP, Vadaparampil ST, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: a statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev. 2009; 18(8):2325–2332 [DOI] [PubMed] [Google Scholar]
- 108. Centers for Disease Control and Prevention Immunization Services Division, Program Operations Branch, Program Annual Report and Progress Assessments. http://www2a.cdc.gov/nip/irar/grantee/granteeInfo.asp Accessed July 27, 2012 [Google Scholar]
- 109. Centers for Disease Control and Prevention. Vaccine-preventable diseases: improving vaccination coverage in children, adolescents, and adults A report on recommendations from the Task Force on Community Preventive Services. MMWR Recomm Rep. 1999; 48(RR-8):1–15 [PubMed] [Google Scholar]
- 110. Horlick G, Shaw FE, Gorji M, et al. Delivering new vaccines to adolescents: the role of school-entry laws. Pediatrics. 2008; 121(Suppl 1):S79–S84 [DOI] [PubMed] [Google Scholar]
- 111. Orenstein WA, Hinman AR. The immunization system in the United States—the role of school immunization laws. Vaccine. 1999; 17(Suppl 3):S19–S24 [DOI] [PubMed] [Google Scholar]
- 112. Immunization Action Coalition State Mandates on Immunization and Vaccine-Preventable Diseases. http://www.immunize.org/laws/Accessed July 27, 2012 [Google Scholar]
- 113. National Conference of State Legislatures HPV Vaccine: State Legislation and Statutes. http://www.ncsl.org/issues-research/health/hpv-vaccine-state-legislation-and-statutes.aspx#2011Accessed July 27, 2012 [Google Scholar]
- 114. Frisch M, Smith E, Grulich A, et al. Cancer in a population-based cohort of men and women in registered homosexual partnerships. Am J Epidemiol. 2003; 157(11):966–972 [DOI] [PubMed] [Google Scholar]
- 115. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004; 54(2):78–93 [DOI] [PubMed] [Google Scholar]
- 116. Arias E, Schauman WS, Eschbach K, et al. The validity of race and Hispanic origin reporting on death certificates in the United States. Vital Health Stat 2. 2008;;(148):1–23 [PubMed] [Google Scholar]
- 117. Howlader N, Ries LA, Stinchcomb DG, et al. The impact of underreported Veterans Affairs data on national cancer statistics: analysis using population-based SEER registries. J Natl Cancer Inst. 2009; 101(7):533–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Centers for Disease Control and Prevention. Human papillomavirus-associated cancers—United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2012; 61(15) 258–261 [PubMed] [Google Scholar]
- 119. Copeland KR, Dorell C, Khare M, et al. Assessment of bias in the National Immunization Survey-Teen: an updated benchmark to the National Health Interview Survey. Paper presented at the American Association for Public Opinion Research Annual Conference May 2010; Chicago, IL [Google Scholar]
- 120. Rauscher GH, Johnson TP, Cho YI, et al. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008; 17(4):748–757 [DOI] [PubMed] [Google Scholar]
- 121. Jemal A, Ward E, Thun M. Declining death rates reflect progress against cancer. PLoS One. 2010; 5(3):e9584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chaturvedi AK, Madeleine MM, Biggar RJ, et al. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009; 101(16):1120–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Simard EP, Pfeiffer RM, Engels EA. Spectrum of cancer risk late after AIDS onset in the United States. Arch Intern Med. 2010; 170(15):1337–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011; 103(9):753–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Donovan B, Franklin N, Guy R, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis. 2011; 11(1):39–44 [DOI] [PubMed] [Google Scholar]
- 126. Read TR, Hocking JS, Chen MY, et al. The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex Transm Infect. 2011; 87(7):544–547 [DOI] [PubMed] [Google Scholar]
- 127. Smith MA, Lew JB, Walker RJ, et al. The predicted impact of HPV vaccination on male infections and male HPV-related cancers in Australia. Vaccine. 2011; 29(48):9112–9122 [DOI] [PubMed] [Google Scholar]
- 128. Kreimer AR, Rodriguez AC, Hildesheim A, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011; 103(19):1444–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Dunne EF, Datta SD, L EM. A review of prophylactic human papillomavirus vaccines: recommendations and monitoring in the US. Cancer. 2008; 113(10 Suppl):2995–3003 [DOI] [PubMed] [Google Scholar]
- 130. Romanowski B, de Borba PC, Naud PS, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009; 374(9706):1975–1985 [DOI] [PubMed] [Google Scholar]
- 131. Gee J, Naleway A, Shui I, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011; 29(46):8279–8284 [DOI] [PubMed] [Google Scholar]
- 132. Goldie SJ, Kuntz KM, Weinstein MC, et al. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA. 1999; 281(19):1822–1829 [DOI] [PubMed] [Google Scholar]
- 133. Chiao EY, Giordano TP, Palefsky JM, et al. Screening HIV-infected individuals for anal cancer precursor lesions: a systematic review. Clin Infect Dis. 2006; 43(2):223–233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.