Abstract
The m.3243A>G is the most prevalent pathogenic mtDNA mutation but little is known about its inheritance. We studied 34 families containing 56 mother-child relations and 82 intersibling relations to investigate its transmission. We found a significant correlation between mother and child heteroplasmy levels (r = 0.679, p < 0.001). In mothers with a heteroplasmy level of below 25% we found 30% offspring without detectable mutation, while in mothers with a heteroplasmy level of above 25%, 100% of the offspring showed the m.3243A>G mutation. Heteroplasmy levels between siblings also correlated (r = 0.512, p < ;0.001), but had limited extra predictive value because of outliers. These new data on inheritance of the m.3243A>G mutation might be of value in counseling patients and preventing transmission of the mutation.
Introduction
Goto et al. first described the adenine to guanine transition at position 3243 of mitochondrial DNA (m.3243A>G) in the MT-TL1 gene encoding tRNALEU(UUR) as the cause of mitochondrial myopathy, encephalopathy, lactate acidosis, and stroke-like episodes (MELAS) syndrome (Goto et al. 1990). Over time, more phenotypic variations of the m.3243A>G mutation have been reported, the most frequent being maternally inherited diabetes and deafness (MIDD) (van den Ouweland et al. 1992). Other variations include cardiac, ocular, and renal involvement (Lev et al. 2004; Lowik et al. 2005; Michaelides et al. 2008).
The m.3243A>G mutation is the most prevalent pathogenic mitochondrial mutation (Greaves and Taylor 2006) with a mutation prevalence ranging from 7.59 to 236/100.000 (Chinnery et al. 2000a; Majamaa et al. 1998; Manwaring et al. 2007).
Several cohort studies have been performed, containing mainly clinical and epidemiological data about carriers of the m.3243A>G mutation (Chinnery et al. 2000a; de Laat et al. 2012; Guillausseau et al. 2001; Katulanda et al. 2008; Ma et al. 2010; Majamaa-Voltti et al. 2006; Majamaa et al. 1998; Manwaring et al. 2007; Parsons et al. 2010) and some containing data on inheritance of the m.3243A>G mutation (Chinnery et al. 2000b; Frederiksen et al. 2006). In contrast to other mitochondrial mutations, sporadic presence of the m.3243A>G mutation is rare (Cree et al. 2009). Providing additional data on inheritance of mitochondrial mutations is essential to prevent transmission of these mutations. Poulton et al. reviewed the different possibilities to prevent transmission of maternally inherited mitochondrial diseases (Poulton et al. 2009). We describe a cohort of 34 families in which 82 carry the m.3243A>G mutation, focusing on 56 mother-child relations and 82 intersibling relations. The main purpose of this study is to indentify female carriers in whom transmission of the m.3243A>G mutation is present and in whom it is not definite.
Methods
All probands are patients of the Nijmegen Center for Mitochondrial Disorders at the Radboud University Nijmegen Medical Centre, diagnosed with the m.3243A>G mutation in muscle or blood. All patients participated in our cohort study for the m.3243A>G mutation (de Laat et al. 2012). This study was approved by the ethics committee of the Nijmegen-Arnhem region. Written informed consent according to the Helsinki agreement was obtained from all parents and patients ≥12 years.
Heteroplasmy levels where determined in urinary epithelial cells (UEC) in all participants using PyrosequencingTM technology (Pyrosequencing, Uppsala, Sweden) as earlier described by Lowik et al. 2005. The pyrosequence reaction of the m.3234A>G mutation had a precision of 1.5%, and the mutation was detected from a heteroplasmy level of 5%. The detection limit for the MELAS mutation (m.3243A>G) was determined by serial dilution of a sample containing this mutation with wild-type mtDNA.
We analyzed the differences in heteroplasmy between mother and child and between familial siblings; the heteroplasmy levels of the older sibling were compared to that of the younger sibling. We used descriptive statistics to present the heteroplasmy levels in our patients, and Pearson’s correlation coefficient was used to evaluate the relationship between the heteroplasmy levels between mother and child and between siblings.
Results
In our cohort of 82 patients from 34 families we identified 56 mother-child relations and 82 intersibling relations.
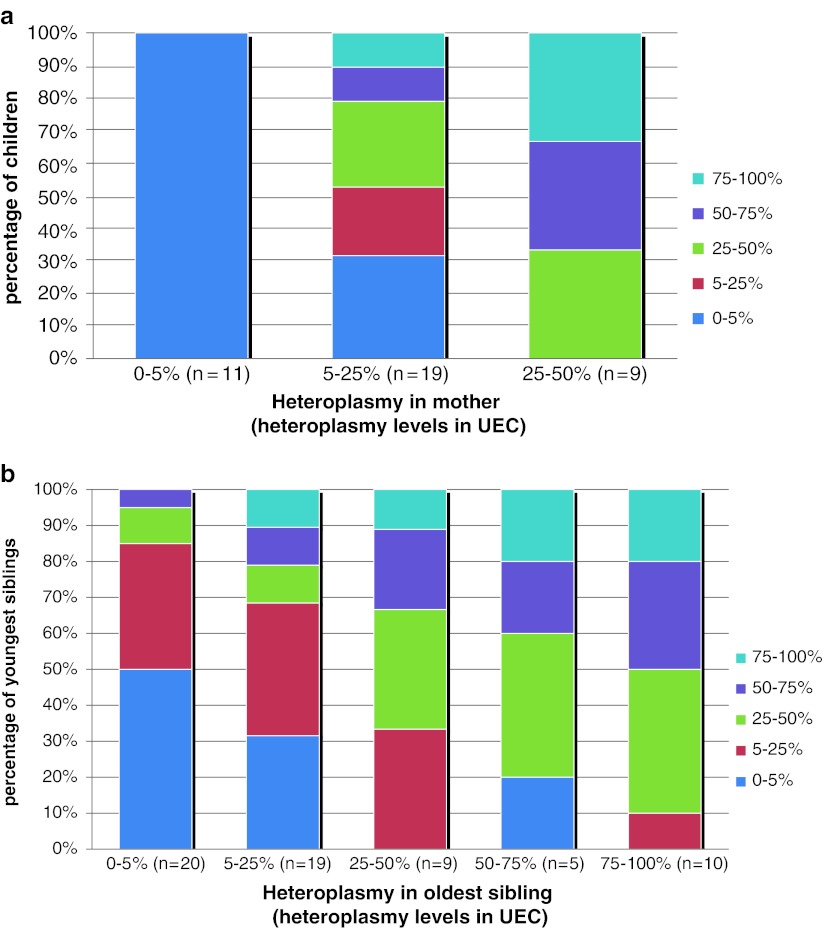
Of the 56 mother-child relations, ten mothers had one child, 14 mothers had two children and 6 mothers had 3 children. A total of 14 mothers that did not carry the m.3243A>G mutation were included, 11 mothers had a detectable mutation load but had a heteroplasmy level of below 25% and 5 mothers had a heteroplasmy level between 25% and 50%. There were no mothers with a heteroplasmy level above 50%. The rate of transmission of the mutation is visualized in Fig. 1a. Here we describe the transmission in more detail. In the mothers lacking the mutation, we found a detectable mutation in three children. These three patients were the only ones in their pedigree that had a detectable mutation; we therefore concluded that these patients were most likely to have a de novo mutation. We excluded these three families, because there was (so far) no inheritance of the mutation in these families. When we excluded these three families, a total of nine mothers were excluded. The five remaining mothers (with an undetectable heteroplasmy level) were sisters of a proband. In four cases, we found a detectable mutation in the mothers of these women. In one case the mother had passed away, so no heteroplasmy level could be determined.
Fig. 1.
(a) Heteroplasmy levels in children divided into three groups depending on the heteroplasmy level of their mother; n gives the number of mother child relations in every group. (b) Heteroplasmy levels in siblings divided in five groups depending on the heteroplasmy level of the older sibling; n gives the number of intersibling relations in every group
The 11 mothers with a detectable mutation load but a heteroplasmy level below 25% had 19 children. Six of these children (32%) had no detectable mutation. In the 13 children where the mutation was present, 4 patients were between 5% and 25%, 5 patients were between 25% and 50%, and 4 patients were above 50% (resp. 61%, 64%, 75% and 93%).
The five mothers with a heteroplasmy level between 25% and 50% had nine children, all with detectable heteroplasmy levels. Three children were between 25% and 50%, three children were between 50% and 75% and three children were above 75%.
A correlation coefficient of 0.679 (p < 0.001) was found between the heteroplasmy levels of mothers and their children.
In our cohort, we indentified 82 intersibling relations. When we excluded the three families with the sporadic mutation, 63 intersibling relations remained. We divided this group into five subgroups depending on the heteroplasmy level of the oldest sibling, (resp. <5%, 5–25%, 26–50%, 51–75% and >75%). The relationship between the heteroplasmy levels in the siblings is indicated in Fig. 1b. Fifty percent of the oldest siblings with an undetectable mutation had a younger sibling with a detectable mutation. In these 10 siblings, the mother had a detectable mutation load in four cases; in the other six cases the heteroplasmy level of the mother was unknown. Of the 19 cases where the older sibling had a heteroplasmy level between 5% and 25%, the younger sibling had an undetectable mutation in 6 cases (32%). In the 24 cases where the older sibling had a heteroplasmy level higher than 25%, one younger sibling (4%) had an undetectable mutation. A correlation coefficient of 0.512 (p < 0.001) was found between the heteroplasmy levels of the siblings.
Discussion
Because of the unique characteristics of mitochondrial inheritance (mitotic segregation, heteroplasmy, and maternal inheritance), genetic counseling in mitochondrial diseases caused by an mtDNA mutation has always been very difficult (Cree et al. 2009). With this study we provide additional information regarding the inheritance of the m.3243A>G mutation. We used UEC to analyze heteroplasmy levels as different studies have shown a good relation between clinical phenotype and heteroplasmy in UEC (Whittaker et al. 2009). Besides, heteroplasmy in UEC is more stable compared to heteroplasmy in lymphocytes (de Laat et al. 2012; Ma et al. 2009). We must keep in mind that although there is a correlation between heteroplasmy levels in UEC and clinical symptoms, there are also patients with high levels of heteroplasmy that do not present with clinical symptoms during childhood. It is hypothesized, but as of yet unknown, whether these “dormant carriers” develop symptoms when they reach adulthood (de Laat et al. 2012).
We found a correlation of r = 0.679 (p < 0.001) between the heteroplasmy levels in mothers and their offspring, indicating that mothers with higher heteroplasmy levels have offspring with higher heteroplasmy levels. However, as indicated in Fig. 1a, there is quite a variance in the transmission of the m.3243A>G mutation. We demonstrated that mothers with a heteroplasmy level of above 25% transmit the mutation to all their offspring, providing clarity for patients. Thirty percent of offspring from mothers who have a heteroplasmy level below 25% will have undetectable heteroplasmy levels of the m.3243A>G mutation (using the current techniques). These findings support the use of such techniques as pre-implantation genetic diagnosis (PGD) to select for unaffected offspring in this group. In mothers with a heteroplasmy level of higher than 25%, all the offspring are affected, making it only possible to select for the embryo with the lowest level of heteroplasmy using PGD. The only options that remain for these women is oöcyte donation or adoption to be 100% sure that there will be no transmission of the m.3243A>G mutation. A future possibility for these women could be nuclear transfer (Poulton et al. 2009).
Besides transmission from mother to child, we also investigated the relationship between heteroplasmy levels of siblings, in order to see if heteroplasmy levels in a child may provide extra information in predicting the heteroplasmy level of further progeny. Figure 1b indicates that the heteroplasmy level in the oldest sibling has some predictive value for younger siblings, but again there are outliers. In the group where the oldest sibling does not have a detectable mutation, there is still a 50% chance that the second sibling will have the mutation, and one patient even had a heteroplasmy level of above 50%. On the other side of the spectrum, we find an almost identical case in which the oldest sibling has a high (above 50%) level of heteroplasmy but the mutation could not be detected in the younger sibling. So, even though there is a correlation between the heteroplasmy levels in siblings (r = 0.512, p < 0.001), the presence of these outliers makes it difficult to offer genetic counseling to these families.
We conclude that the female carriers of the m.3243A>G mutation do not transmit the mutation to all of their offspring. We suggest that mothers with a heteroplasmy level below 25% could consider using techniques like PGD to prevent transmission in case of child wish. Long-term follow-up studies should be performed to examine the stability of the heteroplasmy values, to investigate whether PGD for women with heteroplasmy levels above 25% is feasible.
Acknowledgements
Part of this work was supported by the Energy4All foundation and ZonMW.
Abbreviations
- mtDNA
Mitochondrial DNA
- nDNA
Nuclear DNA
- UEC
Urinary Epithelial Cells
- MELAS
Mitochondrial myopathy, Encephalopathy, Lactate Acidosis, and Stroke-like episodes
- MIDD
Maternally Inherited Diabetes and Deafness
- PGD
Preimplantation Genetic Diagnosis
Conflicts of Interest
Authors report no conflicts of interest.
Key Sentence/Synopsis
In mothers carrying an m.3243A>G mutation with a heteroplasmy level of below 25%, we found that 30% of the offspring were unaffected. This new data on inheritance of the m.3243A>G mutation will be of great help for counseling patients and preventing transmission of the mutation.
Footnotes
Competing interests: None declared
References
- Chinnery PF, Johnson MA, Wardell TM, et al. The epidemiology of pathogenic mitochondrial DNA mutations. Ann Neurol. 2000;48:188–193. doi: 10.1002/1531-8249(200008)48:2<188::AID-ANA8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Chinnery PF, Thorburn DR, Samuels DC, et al. The inheritance of mitochondrial DNA heteroplasmy: random drift, selection or both? Trends Genet. 2000;16:500–505. doi: 10.1016/S0168-9525(00)02120-X. [DOI] [PubMed] [Google Scholar]
- Cree LM, Samuels DC, Chinnery PF. The inheritance of pathogenic mitochondrial DNA mutations. Biochim Biophys Acta. 2009;1792:1097–1102. doi: 10.1016/j.bbadis.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat P, Koene S, van den Heuvel LP, Rodenburg RJ, Janssen MC and Smeitink JA (2012) Clinical features and heteroplasmy in blood, urine and saliva in 34 Dutch families carrying the m.3243A>G mutation. J Inherit Metab Dis [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Frederiksen AL, Andersen PH, Kyvik KO, Jeppesen TD, Vissing J, Schwartz M. Tissue specific distribution of the 3243A->G mtDNA mutation. J Med Genet. 2006;43:671–677. doi: 10.1136/jmg.2005.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348:651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Greaves LC, Taylor RW. Mitochondrial DNA mutations in human disease. IUBMB Life. 2006;58:143–151. doi: 10.1080/15216540600686888. [DOI] [PubMed] [Google Scholar]
- Guillausseau PJ, Massin P, Dubois-LaForgue D, et al. Maternally inherited diabetes and deafness: a multicenter study. Ann Intern Med. 2001;134:721–728. doi: 10.7326/0003-4819-134-9_part_1-200105010-00008. [DOI] [PubMed] [Google Scholar]
- Katulanda P, Groves CJ, Barrett A, et al. Prevalence and clinical characteristics of maternally inherited diabetes and deafness caused by the mt3243A > G mutation in young adult diabetic subjects in Sri Lanka. Diabet Med. 2008;25:370–374. doi: 10.1111/j.1464-5491.2007.02377.x. [DOI] [PubMed] [Google Scholar]
- Lev D, Nissenkorn A, Leshinsky-Silver E, et al. Clinical presentations of mitochondrial cardiomyopathies. Pediatr Cardiol. 2004;25:443–450. doi: 10.1007/s00246-003-0490-7. [DOI] [PubMed] [Google Scholar]
- Lowik MM, Hol FA, Steenbergen EJ, Wetzels JF, van den Heuvel LP. Mitochondrial tRNALeu(UUR) mutation in a patient with steroid-resistant nephrotic syndrome and focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2005;20:336–341. doi: 10.1093/ndt/gfh546. [DOI] [PubMed] [Google Scholar]
- Ma Y, Fang F, Cao Y, et al. Clinical features of mitochondrial DNA m.3243A>G mutation in 47 Chinese families. J Neurol Sci. 2010;291(1–2):17–21. doi: 10.1016/j.jns.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Ma Y, Fang F, Yang Y, et al. The study of mitochondrial A3243G mutation in different samples. Mitochondrion. 2009;9:139–143. doi: 10.1016/j.mito.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Majamaa-Voltti KA, Winqvist S, Remes AM, et al. A 3-year clinical follow-up of adult patients with 3243A>G in mitochondrial DNA. Neurology. 2006;66:1470–1475. doi: 10.1212/01.wnl.0000216136.61640.79. [DOI] [PubMed] [Google Scholar]
- Majamaa K, Moilanen JS, Uimonen S, et al. Epidemiology of A3243G, the mutation for mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes: prevalence of the mutation in an adult population. Am J Hum Genet. 1998;63:447–454. doi: 10.1086/301959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwaring N, Jones MM, Wang JJ, et al. Population prevalence of the MELAS A3243G mutation. Mitochondrion. 2007;7:230–233. doi: 10.1016/j.mito.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Michaelides M, Jenkins SA, Bamiou DE, et al. Macular dystrophy associated with the A3243G mitochondrial DNA mutation. Distinct retinal and associated features, disease variability, and characterization of asymptomatic family members. Arch Ophthalmol. 2008;126:320–328. doi: 10.1001/archopht.126.3.320. [DOI] [PubMed] [Google Scholar]
- Parsons T, Weimer L, Engelstad K, et al. Autonomic symptoms in carriers of the m.3243A>G mitochondrial DNA mutation. Arch Neurol. 2010;67:976–979. doi: 10.1001/archneurol.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton J, Kennedy S, Oakeshott P, Wells D. Preventing transmission of maternally inherited mitochondrial DNA diseases. Bmj. 2009;338:b94. doi: 10.1136/bmj.b94. [DOI] [PubMed] [Google Scholar]
- van den Ouweland JM, Lemkes HH, Ruitenbeek W, et al. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet. 1992;1:368–371. doi: 10.1038/ng0892-368. [DOI] [PubMed] [Google Scholar]
- Whittaker RG, Blackwood JK, Alston CL, et al. Urine heteroplasmy is the best predictor of clinical outcome in the m.3243A>G mtDNA mutation. Neurology. 2009;72:568–569. doi: 10.1212/01.wnl.0000342121.91336.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]