Abstract
Introduction: Trimethylaminuria is a malodour syndrome caused by a functional defect of flavin-containing monoxygenase 3 (FMO3), resulting in accumulation of trimethylamine in body secretions. Recently, (E, E)-2, 4-undecadienal has been shown to deodorize the offensive odour of cooked porcine intestines (chitlins). We tested the deodorizing effect of commercially available (E, E)-2, 4-undecadienal on the odour of trimethylamine (TMA) in solution.
Study Participants: Eleven volunteers among staff of the Children’s Hospital at Westmead, Sydney, Australia.
Methods: This was a study in three stages. In the first stage,12 volunteers sniffed and graded a commercially available trimethylamine at variable concentrations (12.5–10,000 μmol/L). Those who could smell trimethylamine scored the odour of mixtures of (E, E)-2, 4-undecadienal and trimethylamine. Finally, the odour of trimethylamine was graded with increasing concentrations of (E, E)-2, 4-undecadienal (0.1–100 ppm).
Results: All except one could detect the characteristic trimethylamine odour at varying concentrations (12.5–10,000 μmol/L) and reported the odour as offensive and fish like. There was a dose response effect of the ability of (E, E)-2, 4-undecadienal to deodorize the odour of trimethylamine. (E, E)-2, 4-undecadienal at 10 ppm appeared to deodorize the odour of trimethylamine at 1,000 μmol/L without making the former’s odour obvious.
Conclusions: We have demonstrated that (E, E)-2, 4-undecadienal has a deodorizing effect on the offensive odour of trimethylamine in solution. The mechanism of action for this effect and potential for treatment of affected individuals needs further research.
Introduction
Trimethylaminuria (TMAU) (OMIM # 602079) is a metabolic disorder characterized by decreased ability to oxidize and convert dietary-derived trimethylamine (TMA), an aliphatic tertiary amine, to odourless trimethylamine N-Oxide (TMAO) (Humbert et al. 1970; Chalmers et al. 2006). This disorder results in affected patients secreting volatile and malodorous (like rotting fish) TMA in their breath, sweat, urine and other body secretions. This compound can be detected by humans at very low concentrations (<1 ppm) (Willey 1985). Fortunately, accumulation of TMA has no deleterious physical effect but may cause devastating social debilitation and long-term psychiatric consequences (Christodoulou 2012; Mountain et al. 2008). The few therapeutic options for management include dietary restriction of choline- and lecithin-containing foods, avoidance of drugs like carnitine and betaine, copper chlorophyll and/or activated charcoal as binding agents to reduce systemic absorption, probiotics and intermittent antibiotics to balance and/or reduce gut bacterial load (Chalmers et al. 2006; Buby et al. 2004; Treacy et al. 1995).
There is a need to expand the available therapeutic armamentarium. The mechanism of action of chlorophyll in deodorizing TMA odour by forming complexes opens avenues for exploring other compounds (Dashwood et al. 1996). This is intriguing in the context that recently (E, E)-2, 4-undecadienal, a naturally derived compound isolated from coriander leaves, was demonstrated to have a deodorant effect against the offensive odour of cooked porcine large intestine (chitlins) (Ikeura et al. 2010; Kohara et al. 2006).
We decided to test the hypothesis that commercially available (E, E)-2, 4-undecadienal may have a neutralizing effect on the malodour of TMA in solution.
Methods
The study protocol was approved by the Human Research Ethics Committee (HREC), the Children’s Hospital at Westmead, Sydney, Australia.
One hundred and twenty staff members of the Western Sydney Genetics Program were invited in writing to participate in this study. Twelve volunteers (age range 23–57 years, eight females and four males) responded and provided written consent to participate in the study. Five volunteers reported some kind of nasal allergies.
The study was conducted in three stages. In each stage a five-point odour detection scale (given below) was used for volunteers to grade the odour of the TMA alone (stage one) or mixture of TMA and (E, E)-2, 4-undecadienal (stages two and three).
Barely detectable
Mild odour (faint)
Moderately strong odour (obvious)
Strong odour
Very strong repulsive odour
The samples were presented in 60 mL open specimen containers, starting with a distance of about a forearm length from the nose. Subsequently, the container was slowly brought closer to the nose until the volunteer could clearly smell the odour. In order to avoid habituation of odour stimuli, each presentation was followed by a rest period of about 30 s. This coincided with the time taken for opening of jars and communicating the next step to the volunteer. As a rule, each sample was presented only once except in some cases where a volunteer requested to smell again to be sure to quantify the difference in odour intensity. In such a situation, a rest period of about 30 s was given.
In stage one, 12 volunteers were asked to rate increasing concentrations of TMA (12.5−10,000 μmol/L). One volunteer was excluded from entering into the next stage of the study as she could not detect the odour of TMA at any concentration.
In stage two, 11 volunteers rated three concentrations of TMA (1,000, 5,000, and 10,000 μmol/L) as controls, followed by ten mixtures of variable concentrations of TMA and (E, E)-2, 4-undecadienal (Table 1). Volunteers were asked to compare the odour of TMA on its own with the odour of the mixtures, comment on the type of odour (whether it is TMA or some other odour), and report whether it was offensive or non-offensive.
Table 1.
Proportion of volunteers who could smell TMA in mixtures
| Stage | TMA mixturesa(Stage II) | ||||||||||
| Mixture n (%) | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | |
| II | Yes | 4 (36.4) | 4 (36.4) | 1 (9.1) | 5 (45.5) | 4 (36.4) | 3 (27.3) | 7 (63.6) | 4 (36.4) | 3 (27.3) | 11 (100.0) |
| No | 7 (63.6) | 7 (63.6) | 10 (90.9) | 6 (54.5) | 7 (63.6) | 8 (72.7) | 4 (36.4) | 7 (63.6) | 8 (72.7) | 0 | |
| III | Fixed TMA and variable (E, E)-2, 4-undecadienal (Stage III) | ||||||||||
| Concentration n (%) | 0 | 0.1 | 1 | 10 | 25 | 50 | 100 | ||||
| Yes | 11 (100.0) | 10 (90.9) | 9 (81.8) | 4 (36.4) | 0 | 1 (9.1) | 1 (9.1) | ||||
| No | 0 | 1 (9.1) | 2 (18.2) | 7 (63.6) | 11 (100.0) | 10 (90.9) | 10 (90.9) | ||||
aM1–M3 mixtures of 1,000 μmol/L TMA and 1, 10, and 100 ppm (E, E)-2, 4-undecadienal respectively; M4–M6 (5,000 μmol/L TMA and 1, 10, and 100 ppm (E, E)-2, 4-undecadienal, respectively); M7–M9 (10,000 μmol/L TMA and 1, 10, and 100 ppm (E, E)-2, 4-undecadienal, respectively); M10 (10,000 μmol/L TMA and 0.1 ppm (E, E)-2, 4-undecadienal)
In stage three, 11 volunteers rated the odour of a mixture with 1,000 μmol/L of TMA and increasing concentrations of (E, E)-2, 4-undecadienal (0, 0.1, 10, 20, 25, 50, and 100 ppm).
Preparation of the Study Reagents
Trimethylamine hydrochloride was obtained from Sigma-Aldrich, Castle Hill, NSW, Australia. Stock standards were prepared by dissolving in ultra-pure water and stored in sealed containers at room temperature.
(E, E)-2, 4-undecadienal was obtained from Alfa Aesar, Bioscientific, Gymea, NSW, Australia. The compound is an oily liquid at room temperature and varying concentrations were produced from a stock emulsion of 1,000 ppm in ultra-pure water and stored at 4 °C. There was no evidence of the compound coming out of emulsion on storage at the concentrations used.
Mixtures of (TMA and (E, E)-2, 4-undecadienal) in water were prepared at various concentrations from the stocks and stored in sealed containers at room temperature.
Statistical Analysis
The relationship between odour scores and concentrations of TMA and (E, E)-2, 4-undecadienal was examined using Generalized Estimating Equations (GEE) regression. This regression analysis accounts for the potential correlation between odour scores of the same patient (Hanley et al. 2003). The relationship between binary outcomes (proportion detecting TMA or not) and the concentration levels was examined using GEE logistic regression. Compound symmetry covariance matrix was applied in the models to treat repeated measurements as independent in time, but constantly correlated for the odour scores for the same subjects (Little et al. 2000). All analyses were performed using SAS version 9.2 (SAS Institute. SAS: statistical software. 9.1 ed. Cary, North Carolina. SAS Institute, 2003).
Results
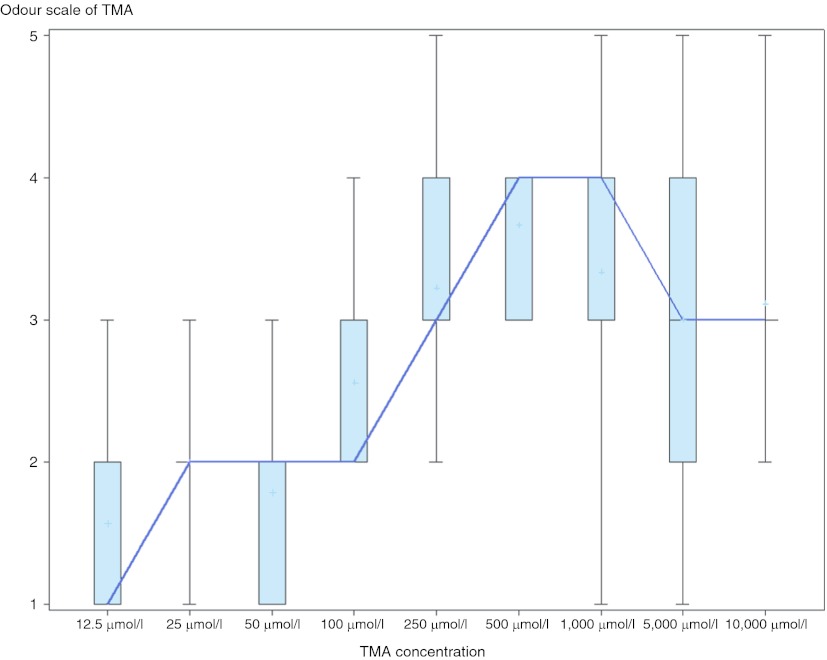
In stage one, all except one test subject could smell TMA at varying concentrations (12.5–10,000 μmol/L). There was a wide variation of odour scores with increasing concentration of TMA indicating inter-observer variation. There was a positive relationship between odour scale and TMA, which plateaued at around a concentration of 1,000 μmol/L (Fig. 1).
Fig. 1.
Boxplot of odour scores for increasing concentration of TMA (Stage I). *Bold line represents median and ‘+’ represents mean
In the second stage of the study, the proportion of subjects who could smell TMA was significantly less in mixtures (M3, M6, M9) with high concentration of (E,E)-2,4-undecadienal (Table 1). There were significantly increased odds of smelling TMA with increasing concentration of TMA, while increasing the concentration of (E, E)-2, 4-undecadienal decreased the odds of smelling TMA in mixtures (Table 2). For an increase of 10 μmol/L TMA, there was a 0.4% increased odds of smelling TMA.
Table 2.
The relationship between the odds of smelling TMA with variable concentration of TMA and (E, E)-2, 4-undecadienal (Stage II)
| ORa | 95 % CI | p-value | |
|---|---|---|---|
| TMA | 1.004 | (1.00–1.01) | 0.003 |
| Undecadienal | 0.79 | (0.66, 0.95) | 0.014 |
aOdds ratios for every 10 unit increase
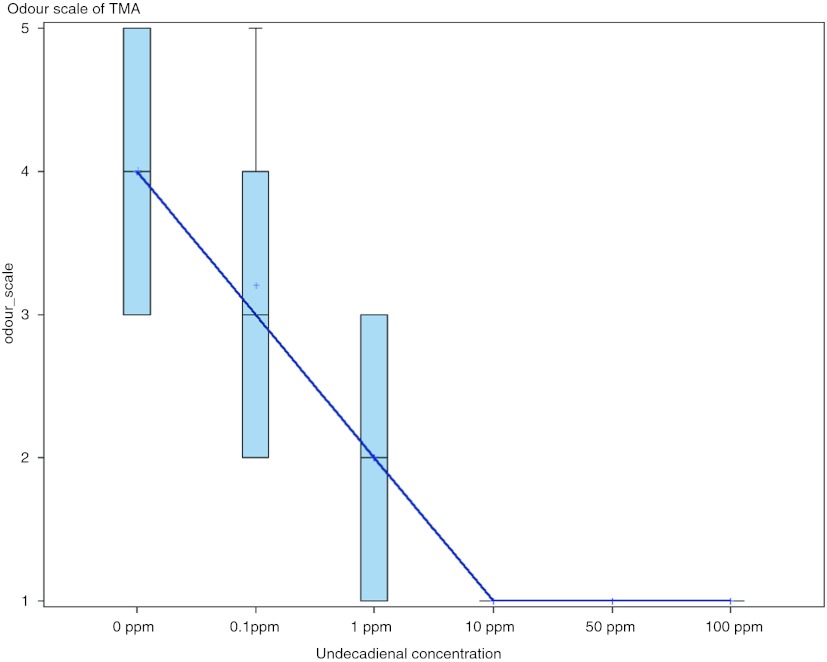
In stage three of the study, keeping the concentration of TMA fixed, the proportion of subjects smelling TMA decreased with increasing concentration of (E, E)-2, 4-undecadienal (Table 1). There was a dose response effect of the ability of (E, E)-2, 4-undecadienal to deodorize the odour of TMA (Fig. 2). Every 10 ppm of (E, E)-2, 4-undecadienal decreased the odour scale of TMA by 0.2 units (95 % CI −0.3, –0.07, p = 0.005). (E, E)-2, 4-undecadienal at a concentration of 10 ppm deodorized the odour of TMA at 1,000 μmol/L without the odour of the (E, E)-2, 4-undecadienal becoming obvious.
Fig. 2.
Odour scale of fixed concentration of TMA (1,000 μmol/L) with increasing concentration of (E, E)-2, 4-undecadienal (Stage III). *Bold line represents median and ‘+’ represents mean
Discussion
We have demonstrated that (E, E)-2, 4-undecadienal has the ability to deodorize the odour of TMA in solution. This is a first such study where this chemical compound has been demonstrated to deodorize the offensive odour of TMA. Previously, the deodorizing ability of (E, E)-2, 4-undecadienal on the malodour associated with cooking porcine intestine (chitlins) has been demonstrated (Kohara et al. 2006; Ikeura et al. 2010). However, the malodour of chitlins does not come from TMA (Kohara et al. 2006). Possible mechanisms for the deodorizing effect of (E, E)-2, 4-undecadienal on TMA include chemical, physical, biological and sensory actions (Ikemoto 1996). Kohara et al. has speculated that the most likely mechanism of action of (E, E)-2, 4-undecadienal (active ingredient in coriander) on the offensive odour of chitlins was a masking effect, that is, hiding the offensive odour with another strong odour, and/or a modification effect, modulating the compound with an offensive odour to a different compound that no longer has the offending odour. They found that the treatment with coriander did not decompose or reduce the main offensive odours. On the other hand, Ikeura et al. has expressed doubts about the masking agent hypothesis, as they used much lower concentrations of (E, E)-2, 4-undecadienal (0.1–100 ppb) at which this compound is odourless.
We used a much higher concentration of (E, E)-2, 4-undecadienal (0.1–100 ppm) at which its specific odour was obvious. In the current study, at higher concentrations of (E, E)-2, 4-undecadienal, the odour of (E, E)-2, 4-undecadienal became obvious (Table 1). Fortunately the odour of (E,E)-2, 4-undecadienal was mostly reported as much less offensive and described differently as “coriander-like”, “oily”, “vegetable oil”, “rancid fat”, “some plant odour” and “paint-like”. This is in contrast to the odour of TMA alone, which was universally reported as offensive and fish-like. The possibility of a chemical reaction between (E,E)-2, 4-undecadienal and TMA warrants further research using chromatographic and other extraction methods.
The plateau effect of intensity of odour of TMA with increasing concentrations is an interesting finding. This could have two possible explanations, saturation of the head space gas concentrations or saturation of the nasal receptors leading to no increase in reported odour strength. Head space concentrations relate to the fact that the concentration of volatile molecules in the air space above a liquid will be determined by TMA’s Henry law constant that is the ratio of concentration in air/concentration in water. The constant for TMA is 1.96 × 102, that is, it much prefers to be in air and the ratio holds until the sample reaches saturation in either phase. As the volume of liquid in our containers was 5 mL and the air volume was 55 mL it is possible that the TMA reached maximum concentration in the air even at sub-saturated aqueous concentrations. Greenman et al. have suggested two-site binding for TMA, which produces high affinity at low concentrations and low affinity binding at higher concentrations (Greenman et al. 2004). This could have given an apparent plateau effect in our study.
A limitation of our study was the inherent variability due to the subjective nature of detecting the TMA odour. Also, this was a pilot study on a small sample of volunteers which might have contributed to wide variation in the odour scale. However, we could clearly demonstrate a robust dose-response relationship of the ability of (E, E)-2, 4-undecadienal to deodorize the odour of TMA.
The results of study have implications for further research into the use of (E, E)-2, 4-undecadienal for treatment of malodour in patients with TMAU. We are not aware of any studies which have demonstrated whether topical use of (E, E)-2, 4-undecadienal cause any local skin irritation. As this is a naturally occurring compound isolated from coriander, it could potentially be tested both as a topical as well as an oral preparation.
Footnotes
Competing interests: None declared
References
- Buby MG, Fisher L, da Costa KA, Thompson D, Mar MH, Zeisel SH. Choline-and betaine-defined diets for use in clinical research and for the management of trimethylaminuria. J Am Diet Assoc. 2004;104:1836–1845. doi: 10.1016/j.jada.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Chalmers RA, Bain MD, Michelakakis Z, Iles RA. Diagnosis and management of trimethylaminuria (FMO3 deficiency) in children. J Inher Metab Dis. 2006;29:162–172. doi: 10.1007/s10545-006-0158-6. [DOI] [PubMed] [Google Scholar]
- Christodoulou J. Trimethylaminuria: an under-recognised and socially debilitating metabolic disorder. J Paediatr Child Health. 2012;48:e153–155. doi: 10.1111/j.1440-1754.2010.01978.x. [DOI] [PubMed] [Google Scholar]
- Dashwood R, Yamane S, Larsen R. Study of the forces of stabilizing complexes between chlorophylls and heterocyclic amine mutagens. Environ Mol Mutagen. 1996;27:211–218. doi: 10.1002/(SICI)1098-2280(1996)27:3<211::AID-EM6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Greenman J, Duffield J, Spencer M, et al. Study on the organoleptic intensity scale for measuring oral malodour. J Dent Res. 2004;83:81–85. doi: 10.1177/154405910408300116. [DOI] [PubMed] [Google Scholar]
- Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: An orientation. Am J Epidemiol. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- Humbert JR, Hammond KB, Hathaway WE, Marcoux JG, O’Brien D. Trimethylaminuria the fish-odour syndrome. Lancet. 1970;1:770–771. doi: 10.1016/S0140-6736(70)90241-2. [DOI] [PubMed] [Google Scholar]
- Ikemoto M. Deodorants of natural plant extracts. J Odor Research and Eng. 1996;27:136–142. [Google Scholar]
- Ikeura H, Kohara K, Xian-Xin L, Kobayashi F, Hayata Y. Identification of (E, E)-2,4-undecadienal from coriander Coriandrum sativum L) as a highly effective deodorant compound against the offensive odor of porcine large intestine. J Agric Food Chem. 2010;58:1014–1017. doi: 10.1021/jf102297q. [DOI] [PubMed] [Google Scholar]
- Kohara K, Kadomoto R, Kozuka H, Sakamoto K, Hayata Y. Deodorizing effect of coriander on the offensive odor of the porcine large intestine. Food Sci Technol Res. 2006;12:38–42. doi: 10.3136/fstr.12.38. [DOI] [Google Scholar]
- Little RC, Pendargast J, Natarajan R. Modelling covariance structures in the analysis of repeated measures data. Stat Med. 2000;19:1793–1819. doi: 10.1002/1097-0258(20001130)19:22<3140::AID-SIM610>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Mountain H, Brisbane JM, Hooper AJ, Burnett JR, Goldblatt J. Trimethylaminuria (fish malodour syndrome): a “benign” genetic condition with major psychosocial sequelae. Med J Aust. 2008;189:468. doi: 10.5694/j.1326-5377.2008.tb02126.x. [DOI] [PubMed] [Google Scholar]
- Treacy E, Johnson D, Pitt JJ, Danks DM. Trimethylaminuria, fish odour syndrome: a new method of detection and response to treatment with metronidazole. J Inher Metab Dis. 1995;18:306–312. doi: 10.1007/BF00710420. [DOI] [PubMed] [Google Scholar]
- Willey GR. Trimethylamine-a pungent experience. Educ Chem. 1985;22:178–181. [Google Scholar]