Abstract
The history of the Newborn Screening Program in Mainland China begins in 1981, when a pilot plan was developed that demonstrated the feasibility of its implementation. It has so far focused on the detection of congenital hypothyroidism (CH) and phenylketonuria (PKU) to prevent or reduce mental and physical developmental retardation in children. Throughout this period, a total of 35,795,550 dried blood samples (DBS) of newborns (NB) have been analyzed for PKU, and 35,715,988 for CH. During this period, 3,082 cases with PKU have been diagnosed, resulting in an incidence of 1 case per 11,614 (95% confidence interval 11,218–12,039) live births. In relation to CH, 17,556 cases have been confirmed, arriving at an incidence of 1 case per 2,034(95% confidence interval 2,005–2,065) live births. The biggest challenge for universal newborn screening is still to increase coverage to mid-western area. In Mainland China, MS/MS newborn screening started in 2004. In a pilot study, 371,942 neonates were screened, and 98 cases were detected with one of the metabolic disorders, and the collective estimated prevalence amounted to 1 in 3795 (95% confidence interval 3,168–4,732) live births, with a sensitivity of 98.99%, a specificity of 99.83%, and a positive predictive value of 13.57%. The most important is to get the government’s policy and financial support for expanded screening.
Universal Newborn Screening
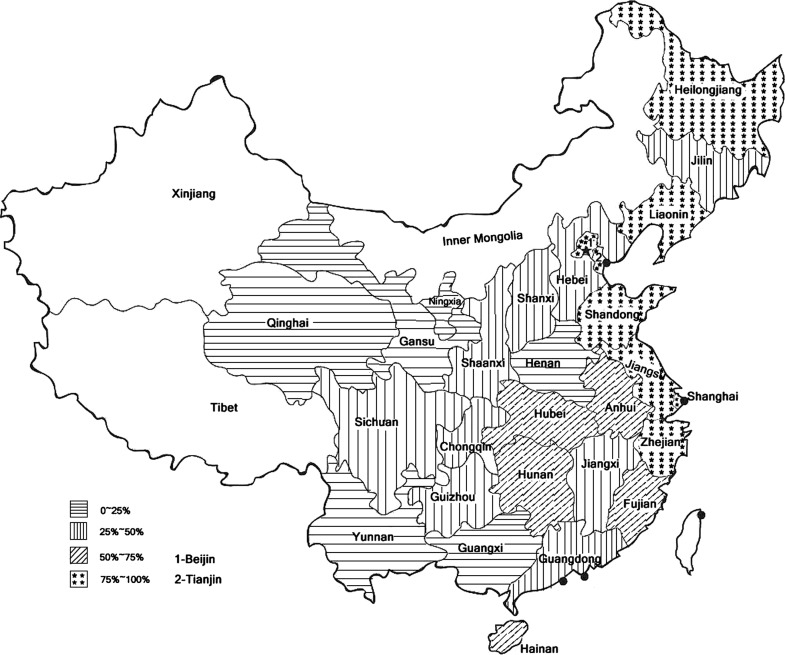
In Mainland China, the Newborn Screening Program was initiated in 1981, when a pilot plan was developed that demonstrated the feasibility of its implementation. With assistance from Robert Guthrie (USA) and Hiroshi Naruse (Japan), a collaborating integrated screening program was started in Shanghai in October 1981 with 14 maternity hospitals participating (Padilla and Therrell 2007). From 1992 to 1993, the World Health Organization (WHO) and the Ministry of Public Health sponsored a cooperative project to pilot NBS in seven major cities (Gu and Chen 1999). Presidential Order 33 was promulgated in October 1994, and the Law of the People’s Republic of China on Maternal and Infant Health Care became enforceable on June 1, 1995 (Padilla and Therrell 2007). Since 1998, laboratory quality control in neonatal screening has been carried out by the National Center for Clinical Laboratory (NCCL) (Zhan et al. 2009). The NCCL is authorized to hold annual meetings for quality evaluation and monitoring of activities among laboratories. As of 2009, three national congresses for NBS have been held. In 2002, there were only 46 centers for screening, but by the end of 2009, 179 centers in 30 provinces have conducted neonatal screening. The coverage increased from 3.86% in 2003 to 59.01% in 2009 (Fig. 1). However, regional differences are still significant. In 2007, the rates of coverage in the eastern, middle, and western regions were about 84.90%, 30.41%, and 19.82%, respectively (Cao et al. 2009). Many challenges have faced and continue to face Mainland China in implementing newborn screening, including differences in language and culture, extremes in geography (large numbers of mountainous regions), and poor economies and education. It must be indicated that in many of the provinces of the middle and western regions, the number of births outside of hospitals approaches 30% (Fig. 2). In 2000, the minimal requirement for newborn screening was defined by law in China that includes the screening for congenital hypothyroidism and phenylketonuria (Zheng et al. 2010). Apart from CH and PKU, glucose-6-phosphate dehydrogenase deficiency is screened in some provinces in South China (Table 1). In some regions, such as Shanghai, Chongqing, and Yunnan, screening for congenital adrenal cortical hyperplasia has been started (Table 1). As of 2009, four provinces have achieved screening free. In the remaining provinces, screening charges range from US $7.43 to $18.52.
Fig. 1.
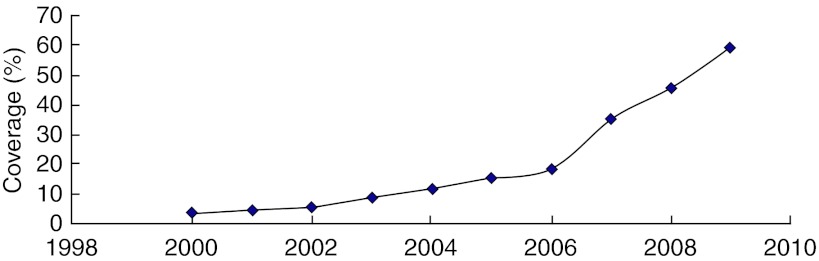
The coverage of universal newborn screening in Mainland China
Fig. 2.
The Mainland China region
Table 1.
Program demographics in Mainland China Universal Newborn Screening Programs
| Province | Births(000) 2009a | Date NBS began | Coverage 2009b | Screened conditions | Cost in USDb | |||
|---|---|---|---|---|---|---|---|---|
| PKU | CH | CAH | G6PD | |||||
| Anhui | 800.6 | 1996 | 59.52% | + | + | 7.43 | ||
| Beijing | 141.5 | 1989 | 98.16% | + | + | Free | ||
| Chongqing | 283.1 | 1996 | 31.15% | + | + | + | + | 17.03 |
| Fujian | 442.5 | 1998 | 62.76% | + | + | 8.51 | ||
| Gansu | 350.9 | 2000 | 11.32% | + | + | 9.26 | ||
| Guangdong | 1135.4 | 1989 | 45.96% | + | + | + | 17.80 | |
| Guangxi | 688.1 | 2007 | 12.15% | + | + | + | + | 18.52 |
| Guizhou | 254.1 | 2007 | 27.84% | + | + | Free | ||
| Hainan | 126.6 | 2007 | 59.45% | + | + | + | Free | |
| Henan | 1086.3 | 1997 | 8.64% | + | + | 7.74 | ||
| Hebei | 909.5 | 1997 | 49.45% | + | + | 7.74 | ||
| Heilongjiang | 286.2 | 1999 | 92.21% | + | + | 8.51 | ||
| Hubei | 542.3 | 1993 | 73.51% | + | + | 7.74 | ||
| Hunan | 835.9 | 1996 | 56.77% | + | + | 7.74 | ||
| Inner Mongolia | 231.8 | 2007 | – | + | + | 7.43 | ||
| Jilin | 183.3 | 2007 | 35.67% | + | + | 7.74 | ||
| Jiangsu | 737.7 | 1985 | 78.96% | + | + | + | 10.06 | |
| Jiangxi | 614.7 | 2005 | 34.76% | + | + | 8.05 | ||
| Liaoning | 261.7 | 2002 | 85.16% | + | + | 8.51 | ||
| Ningxia | 90.7 | 2007 | 12.61% | + | + | 7.74 | ||
| Qinghai | 80.8 | 2009 | 8.92% | + | + | 7.74 | ||
| Shaanxi | 386.3 | 2006 | 34.27% | + | + | 7.74 | ||
| Shandong | 1107.9 | 1992 | 96.45% | + | + | 8.51 | ||
| Shanxi | 372.5 | 2004 | 26.30% | + | + | 7.74 | ||
| Shanghai | 165.9 | 1981 | 97.79% | + | + | + | + | Free |
| Sichuan | 748.9 | 1992 | 31.45% | + | + | 7.74 | ||
| Tianjin | 101.9 | 1989 | 87.61% | + | + | Free | ||
| Tibet | 44.4 | – | – | – | – | – | – | – |
| Xinjiang | 345.2 | 2003 | – | + | + | 11.57 | ||
| Yunnan | 572.7 | 1998 | 3.64% | + | + | + | + | 15.48 |
| Zhejiang | 529.4 | 1998 | 97.17% | + | + | 8.51 | ||
ahttp://www.stats.gov.cn/tjsj/ndsj/2010/indexch.htm
bSome information came from personal communication (e-mail or phone)
The sample for newborn screening is collected between 48 and 72 h after birth. The staff will collect a few drops of blood onto a screening card by pricking baby’s heel. This card is then sent to the screening laboratory for testing. PKU screening was originally performed with bacteria inhibition assay (BIA). At present, most laboratories employ fluorometric method for the measurement of phenylalanine concentrations, whereas enzymatic colorimetric method is also used in some laboratories. The cutoff value was 120 μmol/L for hyperphenylalaninemia, and differentiation between PKU and tetrahydrobiopterin (BH4) deficiency was performed in majority of patients. Serum phenylalaninemia concentrations higher than 120 μmol/L required reexamination. PKU patients were confirmed by retest’s results (fluorometric method: serum phe > 120 μmol/L; bacteria inhibition assay: serum phe > 240 μmol/L. In CH screening, blood thyroid-stimulating hormone (TSH) was quantified by radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), enzymatic immunofluorescence assay (EFIA), or dissociation-enhanced lanthanide fluoroimmunoassay (DELFIA). The cutoff value was 10 IU/L for CH screening. Before 1998, laboratories of various screening centers in China employed RIA, which later was replaced by DELFIA (Gu et al. 2008). CH patients were confirmed by thyroid function (serum thyroid-stimulating hormone elevated and serum-free thyroxine reduced). Throughout this period, a total of 35,795,550 newborns (NB) have been analyzed for PKU and 35,715,988 for CH. During this period, 3,082 cases with PKU have been diagnosed, resulting in an incidence of 1 case per 11,614 (95% confidence interval 11,218–12,039) live births, an average age of diagnosis of 21 ± 11.2 days. In relation to CH, 17,556 cases have been confirmed, arriving at an incidence of 1 case per 2,034(95% confidence interval 2,005–2,065) live births, with average age of diagnosis of 14.7 ± 7.8 days.
Our results have a number of limitations. The serum phe above 360 μmol/L treatment was recommended in China. Three thousand eighty-two cases of PKU were confirmed rather than requiring treatment patients. We did not know how many patients need treatment. In additional, 3,082 cases of PKU included “classical” PKU, mild HPA, and defects of BH4 metabolism. In the data collection process, we did not obtain accurate classification information. Finally, if the thyroid function returns to normal before 3 years old, CH patients will be recommended to stop treatment. Almost one-third of the CH patients can stop treatment. Unfortunately, we also did not obtain accurate dates.
Newborn Screening Using Tandem Mass Spectrometry
By now, high screening rates are seen in a small number of economically developed regions, such as Beijing, Shanghai, and Zhejiang (Table 1). Due to the good results of the Universal Newborn Screening Program, the local government is evaluating the initiation of an extended pilot program, to introduce new target diseases. In Mainland China, MS/MS newborn screening started in 2004 (Gu et al. 2008). Since 2004, the coordination of screening services has taken place in four newborn screening centers, namely, Children’s Hospital Zhejiang University School of Medicine, Xinhua Hospital, Center for Clinical Laboratory Development, Chinese Academy of Medical Science, and Guangzhou Women and Children’s Medical Center. Each of these four centers started individual voluntary fee-paying programs for MS/MS screening. The charge for routine MS/MS screening is US $50–100 per infant. As of 2010, almost 800,000 neonates were screened. In a pilot study, 371,942 neonates were screened, and 98 cases were detected with one of the metabolic disorders, and the collective estimated prevalence amounted to 1 in 3,795 (95% confidence interval 3,168–4,732) live births, with a sensitivity of 98.99%, a specificity of 99.83%, and a positive predictive value of 13.57%. Up to now, one false-negative case of hyperphenylalaninemia has been confirmed (cases should be added with the follow-up in the future).The most common inborn error was hyperphenylalaninemia (HPA). Methylmalonic acidemia was the second most common disorder (Table 2). While newborn screening is strongly recommended for all babies, participation is voluntary. It will ask a parent to provide written consent for the screening test before sample collection.
Table 2.
Confirmed diagnoses in the China Newborn Screening Program
| Disorders | Diagnoses |
|---|---|
| Hyperphenylalaninemia | 34 |
| Maple syrup urine disease | 4 |
| Tyrosinemia | 3 |
| Homocystinuria | 3 |
| Argininemia | 2 |
| Hyperammonemia-hyperornithinemia-homocitrullinuria syndrome | 1 |
| Isovaleric acidemia | 4 |
| Glutaric acidemia type I | 2 |
| Methylmalonic aciduria | 10 |
| Propionic acidemia | 2 |
| 3-Methylcrotonyl-CoA carboxylase deficiency | 9 |
| 2-Methylbutyryl-CoA dehydrogenase deficiency | 3 |
| Medium-chain acyl-CoA dehydrogenase deficiency | 4 |
| Carnitine transporter defect | 9 |
| Trifunctional protein deficiency | 2 |
| Short-chain acyl-CoA dehydrogenase deficiency | 4 |
| Very-long-chain acyl-CoA dehydrogenase deficiency | 2 |
| Total | 98 |
Conclusions and Perspectives
The National Newborn Screening Program in Mainland China has managed some success, obtaining in 30 years the diagnosis and prevention of mental retardation in 20,638 children. Among the regions that lack total coverage, the obstacles most often cited are poor economies, insufficient health education, lack of government support, early hospital discharge, and large numbers of out-of-hospital births. The biggest challenge is still to increase coverage to the entire country, especially in the mid-western area. In additional, some of the local government (e.g., Beijing, Shanghai, Tianjin, and Shanxi) provided free milk powder treatment to babies with PKU. Each baby with the disease will receive such milk powder for free for 3–6 years. A family can save US $3,000 a year as a benefit of the program, a significant amount for poor families. However, most of patients need afford by families. This is a heavy burden for some families. Three points we would like to suggest: First, the government has to give policy support to appropriate disease screening and treatment. Second is to expand the service network and improve the diagnosis and treatment of medical personnel. Education of the screening staff needs to be improved. Especially, mass spectrometry screening is not just establishing the technology, but far more important are the interpretation of the data and the maintenance of high quality over time. This can only be achieved with well-trained staff. Communication between screening centers and doctors should be improved. Delays in taking of the second sample or in the initiation of treatment are thus inevitable. Last is to develop people’s perceived screening awareness and consider the cost afforded by the National Health Insurance. Screening should be free to avoid bias in screening and allow general access for the population (at least for PKU and TSH screening).Nevertheless, the challenges for the future are very important, since to start an extended newborn screening program implies increasing the number of diseases screened for greater biochemical and clinical complexity. The most important is to get the government’s policy and financial support for expanded screening.
Acknowledgments
We are grateful to the individuals for providing their screening data.
Synopsis: Past, Present, and Future of Newborn Screening in Mainland China
In most economically developed countries, blood spot newborn screening (NBS) using biochemical markers to detect certain congenital conditions is a public health activity aimed at the early identification and treatment/management of affected newborns. As an integral part of maternal and child health care in Mainland China, it has so far focused on the detection of congenital hypothyroidism (CH) and phenylketonuria (PKU) to prevent or reduce mental and physical developmental retardation in children. In our country, NBS and other infant screening are just emerging as a priority. This chapter will focus on NBS in the Mainland China, a region of vastly differing newborn screening priorities.
Footnotes
Competing interests: None declared
References
- Cao Y, Yuan P, Wang YP, Mao M, Zhu J. The profile of newborn screening coverage in China. J Med Screen. 2009;16(4):163–166. doi: 10.1258/jms.2009.009088. [DOI] [PubMed] [Google Scholar]
- Gu XF, Chen RG. Current status of neonatal screening in China. J Med Screen. 1999;6:186–187. doi: 10.1136/jms.6.4.186. [DOI] [PubMed] [Google Scholar]
- Gu XF, Wang ZJ, Ye J, et al. Newborn screening in China: phenylketonuria, congenital hypothyroidism and expanded screening. Ann Acad Med Singapore. 2008;37(Suppl 3):107–110. [PubMed] [Google Scholar]
- Padilla CD, Therrell BL. Newborn screening in the Asia Pacific region. J Inherit Metab Dis. 2007;30(4):490–506. doi: 10.1007/s10545-007-0687-7. [DOI] [PubMed] [Google Scholar]
- Zhan JY, Qin YF, Zhao ZY. Neonatal screening for congenital hypothyroidism and phenylketonuria in China. World J Pediatr. 2009;5(2):136–139. doi: 10.1007/s12519-009-0027-0. [DOI] [PubMed] [Google Scholar]
- Zheng S, Song M, Wu L, Yang S, Shen J, Lu X, et al. China: public health genomics. Public Health Genomics. 2010;13:269–275. doi: 10.1159/000240969. [DOI] [PubMed] [Google Scholar]