Abstract
The present work presents a “from gene defect to clinics” pathogenesis study of a patient with a hitherto unreported mutation in the CPT1A gene. In early childhood, the patient developed a life-threatening episode (hypoketotic hypoglycemia, liver cytolysis, and hepatomegaly) evocative of a mitochondrial fatty acid oxidation disorder, and presented deficient fibroblast carnitine palmitoyltransferase 1 (CPT1) activity and homozygosity for the c.1783 C > T nucleotide substitution on exon 15 of CPT1A (p.R595W mutant). While confirming CPT1A deficiency, whole blood de novo acylcarnitine synthesis and the levels of carnitine and its esters formally linked intracellular free-carnitine depletion to intracellular carnitine esterification. Sequence alignment and modeling of wild-type and p.*R595W CPT1A proteins indicated that the Arg595 targeted by the mutated codon is phylogenetically well conversed. It contributes to a hydrogen bond network with neighboring residues Cys304 and Met593 but does not participate in the catalysis and carnitine pocket. Its replacement by tryptophan induces steric hindrance with the side chain of Ile480 located in α-helix 12, affecting protein architecture and function. This hindrance with Ile480 is also originally described with tryptophan 304 in the known mutant p.C304W CPT1A, suggesting that the mechanisms that invalidate CPT1A activity and underlie pathogenesis could be common in both the new (p.R595W) and previously described (p.C304W) mutants.
Introduction
Mitochondria play an essential role in life and achieve a wide spectrum of cell functions. One of these functions is the cell energy yield, and in this respect, long-chain fatty acids represent a main source of energetic substrates for mammalian mitochondria. Mitochondrial long-chain fatty acid oxidation is a process physiologically dependent on l-carnitine and the production of carnitine esters as shuttle forms of CoA esters (Houten and Wanders 2010). An adequate appraisal of the manner by which the esterification of carnitine occurs in both healthy cells and disease may improve the understanding of mitochondrial β-oxidation disorders and diagnosis, and beyond the scope of these disorders, any diseases or environmental factors affecting mitochondrial fatty acid oxidation.
Carnitine palmitoyltransferase (CPT) 1 catalyzes the rate-limiting step of mitochondrial fatty acid oxidation. This enzyme exhibits a catalytic site on the inner aspect of the mitochondrial outer membrane and a malonyl-CoA sensitive site (Cook et al. 1994; Morillas et al. 2002, 2003) on the cytosolic surface of this membrane (Kashfi and Cook 1999; Murthy and Pande 1987). The malonyl-CoA concentration is enhanced upon high glucose intake (postabsorptive period) and is involved in fatty acid synthesis. Its inhibitory action against CPT1-driven fatty acid oxidation prevents de novo formed fatty acids from being degraded by β-oxidation and tilts the fatty acid oxidation/esterification balance in favor of esterification. CPT1 exists as two main, liver (CPT1A or L-CPT I) and muscle (CPT1B or M-CPT I), isoforms (Esser et al. 1993; Yamazaki et al. 1995). A third isoform has been recently described in the brain (CPT1C) (Price et al. 2002). CPT1A is expressed in the liver, kidney, brain, pancreas, leukocytes, fibroblasts, and embryonic tissues whereas CPT1B is found in muscle and heart, and brown adipose tissue (Bonnefont et al. 2004). CPT1C is restricted to the central nervous system and its exact role is still unclear (Sierra et al. 2008; Wolfgang et al. 2006), although exciting developments on its role in the hypothalamic control of food intake have recently emerged (Wolfgang and Lane 2011).
The CPT2 gene is distinct from CPT1 genes and encodes a protein, which physiologically converts the acylcarnitines entering mitochondrial matrix back into CoA esters, thus supplying local acyl-CoA β-oxidation (Houten and Wanders 2010). In contrast to CPT2 deficiency (OMIM 600650) which has been described in many patients, less than 30 cases of CPT1 deficiency (OMIM 600528) have been reported, with no 1B or 1C isoform deficiencies reported in humans to date. The CPT1A protein is a monomer of 773 amino acids generated by a 2.319 kb transcript encoded by a gene located on chromosome 11 at the q13.1–q13.5 locus (Britton et al. 1997). Mutations invalidating protein function are often single point mutations and occur throughout the entire CPT1A gene sequence (Bonnefont et al. 2004).
Materials and Methods
Solvents, Reagents, and Internal Calibrators
Chemical reagents and solvents were of HPLC grade from Merck, acetonitrile from JT Baker, 1-butanol and methanol from Prolabo, and stable isotopes including [16-2H3, 15-2H2]-palmitate (substrate), [16-2H3]-pamitoylcarnitine, [8-2H3]-octanoylcarnitine, and [3-2H3]-propionylcarnitine (internal standards) from CDN-Isotopes Inc. l-carnitine and other chemicals were from Sigma (St Louis, MO).
Organic Acids, Acylglycines, Carnitine, and Acylcarnitines
Trimethylsilyl derivatives of organic acids were quantified by capillary gas chromatography coupled to mass spectrometry (GC-MS); total and free-carnitine levels were determined as previously described (Fontaine et al. 1989; Vockley and Whiteman 2002). Acylcarnitine species in body fluids were studied by fast atom bombardment (FAB)–MS or MS–MS analyses of Guthrie cards within 72 h following blood spotting of the filter paper (Fontaine et al. 1989, 1996; Dessein et al. 2009).
Enzyme Studies on Cultured Fibroblasts
Oxidation of 14C labeled fatty acids and CPT1 were assayed as described previously (Vianey-Saban et al. 1993).
Gene Studies
The CPT1A gene was studied by sequencing its genomic DNA throughout the 19 exons and the flanking intronic regions.
De Novo Synthesis of Acylcarnitines in Whole Blood Samples
De novo synthesis of acylcarnitines in whole blood samples incubated with deuterated palmitate was monitored by electrospray ionization tandem mass spectrometry (ESI/MS–MS) essentially as described previously (Dessein et al. 2009). This procedure was also adapted as a quantitative assay of CPT1 in whole samples from the patient and controls which were analyzed simultaneously over short incubation periods.
Homology Modeling
The structural model for rat CPT1A was obtained by using homology modeling procedures based on multiple sequence alignment of the carnitine acyltransferase family of proteins, including the known 3D-structure of mouse carnitine acetyltransferase (PDB accession number 1NDB) (Morillas et al. 2004). This model included most of the protein sequence (residues 166–772) and was built up with Geno3D (Combet et al. 2002). The protein structure and predicted features of R585W and C304W CPT1 mutants were studied with Swiss Pdb-Viewer (http://www.expasy.org/spdbv).
Case Report
The patient was born normally from consanguineous parents. Familial history included sudden infant death of a 9-month-old brother presenting with severe hepatomegaly of unknown etiology. At 3 years of age, the patient presented with a gastroenteritis which developed within a few days into a hypotonic comatose state prompting hospitalization. On admission, a type II coma was recorded and a severe hepatomegaly was observed. Biological investigations revealed hyponatremia, and hypoglycemia (blood glucose below 0.20 g/l) without urinary ketosis. The patient also presented with blood ASAT and ALAT levels at 590 and 355 IU/l, respectively (upper control values at 30 IU/l). Urinary organic acids were normal without any increase in dicarboxylic fatty acids. The patient’s clinical status improved progressively. The patient was diagnosed as CPT1 deficient at the age of 4 years and was given a low-fat diet (see below). The clinical outcome included psychomotor delay, speaking difficulties and cryptogenic partial epilepsy (controlled by Trileptal). Schooling was followed in a medical and educational institute. At 9 years, the patient experienced another episode of decompensation during the course of gastroenteritis with vomiting, diarrhea, and anorexia. The patient became confused with cerebral hyperexcitability and was hospitalized. A moderate hepatomegaly was observed. Blood ASAT (63 UI/l) but not ALAT (23 UI/l) levels were moderately increased. Other biological parameters including glycemia, uremia, blood electrolytes, and ammoniemia were normal. Myolysis, which was revealed by creatine kinase levels rising to 3,614 U/l and LDH levels to 478 UI/l, also developed without, however, renal failure (normal creatininemia). The patient recovered after 3 days of treatment essentially based on oxygenotherapy and infusion of glucose (10 mg/kg/min). The patient is now adult, retarded and exhibits language difficulties. Treatment is nutritional and consists of an avoidance of fasting and a limited supply of lipids contributing to 21.5% of dietary energy and composed by 54% and 46% of medium- and long-chain fatty acid-containing triglycerides, respectively. The patient’s lipid profile (serum cholesterol, cholesterol HDL, cholesterol LDL, HDL/LDL + VLDL ratio and triglycerides) has always been normal (the patient was, however, prescribed a low-fat diet immediately after diagnosis). Nonesterified fatty acid levels were also repeatedly normal in blood.
Results
Biological Explorations
The patient’s fasting glycemia was normal (0.89 g/l) and associated with elevated plasma concentrations of free fatty acids (2 mmol/l) without a rise in ketones (acetoacetate and β-hydroxybutyrate at 0.00–0.01 mmol/l), thus suggesting a defect in fatty acid oxidation. Medium-chain acyl-CoA dehydrogenase deficiency was a priori discarded after a normal 3-phenylpropionate (25 mg/kg) loading test and the absence of abnormal organic acids. Deficient long-chain fatty acid oxidation was supported by the failure of loads of long-chain fatty acids to increase blood ketone levels.
Biochemical Explorations on Cultured Skin Fibroblasts
The analysis of the oxidation of radiolabeled fatty acids in skin fibroblasts indicated that long- and not medium-chain fatty acid oxidation was deficient (7–17% of control values, Table 1). Fibroblast mitochondrial CPT 1 and 2 activities were determined in intact and disrupted mitochondria, respectively, via a procedure (Demaugre et al. 1988) in which measurements of tritiated palmitoyl-l-carnitine produced from tritiated l-carnitine and palmitoyl-CoA (for CPT1) and tritiated l-carnitine produced from a tritiated palmitoyl-l-carnitine and CoA (for CPT2) accounted for CPT activities (Table 1). CPT1 activity, assayed as malonyl-CoA-sensitive CPT in intact mitochondria, was 7% of control values. In contrast, CPT2, assayed as soluble CPT activity after membrane disruption, was not deficient in patient (Table 1). Because fibroblasts produce only the CPT1A isoform, the patient was diagnosed as suffering from liver CPT1 deficiency. This diagnosis was further corroborated by biochemical and gene studies on blood (vide infra).
Table 1.
Oxidation of 14C-labeled fatty acids and mitochondrial carnitine palmitoyltransferase activities in cultured skin fibroblasts
| Substrate(s) | Patient | Simultaneous controls (n = 3) | Controls (n = 46) mean ± SD |
|---|---|---|---|
| Rates of oxidation of 14C-labeled fatty acids nmol 14CO2/h/106 cells [% simultaneous control values] | |||
| [1-14C]-palmitate | 0.08 [7%] | 0.65–1.37 | 0.56 ± 0.13 |
| [1-14C]-octanoate | 0.60 [77%] | 0.85–1.04 | 0.54 ± 0.14 |
| [1,4-14C]-succinate | 2.93 | 2.34–3.74 | 2.29 ± 0.41 |
| Carnitine palmitoyltransferase activities (nmol/min.mg protein) | |||
| Intact mitochondria | |||
| Palmitoyl-CoA +L-[methyl3H]-carnitine | |||
| Without Malonyl-CoA | 0.50 | 1.65–2.30–2.80 | 1.69 ± 0.60 |
| With Malonyl CoA | 0.40 | 0.16–0.28–0.60 | 0.26 ± 0.14 |
| CPT1 activity | 0.10 [7%] | 1.49–2.02–2.20 | 1.43 ± 0.44 |
| Disrupted mitochondria | |||
| Palmitoyl-l-[methyl3H] carnitine + CoASH | |||
| CPT2 activity | 3.13 | 2.13–2.24–3.01 | 2.79 ± 1.17 |
Fatty acid and succinate oxidation rates were measured in intact cultured fibroblasts by 14CO2 release from 14C labeled substrates. Carnitine palmitoyltransferases 1 (CPT1) and 2 (CPT2) were assayed in mitochondrial preparations by production of tritiated palmitoyl-l-carnitine and tritiated l-carnitine, respectively. CPT1 was measured as the malonyl-CoA-sensitive activity in intact mitochondria (activity without malonyl-CoA minus that under malonyl-CoA) and CPT2 as the activity upon membrane disruption. Each result is the mean of three separate experimental determinations in patient samples and simultaneous controls; reference control values expressed as means ± SD and residual fatty acid oxidation and CPT1 in patient samples appear between brackets as percentage values
Serum and Whole Blood Carnitine and Acylcarnitine Levels
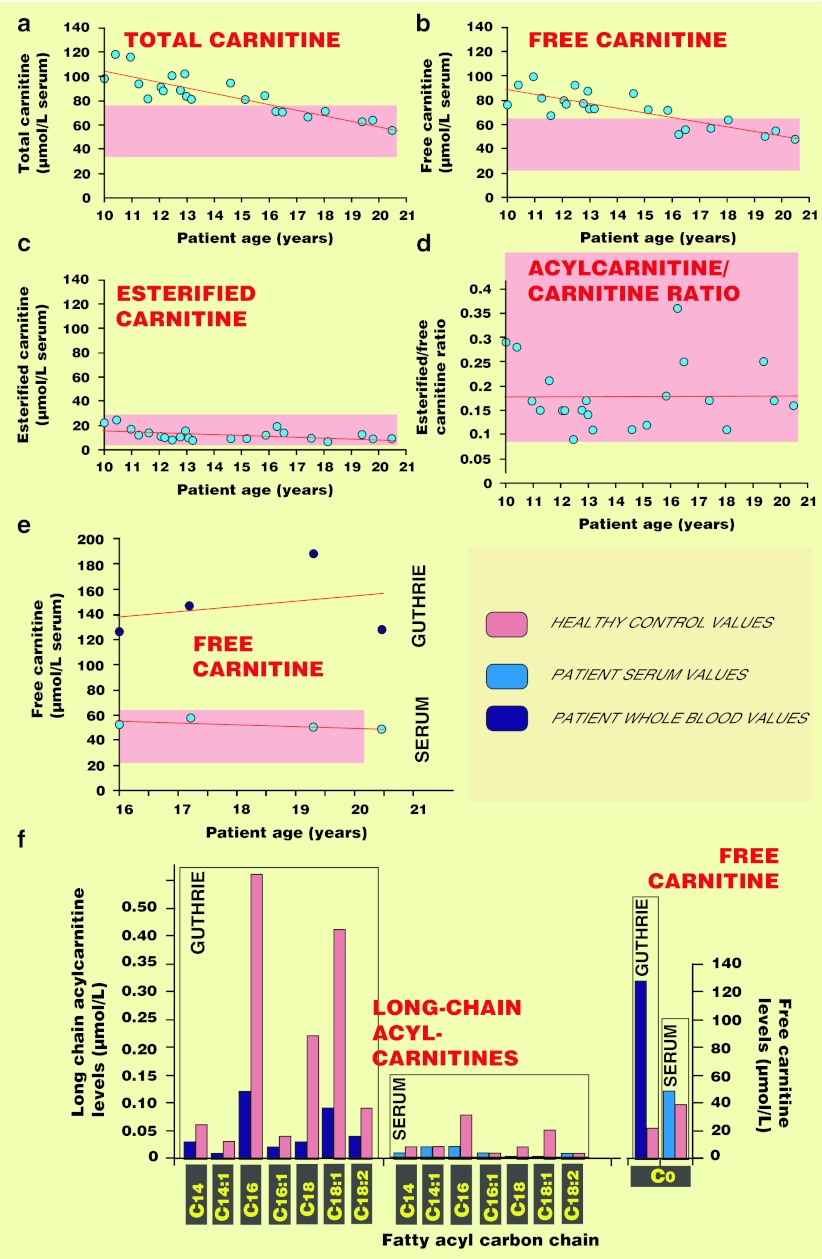
At 3 years of age, the patient’s levels of total carnitine had increased to 89 μmol/l, free and esterified carnitine levels were at 66 and 23 μmol/l, respectively, and the esterified: free-carnitine ratio was 0.35. Figure 1a–d shows the evolution of the patient’s serum carnitine concentrations over a life period of 10 years. The patient’s serum total and free-carnitine levels decreased progressively with time without an increase in the levels of esterified carnitine. Free l-carnitine, as measured in whole blood samples via spot deposit on Guthrie cards, was consistently above the upper limit even when serum values were normal (Fig. 1e). The drop in long-chain acylcarnitine levels was also better “visualized” in whole blood (Fig. 1f), also contributing to enhance whole blood free carnitine : long-chain acylcarnitine ratio.
Fig. 1.
Measurements of carnitine and acylcarnitine levels in serum and whole blood. Levels of total and free carnitine along with acylcarnitines (esterified carnitine) were measured in serum from the patient (a–d) at various time points during the last decade. Concomitant measurements in serum and whole blood (blood spots on Guthrie cards) drawn from the patient at similar time points were further compared, for their concentration of free carnitine (e) and individual long-chain acylcarnitines (f). Samples from a healthy control were run concomitantly. C0 fatty acyl carbon chain indicates the absence of esterification of carnitine and thus refers to free carnitine
De Novo Synthesis of Acylcarnitines by Whole Blood Samples
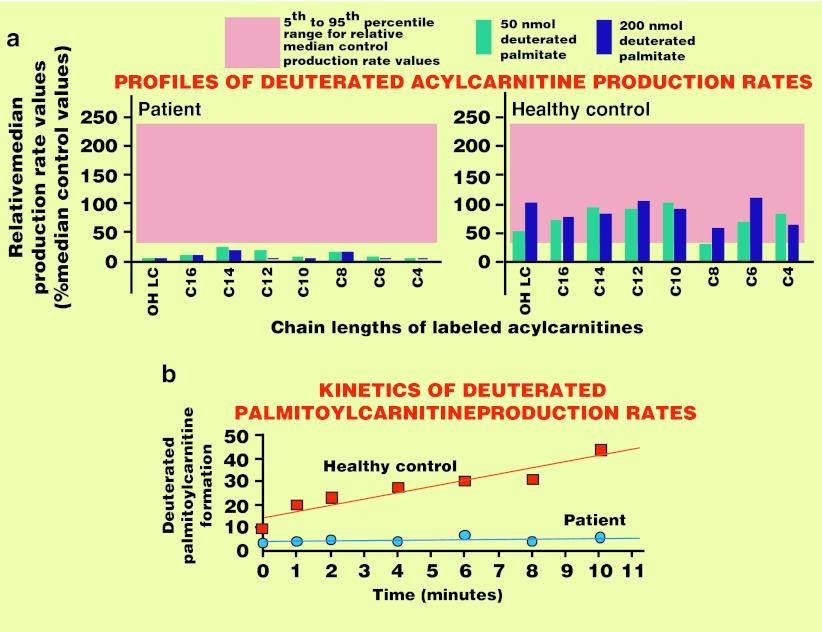
Relative median [D5]-acylcarnitine production rates generated from a 6-h incubation of patient whole blood samples with [16-2H3, 15-2H2]-[D5]-palmitate are presented in Fig. 2a. Compared to the profile of a healthy control generated in the same run and to median reference values from fifty healthy controls, the patient’s profile exhibited deficient production rates for all acylcarnitine species. As well as the data collected after the 6-h incubation period which allowed the qualitative assessment of CPT1 function, short incubation periods were also implemented to quantitatively assay CPT1. As illustrated in Fig. 2b, the patient’s rate of deuterated palmitoylcarnitine formation over the first 10-min period was only 10% of the rate observed in a simultaneous run with a healthy control.
Fig. 2.
Determination of deuterated acylcarnitine production rates in whole blood samples (a) The profiles of the rates at which individual deuterated acylcarnitines were formed from deuterated palmitate were determined simultaneously in whole blood samples from the patient and a healthy control. Weighted reference range values (5th to 95th percentile interval, n = 52) are given in the pink colored backgrounds. These profiles are usually determined to identify an impediment of mitochondrial fatty acid oxidation and to locate the site of the underlying enzyme failure. The blockade is here upstream to mitochondrial long-chain acylcarnitine formation, explaining why formation of all the individual acylcarnitines is strongly reduced and is thus consistent with a defect in the type 1 carnitine palmitoyltransferase, which physiologically produces long-chain acylcarnitines. (b) CPT1 is assayed by the ability of whole blood to generate deuterated palmitoylcarnitine from deuterated palmitate over short incubation time periods. Results are given as mean values of three separate determinations. It is assumed that the acyl-CoA synthetase, which precedes action of CPT1 is not rate limiting, a view corroborated by similar extents to which patient CPT1 is deficient in the present measurements and those performed on skin fibroblasts (see Table 1)
Genetic Studies
Sequencing of the 19 exons and flanking intronic regions of the CPT1A gene indicated that the patient was homozygous for a c.1783 C > T nucleotide substitution in exon 15 in which the well conserved arginine at position 595 was replaced by a tryptophan (p.R595W mutant). This mutation was identified in the patient at adulthood (22 years). Heterozygosity of both parents for the same mutation has subsequently been demonstrated by sequence analysis of genomic DNA samples.
Structural Analysis
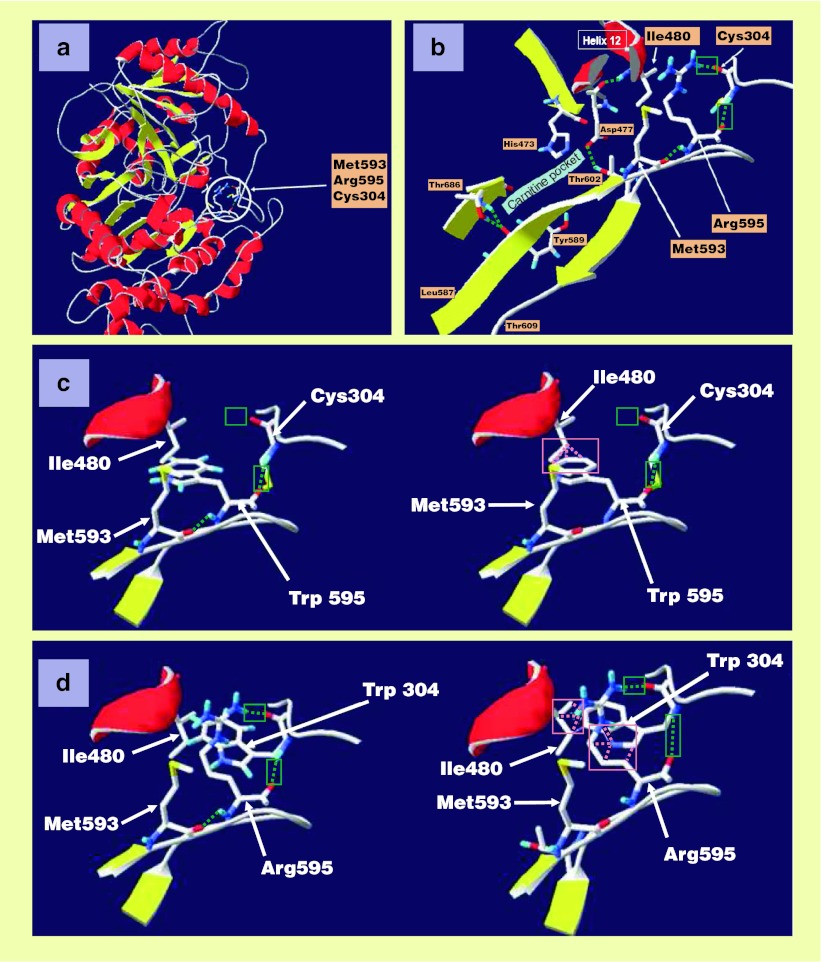
To assess disease-driven changes in protein structure, molecular modeling of the patient’s R585W CPT1 protein was carried out, and in the wake of this study, modeling of another protein mutant described in the literature, C304W CPT1, was also performed. A ribbon plot picture of wild-type CPT1A (residues 166–772) highlighting the localization of Arg595 and neighboring residues Met 593 and Cys304 is given in Fig. 3a. The region around Arg595 and amino acids playing a role in catalysis (His 473, Asp477, Thr 602, Tyr 589, and Thr 686) (Morillas et al. 2004) is detailed in Fig. 3b. Arg595 is not directly involved in the catalysis and carnitine pocket, although it does contribute to a hydrogen bond network with the neighboring residues Cys304 and Met593 (Fig. 3c). Its replacement by a tryptophan (p.Arg595Trp) disrupts the bond network by removing one of the two hydrogen bonds normally developed with Cys304 (Fig. 3c). Moreover, the bulky tryptophan side chain induces steric hindrance (“clashes”) with, and hence shifts, the side chain of Ile480 located in α-helix 12 (Fig. 3c). Such a twist may not only displace the α-helix 12 and destabilize protein architecture but may also affect the conformation of the carnitine pocket, and hence protein function. Interestingly, transfection studies for another CPT1A mutation, affecting one of the partners of Arg595, namely Cys304 (p.C304W mutant), evidenced lowered protein levels and abolished enzyme activity (Brown et al. 2001). These modeling studies show here for the first time that, although it maintains the two hydrogen bonds with Arg595, replacement of Cys304 by Trp304 introduces steric hindrance between the tryptophan and Arg595 and importantly also the side chain of Ile480 (Fig. 3d), suggesting that common pathogenesis mechanisms lead to the loss of CPT1A activity in the present R595W mutant and the previously described C304W mutant.
Fig. 3.
Modeling of wild-type and mutant CPT1A proteins. Wild-type protein structure and features are highlighted along with steric hindrance emerging between side chain of natural Ile480 and local tryptophan generated by a mutated codon. (a) Ribbon representation of the CPT1A homology model illustrates α-helices in red and β-strands in yellow. Arg595 and its two partners Met593 and Cys304 are drawn in stick representation within the blank circle. (b) An enlarged view of Arg595 and its neighboring residues stresses that Arg595 is at distance from the carnitine pocket, which contains the amino acids involved in catalysis (His 473, Asp477, Tyr 589, Thr 602, Thr686). Arg595 is located in the middle of a long stretch of conserved amino acid residues going from Leu587 to Thr609 and, via participation to a hydrogen bond network with Cys304 and Met593, stabilizes protein structure and hence protein function (hydrogen bonds are represented by green dash lines). (c) Replacement of Arg595 by Trp595 disrupts one hydrogen bond with Cys304 (left part of the panel) and causes several clashes (steric hindrances) with the side chain of Ile480 as represented in the right part of this panel by pink dash lines. (d) Replacement of Cys304 by Trp304 does not disrupt the two hydrogen bonds with Arg595 (left part) but, however, introduces clashes with Arg595 and also with the side chain of Ile480 (pink dash lines in the right part of the panel)
Discussion
The present work highlights the successive steps involved in pathogenesis from the gene defect to the patient’s clinical picture. The gene defect generates a protein mutant in which the tryptophan replacing Arg595 causes steric hindrance with Ile480. The latter is subsequently displaced and it is predicted that this leads to a shift in the position of the α-helix 12 in which Ile480 is located. This represents a coherent basis for disruption of normal protein structure and function, and, as presently assessed, CPT1 activity. Interestingly, evidence for steric hindrance due to a tryptophan residue also impacting the Ile480 side chain is also provided here for another protein mutant, R304W CPT1, previously shown to be unstable and inactive (Brown et al. 2001). The ultimate collapse in CPT1 activity gave rise in our patient to a clinical picture evocative of a mitochondrial fatty acid oxidation disorder and consisting of hypoketotic and hypoglycemic life-threatening episodes sometimes fatal in early childhood (Vockley 1994; Vockley and Whiteman 2002) along with hepatomegaly developing during these metabolic attacks. These episodes triggered by catabolic states (starvation, fever) are metabolically understood as resulting from a failure of mitochondrial fatty acid oxidation to produce the ketones and reducing equivalents (NADH + H+) needed to direct glyceraldehyde 3-P dehydrogenase activity toward glucose formation in liver. A clinical feature which remains unexplained is myolysis. Initially suspected in a patient with a CPT1A mutation common to Alaskan populations, the pathophysiology of this muscular lesion still remains enigmatic considering the normal activity of the M-CPT1 isoform in liver CPT1 deficiency (Bonnefont et al. 2004; Haworth et al. 1992; Olpin et al. 2001; Yamamoto et al. 1994). Moreover, the Inuit myopathy case is no longer regarded as being associated with the Inuit CPT1A mutation (Bennett et al. 2005). Therefore, muscle signs in our patient might be unique even if, at the present time, the exact link with deficient liver CPT1 activity remains to be elucidated. Mental retardation is not usually observed in fatty acid oxidation disorders suggesting that, in our patient, it most likely reflected CNS damage caused by the hypoglycemic episodes, which developed before adequate preventive/therapeutic measures were taken.
The present data provide interesting clues linking intracellular carnitine esterification to intracellular free carnitine depletion. Intracellular carnitine esterification was assessed by determining de novo synthesis rates of acylcarnitines in the patient and controls. In the patient, the rate of intracellular carnitine esterification, i.e. acylcarnitine formation, was strongly reduced. Intracellular free carnitine concentrations were assessed by comparing serum (a cell-free medium) to whole blood (serum plus cells) carnitine levels. Interestingly, although the patient’s free carnitine levels were either above or in normal range values in serum, they were, when measured, consistently increased in whole blood samples, indicating that intracellular free carnitine concentrations are increased under CPT1 deficiency. Therefore, the diagnostic value of whole blood is better than that of serum to account for increased levels in free carnitine linked to CPT1A deficiency.
Acknowledgments
The authors thank the patient, the patient’s parents and laboratory volunteer controls for participating in this study. Written informed consent was obtained from the patient and the patient’s parents for the present report. The authors gratefully acknowledge J.C. Vienne, I. Kumorek, F. Hottevart and A. Kerkove for their technical assistance. This work was supported by grants from the French Ministère de la Santé (PHRC 2003R/1903) and FMO (Fédération des Maladies Orphelines).
Synopsis
Features of a new c.1783 C > T nucleotide substitution in CPT1A exon 15 (p.R595W) include deficient palmitoylcarnitine formation from deuterated palmitate (assessed by a novel and safe in situ assay), molecular pathogenesis mechanisms that also apply partially to the CPT1A C304W mutant (steric hindrance with Ile 480 and α-helix 12), and a link between intracellular carnitine depletion and esterification.
Competing Interest
None declared
Footnotes
Competing interests: None declared
M. Fontaine and A.F. Dessein are co-first authors.
References
- Bennett MJ, Narayan SB, Santani AB (2005) Carnitine palmitoyltransferase 1A. In: Pagon RA, Bird TD, Dolan CR, Stephens K (eds) Gene reviews [Internet]. University of Washington, Seattle, WA, USA [updated 2010]
- Bonnefont JP, Djouadi F, Prip-Buus C, Gobin S, Munnich A, Bastin J. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspect Med. 2004;25:495–520. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Britton CH, Mackey DW, Esser V, et al. Fine chromosome mapping of the genes for human liver and muscle carnitine palmitoyltransferase I. Genomics. 1997;40:209–211. doi: 10.1006/geno.1996.4539. [DOI] [PubMed] [Google Scholar]
- Brown NF, Mullur RS, Subramanian I, et al. Molecular characterization of L-CPT I deficiency in six patients: insights into function of the native enzyme. J Lipid Res. 2001;42:1134–1142. [PubMed] [Google Scholar]
- Combet C, Jambon M, Deléage G, Geourjon C. Geno3D: automatic comparative molecular modelling of protein. Bioinformatics. 2002;18:213–214. doi: 10.1093/bioinformatics/18.1.213. [DOI] [PubMed] [Google Scholar]
- Cook GA, Mynatt RL, Kashfi K. Yonetani-Theorell analysis of hepatic carnitine palmitoyltransferase-I inhibition indicates two distinct inhibitory binding sites. J Biol Chem. 1994;269:8803–8807. [PubMed] [Google Scholar]
- Demaugre F, Bonnefont JP, Mitchell G, et al. Hepatic and muscular presentations of carnitine palmitoyl transferase deficiency: two distinct entities. Pediatr Res. 1988;24:308–311. doi: 10.1203/00006450-198809000-00006. [DOI] [PubMed] [Google Scholar]
- Dessein AF, Fontaine M, Dobbelaere D, et al. Deuterated palmitate-driven acylcarnitine formation by whole-blood samples for a rapid diagnostic exploration of mitochondrial fatty acid oxidation disorders. Clin Chim Acta. 2009;406:23–26. doi: 10.1016/j.cca.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Esser V, Britton CH, Weis BC, Foster DW, McGarry JD. Cloning, sequencing, and expression of a cDNA encoding rat liver carnitine palmitoyltransferase I. Direct evidence that a single polypeptide is involved in inhibitor interaction and catalytic function. J Biol Chem. 1993;268:5817–5822. [PubMed] [Google Scholar]
- Fontaine M, Porchet N, Largilliere C, et al. Biochemical contribution to diagnosis and study of a new case of D-glyceric acidemia/aciduria. Clin Chem. 1989;35:2148–2151. [PubMed] [Google Scholar]
- Fontaine M, Briand G, Vallée L, et al. Acylcarnitine removal in a patient with acyl-CoA beta-oxidation deficiency disorder: effect of L-carnitine therapy and starvation. Clin Chim Acta. 1996;252:109–122. doi: 10.1016/0009-8981(96)06323-1. [DOI] [PubMed] [Google Scholar]
- Haworth JC, Demaugre F, Booth FA, et al. Atypical features of the hepatic form of carnitine palmitoyltransferase deficiency in a Hutterite family. J Pediatr. 1992;121:553–557. doi: 10.1016/S0022-3476(05)81143-6. [DOI] [PubMed] [Google Scholar]
- Houten SM, Wanders RJ (2010) A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J Inherit Metab Dis 33:469–477. http://www.expasy.org/spdbv [DOI] [PMC free article] [PubMed]
- Kashfi K, Cook GA. Topology of hepatic mitochondrial carnitine palmitoyltransferase I. Adv Exp Med Biol. 1999;466:27–42. doi: 10.1007/0-306-46818-2_3. [DOI] [PubMed] [Google Scholar]
- Morillas M, Gómez-Puertas P, Rubi BA, et al. Structural model of a malonyl-CoA-binding site of carnitine octanoyltransferase and carnitine palmitoyltransferase I: mutational analysis of malonyl-CoA affinity domain. J Biol Chem. 2002;277:11473–11480. doi: 10.1074/jbc.M111628200. [DOI] [PubMed] [Google Scholar]
- Morillas M, Gómez-Puertas P, Bentebibel A, et al. Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase 1 critical for malonyl-CoA- inhibition. Mutation of methionine 593 abolishes malonyl-CoA inhibition. J Biol Chem. 2003;278:9058–9063. doi: 10.1074/jbc.M209999200. [DOI] [PubMed] [Google Scholar]
- Morillas M, López-Viñas E, Valencia A, et al. Structural model of carnitine palmitoyltransferase I based on the carnitine acetyltransferase crystal. Biochem J. 2004;379:777–784. doi: 10.1042/BJ20031373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy MS, Pande SV. Malonyl-CoA binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc Natl Acad Sci USA. 1987;84:378–382. doi: 10.1073/pnas.84.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olpin SE, Allen J, Bonham JR, et al. Features of carnitine palmitoyltransferase type I deficiency. J Inherit Metab Dis. 2001;24:35–42. doi: 10.1023/A:1005694320063. [DOI] [PubMed] [Google Scholar]
- Price NT, van der Leij FR, Jackson VN, et al. A novel brain-expressed protein related to carnitine palmitoyltransferase 1. Genomics. 2002;80:433–442. doi: 10.1006/geno.2002.6845. [DOI] [PubMed] [Google Scholar]
- Sierra AY, Gratacós E, Carrasco P, et al. CPT1c is localized in endoplasmic reticulum of neurons and has carnitine palmitoyltransferase activity. J Biol Chem. 2008;283:6878–6885. doi: 10.1074/jbc.M707965200. [DOI] [PubMed] [Google Scholar]
- Vianey-Saban C, Mousson B, Bertrand C, et al. Carnitine palmitoyl transferase I deficiency presenting as a Reye-like syndrome without hypoglycaemia. Eur J Pediatr. 1993;152:334–338. doi: 10.1007/BF01956748. [DOI] [PubMed] [Google Scholar]
- Vockley J. The changing face of disorders of fatty acid oxidation. Mayo Clin Proc. 1994;69:249–257. doi: 10.1016/S0025-6196(12)61064-7. [DOI] [PubMed] [Google Scholar]
- Vockley J, Whiteman DA. Defects of mitochondrial beta-oxidation: a growing group of disorders. Neuromuscul Disord. 2002;12:235–246. doi: 10.1016/S0960-8966(01)00308-X. [DOI] [PubMed] [Google Scholar]
- Wolfgang MJ, Lane MD. Hypothalamic malonyl-CoA and CPT1c in the treatment of obesity. FEBS J. 2011;278:552–558. doi: 10.1111/j.1742-4658.2010.07978.x. [DOI] [PubMed] [Google Scholar]
- Wolfgang MJ, Kurama T, Dai Y, et al. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proc Natl Acad Sci USA. 2006;103:7282–7287. doi: 10.1073/pnas.0602205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Abe H, Kanazawa M et al (1994) Clinical, biochemical and molecular studies in fatty acid oxidation disorders (1): a Japanese case with carnitine palmitoyltransferase I deficiency presenting familial Reye-like episode. In: VI International congress inborn errors of metabolism, Milano, Italy, 27–31 May 1994
- Yamazaki N, Shinohara Y, Shima A, Terada H. High expression of a novel carnitine palmitoyltransferase I like protein in rat brown adipose tissue and heart: isolation and characterization of its cDNA clone. FEBS Lett. 1995;363:41–45. doi: 10.1016/0014-5793(95)00277-G. [DOI] [PubMed] [Google Scholar]