Abstract
Background: Since 2008 patients with BH4-sensitive phenylketonuria can be treated with sapropterin dihydrochloride (Kuvan®) in addition to the classic phenylalanine (Phe) restricted diet. The aim of this study was to evaluate the nutritional changes and micronutrient supply in patients with phenylketonuria (PKU) under therapy with tetrahydrobiopterin (BH4).
Subjects and Methods: 19 children with PKU (4–18 years) and potential BH4-sensitivity were included, 14 completed the study protocol. Dried blood Phe concentrations as well as detailed dietary records were obtained throughout the study at preassigned study days.
Results: Eight patients could increase their Phe tolerance from 629 ± 476 mg to 2131 ± 1084 mg (P = 0.006) under BH4 while maintaining good metabolic control (Phe concentration in dried blood 283 ± 145 μM vs. 304 ± 136 μM, P = 1.0), therefore proving to be BH4-sensitive. They decreased their consumption of special low protein products and fruit while increasing their consumption of high protein foods such as processed meat, milk and dairy products. Intake of vitamin D (P = 0.016), iron (P = 0.002), calcium (P = 0.017), iodine (P = 0.005) and zinc (P = 0.046) significantly declined during BH4 treatment while no differences in energy and macronutrient supply occurred.
Conclusion: BH4-sensitive patients showed good metabolic control under markedly increased Phe consumption. However, the insufficient supply of some micronutrients needs consideration. Long-term multicenter settings with higher sample sizes are necessary to investigate the changes of nutrient intake under BH4 therapy to further evaluate potential risks of malnutrition. Supplementation may become necessary.
Introduction
Phenylketonuria (PKU, OMIM 261600), one of the most common inborn errors of metabolism, is caused by mutations in the gene encoding phenylalanine hydroxylase (PAH, EC 1.14.16.1), leading to deficient enzyme activity. More than 500 mutations in the PAH gene have been identified (http://www.PAHdb.mcgill.ca). PAH is primarily expressed in the liver, its activity depending on tetrahydrobiopterin (BH4) as a cofactor. The enzyme catalyses the irreversible hydroxylation of phenylalanine (Phe) to tyrosine (Tyr). In PAH deficient patients, Phe accumulates in blood and tissues while a deficiency of Tyr develops. In untreated patients, this results in severe, irreversible psychomotor retardation. Newborn screening and initiation of dietary treatment within the first weeks of life nowadays allow for a normal neurocognitive development (Scriver et al. 1998). The treatment of PKU consists in dietary restriction of Phe and therefore natural protein and a supplementation with Phe free amino acid mixtures (AAM). Next to essential amino acids except for Phe the AAM contain energy, carbohydrates and fat as well as vitamins, minerals and trace elements as currently recommended by the national councils (for the German speaking countries, DACH 2000). The extent of Phe restriction depends on the patients` individual Phe tolerance, which depends on residual PAH activity as well as the patients` age and weight (Scriver et al. 1998). Phe tolerance is determined by regularly monitored dried blood Phe concentration. Protein rich foods such as meat or dairy products, which are also important sources for other nutrients such as iron, vitamin B12 or calcium, must be strictly avoided. Most natural food has to be replaced by special low protein products.
Phe restriction and AAM supply are well established as an efficient and safe treatment for PKU patients. However, this therapy remains a great challenge and burden for the patients and their families. In the classical treatment of PKU it is necessary to plan meals exactly and to calculate the Phe content of all foods. Only recently, some specialized centres allow the unrestricted consumption of fruits and vegetables under certain conditions (Macdonald et al. 2003; Rohde et al. 2012; Zimmermann et al. 2012). However, the individual Phe tolerance has to be respected. Moreover, the AAM have an unpleasant taste. As a consequence patients often show a low diet adherence, particularly older children and adolescents (Macdonald 2000; Macdonald et al. 2010). Especially during adolescence many patients drop out of specialized care (Mütze et al. 2011). Discontinuation of dietary treatment bears a high risk of nutrient deficiencies, cognitive and emotional dysfunction as well as behavioral problems. Therefore, life-long diet is strongly recommended (Stemerdink et al. 2000; Burgard et al. 1997; Weglage et al. 1992).
In recent years, BH4 supplementation has been investigated as a new therapeutic tool in PKU treatment. It was shown that in some PKU patients BH4 enhanced the residual PAH activity and partially restored Phe oxidation (Muntau et al. 2002). As a result, blood Phe concentrations decrease while Phe tolerance can increase up to 2- to 3-fold (Hennermann et al. 2005; Lambruschini et al. 2005; Burlina and Blau 2009; Trefz et al. 2009). This now allows some patients to consume protein rich foods. The dosage of Phe free AAM can be reduced. Due to the higher Phe tolerance, quality of life as well as therapy adherence may be enhanced (Ziesch et al. 2012). However, BH4 monotherapy seems to be sufficient in only few cases (Lambruschini et al. 2005).
Available reports on BH4 supplementation have focused on the identification of patients who may benefit from BH4, and the quality of metabolic control (Bélanger-Quintana et al. 2005; Fiori et al. 2005; Burton et al. 2007, 2010; Burlina and Blau 2009; Trefz et al. 2009). The small number of studies investigating the nutrition of PKU patients under BH4 treatment revealed heterogeneous results (Lambruschini et al. 2005; Singh et al. 2010).
The aim of this study was to investigate eating habits of BH4-sensitive patients under BH4 therapy compared to the classical Phe restricted diet. We evaluated food and nutrient intake in order to examine whether BH4 treated PKU patients are adequately supplied with critical nutrients under relaxed dietary conditions.
Study Design
This open, monocentric, prospective intervention trial follows the principles of the Declaration of Helsinki and ICH/GCP. It was approved by the University of Leipzig’s ethics committee (registration-number 087-2009-20042009). All included patients and/or their guardians gave written informed consent.
Inclusion was restricted to patients aged 4–18 years with PKU and potential BH4 sensitivity. Patients with a BH4 deficiency as well as additional diseases or abnormal signs in the general or neurological examination, a need for concomitant medication except the Phe free AAM, an implementation of other diets except the Phe restricted diet and an existing or planned pregnancy were excluded.
The study design is shown in Fig. 1. In each of the four study periods the patients were asked to send dried blood samples and dietary records to the hospital on preassigned study days.
Fig. 1.
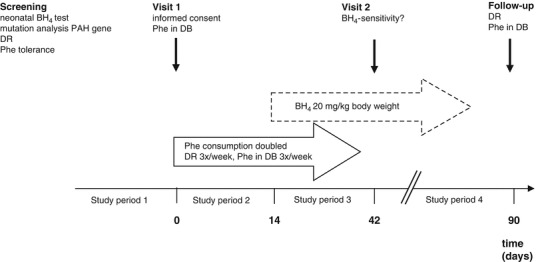
Study design of BH4-intervention trial in PKU patients. BH 4 tetrahydrobiopterin, PAH phenylalanine hydroxylase, Phe phenylalanine, DB dried blood, DR dietary record
Study period 1 consisted in the reevaluation of the current dietary treatment by review of Phe concentrations in dried blood one year prior to enrolment and determination of current Phe tolerance using a 3 day dietary record (days –3 to 0). In study period 2 (days 0 to 14), the patients were instructed to double their daily Phe intake from natural protein (from any food) to achieve Phe concentrations ≥ 600 μmol/l. No standardized meals were given throughout the study. The intake of the Phe free AAM was continued. Dietary records were performed three times a week and dried blood samples for analyses of Phe concentrations were obtained. In study period 3 (day 14 to 42), the patients received sapropterin dihydrochloride (Kuvan®, Merck Serono) at 20 mg per kg body weight daily, administered as single oral dose in the morning, while continuing the doubled Phe intake. On day 42, patients were defined as BH4-sensitive if they showed a reduction in Phe concentration of ≥ 30 % and/or an increase of Phe consumption by ≥ 100 % while Phe concentrations remained within the therapeutic range. All other patients were classified as BH4-resistant. During study period 4 (days 43 through 90 = follow-up), BH4-sensitive patients continued BH4 treatment. During follow up, every actual Phe concentration was evaluated and followed by personal contact with the patient or the parents to advise on further increase of Phe consumption. Once the patients had reached an adequate protein consumption from natural foods (DACH 2000) AAM dosage was reduced.
Subjects and Methods
Subjects
A total of 41 patients (4–18 years), treated at the outpatient clinic for inborn metabolic diseases at the University Hospital in Leipzig, Germany, were screened for participation. They were tested for potential sensitivity to BH4 by determination of their PAH mutation and reevaluation of the neonatal BH4-test. In 20 of the patients BH4-sensitivity seemed to be implausible. The other (n = 21), including those whose results were incomplete, were offered to test BH4 (as sapropterin dihydrochloride, Kuvan®) as a potential additional treatment. Two of the patients declined participation. Nineteen patients were included into the study.
Of these, 18 completed the protocol. One patient refused to take the medication and terminated study participation. Four other patients ignored essential parts of the study protocol and had to be excluded from the analysis. All participants had been diagnosed with PKU by newborn screening and dietary treatment had been initiated within the first 2 weeks of life. All but four underwent a test for BH4-sensitivity when first diagnosed. None of them showed a deficiency in tetrahydrobiopterin (Kaufman et al. 1978; Blau et al. 2011). All patients were regularly followed in the outpatient clinic for inborn metabolic diseases and their reliable adherence to a Phe restricted diet had been recorded. The dosage of substitution with synthetic amino acids followed the current recommendations for protein intake in children (DACH 2000) with an added surplus of 20 % to account for possible differences in biological value relative to natural protein.
Methods
Assessment of Plasma Phe Concentrations
Patients took samples of capillary whole blood on filter paper at preassigned study days. All parents were familiar with the proper technique, having been trained during the patients` newborn period. Phe and Tyr concentrations in dried blood were determined by liquid chromatography/tandem mass spectrometry (LC-MS/MS) as previously described (Ceglarek et al. 2002).
Assessment of Food and Nutrient Intake
The patients and/or their guardians performed dietary records throughout the study on preassigned study days. All foods and beverages, including special low protein foods as well as the AAM, were weighed and documented. All ingested food was allocated to the following food groups: bread and cereal products, potatoes/ rice and pasta, vegetables, fruit, milk and dairy products, other food of animal origin (meat and processed meat, fish, egg), edible fat (butter, margarine, oil), sweets and snacks, special low-protein products. This classification was chosen according to the recommendations for age-based food consumption issued by the German Research Institute of Child Nutrition (Kersting and Alexy 2005).
Nutritional analysis was performed using the Food and Control Management System “Diät 2000” based on the updated version of the Bundeslebensmittelschlüssel (Hartmann 2009). Additional information on AAM, low protein foods as well as other processed foods provided by the manufacturer was added to the database. Besides Phe intake (mg/day) the following nutrients were calculated as percentage of regular daily allowance, recommended for the German speaking countries (DACH 2000): energy, protein, carbohydrate, fat, vitamin C, vitamin D, vitamin B12, iron, calcium, iodine and zinc.
Mean nutrient supply and food consumption of BH4-sensitive patients under the new therapeutic regime was compared to the classical dietary treatment and to the current recommendations for nutrient intake (DACH 2000). In addition, mean nutrient supply was compared to data from a cohort of age-matched healthy German children (Mensink et al. 2007).
Statistical Analysis
All procedures were performed using SPSS for Windows 17 (SPSS Inc., Chicago, Illinois).
To reduce the number of variables, laboratory data from each study period were averaged and used for analyses. If normality of distribution could be assured, longitudinal changes in nutrient supply over the four study periods were analysed by Wilk’s multivariate analysis of variance (MANOVA) with “time” as the within-subject factor with four levels (study periods 1 through 4). If Wilk’s analysis yielded a significant effect of “time”, this was followed by repeated (sequential) contrasts to locate pairs with significant changes over time. In analogy, data that were not normally distributed were analysed by Friedman test and Wilcoxon test.
Significance was accepted for P < 0.05. Data are given as mean ± standard deviation (SD) unless otherwise stated.
Results
Patient Characteristics
A total of 14 patients (6 females, 8 males) could be considered in the analysis of metabolic control and Phe intake. All of them had a normal body weight with a mean BMI-SDS of 0.21 ± 1.0. In one of these patients no data of nutrient supply under classical therapy except for Phe consumption were available. Thus, this patient had to be excluded from the nutrition analyses. No adverse events were observed throughout the study.
Metabolic Control and Phe Consumption
The data of Phe concetration in dried blood, Phe and natural protein consumption are presented in Table 1. Over the last year prior to study entry all included patients showed good metabolic control. Eight patients proved to be BH4-sensitive. In the other six patients, BH4 therapy was not effective. Only BH4-sensitive patients stayed on BH4 therapy during follow-up. They continued to show good metabolic control with stable dried blood Phe concentrations within the therapeutic range that did not differ from study entry (see Table 1, P = 1.0).
Table 1.
Phe concentration in dried blood, Phe and natural protein consumption of the patients throughout the study
| Mean-Age (range) | Phe concentration (μmol/l) in dried blood (mean ± SD) |
Phe decrease on day 42 (%) | Phe (mg/day) and natural protein (g/day) consumption (mean ± SD) | Increase of Phe intake on day 42 (%) | Increase of Phe intake on day 90 (%) | DACH RDA (g/day) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SP1 | SP2 | SP3 | SP4 | SP3 vs SP2 | SP1 | SP2 | SP3 | SP4 | SP3 vs SP1 | SP4 vs SP1 | |||||
| All patients ( n = 14) | 10 (4–16) | 364 (169) | 516 (241) | 420 (245) | 440 (204) | 25.8 | 550 (378) – |
1098 (611) – |
1099 (611) – |
1442 (1153) – |
112.4 | 164.1 | |||
| ID | Sex | Age (years) | |||||||||||||
| BH 4 -sensitive patients ( n = 8) | 2 | m | 6 | 100 (88) | 273 (75) |
225 (109) | 215 (44) | 17.6 | 351 (4) 10.5 (1.2) |
1062 (179) 24.7 (4.6) |
1103 (143) 24.3 (2.8) |
1039 (87) 23.7 (2.2) |
214.1 | 195.8 | 18 |
| 5 | m | 16 | 215 (42) | 233 (39) |
131 (46) | 198 (49) | 43.7 | 733 (21) 18.9 (2.4) |
1739 (318) 37.5 (11.6) |
1526 (244) 35.8 (5.2) |
3048 (641) 69.6 (16.5) |
108.1 | 315.7 | 60 | |
| 8 | m | 8 | 201 (58) | 231 (55) |
158 (37) | 186 (36) | 31.6 | 657 (64) 16.0 (2.9) |
1360 (39) 32.0 (2.1) |
1521(206) 34.9 (5.6) |
3162 (355) 69.0 (7.3) |
131.5 | 381.0 | 24 | |
| 9 | f | 16 | 520 (133) | 618 (61) |
386 (103) | 487 (183) | 37.5 | 432 (23) 11.4 (0.5) |
1557 (643) 35.8 (12.5) |
1556 (607) 34.4 (13.4) |
2851 (1245) 65.3 (29.4) |
260.0 | 559.6 | 46 | |
| 14 | f | 8 | 203 (48) |
269 (88) |
163 (23) | 164 (47) | 39.4 | 385 (2) 9.6 (0.5) |
787 (9) 17.9 (1.1) |
790 (48) 17.9 (1.5) |
1566 (132) 34.8 (2.7) |
105.3 | 306.8 | 24 | |
| 24 | f | 11 | 278 (122) | 375 (195) | 259 (68) | 377 (126) | 39.9 | 382 (10) 9.3 (0.1) |
968 (85) 22.3 (2.3) |
1019 (44) 23.4 (1.1) |
1298 (18) 29.1 (2.2) |
167.1 | 240.1 | 35 | |
| 25 | m | 5 | 277 (98) |
427 (165) | 199 (74) | 308 (59) | 53.4 | 342 (47) 8.6 (0.9) |
728 (60) 16.8 (1.2) |
759 (61) 17.6 (1.8) |
704 (33) 16.0 (1.1) |
121.8 | 105.8 | 18 | |
| 26 | f | 15 | 475 (54) |
589 (52) |
381 (88) | 498 (99) | 35,3 | 1750 (0) 38.8 (0) |
2777 (477) 88.9 (9.9) |
2810 (826) 88.0 (20.5) |
3380 (249) 76.2 (5.3) |
60.8 | 93.2 | 46 | |
| Total mean (SD) |
283
(145) a |
376
(157) a |
238
(99) a |
304
(136) |
37,3 |
629
(476) b |
1372
(679) b |
1386
(661) b |
2131
(1084) b |
146.1 | 274.8 | n.a. | |||
| MANOVA (P) | 0.018* | 0.006* | |||||||||||||
Abbreviations: SP study period, SP 1 Evaluation of patients (neonatal BH4 test, Phe tolerance), SP 2 Phe challenge, SP 3 Phe challenge + BH4 intake, SP 4 Follow-up, DACH-RDA regular daily allowance, recommended for the German speaking countries (DACH 2000), n.a. not applicable, f female, m male
aSignificant difference between period 1 and 2 (P = 0.016) and period 2 and 3 (P = 0.007)
bSignificant difference between period 1 and 2 (P = 0.001); period 1 and 3 (P = 0.001) and period 1 and 4 (P = 0.009)
*Significant effect of "time" yielded by Wilkçs multivariate analysis of variance (MANOVA)
In BH4-resistant patients we found a slight increase of Phe intake and a significantly higher Phe concentration in dried blood during follow-up compared to study entry (621 μmol/l ± 117 vs 474 μmol/l ± 141 at study entry; P = 0.003). It took them another three months to regain the original metabolic control (data not shown).
Food Consumption of BH4-Sensitive Patients
Figure 2 shows the food consumption of BH4-sensitive patients under classical dietary treatment compared to BH4 therapy. The serving sizes of consumed food remained unchanged throughout the study. In contrast, remarkable changes with regard to food choice could be observed. Before study entry all BH4-sensitive patients followed a Phe restricted diet, having some characteristics of a vegan diet. Due to the partially very low Phe tolerance they mainly consumed fruit and vegetables. A further important share of total food consumption was spent on special low protein products, mainly low protein sausages, bread, pasta, rice, potato products. In addition, the patients consumed a relatively large amount of sweets (in particularly jelly beans), jelly and sweet powdered instant drinks. No differences compared to BH4-resistant patients could be revealed.
Fig. 2.
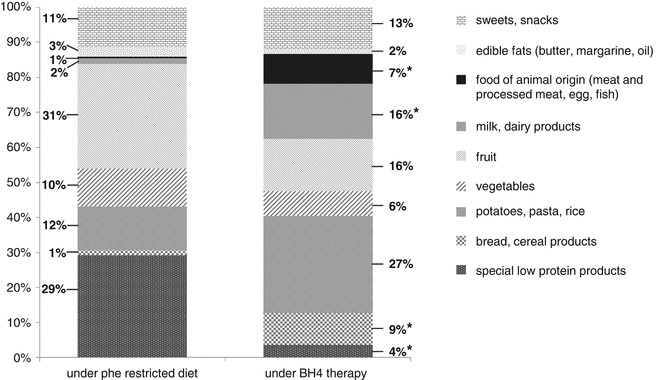
Food consumption of BH4-sensitive patients on classical dietary treatment (left bar) compared to BH4 therapy during follow-up (right bar). Shown are the shares of food groups (%) of total food consumption (*significant difference between period 1 and 4: bread (P = 0.022), dairy products (P = 0.002); potatoes, pasta and rice (P = 0.021), food of animal origin (meat, processed meat, fish, egg, P = 0.016); special low protein products (P = 0.009))
From study period 2 onwards all BH4-sensitive patients increased their consumption of foods with a higher protein content such as bread, pasta, rice and dairy products as well as meat, egg and fish. The mean fruit and vegetable consumption under BH4 administration dropped compared to classical treatment, but no significance was found (P = 0.274 and P = 0.139, respectively). In contrast, the intake of edible fats as well as sweets and snacks remained stable. The consumption of special low protein products declined significantly.
Macronutrient Intake of BH4-Sensitive Patients
Under classical treatment (study period 1) as well as in study periods 2 and 3 all BH4-sensitive patients regularly took an AAM. During follow-up six of the eight BH4-sensitive patients could end any AAM supply. In the other two BH4-sensitive patients the dosage could be reduced.
Under classical treatment (study period 1) total protein intake (sum of synthetic protein from AAM and intact protein from natural food) was markedly above the recommended range (165 ± 38 %). Total protein intake remained stable throughout the study, but the proportions of synthetic and natural protein changed. In study period 1 the intake of synthetic protein from AAM was 120 ± 28 % compared to 45 ± 13 % from intact natural protein of the current recommendation (DACH 2000). By doubling the Phe intake in study periods 2 and 3 the patients ingested twice as much natural protein and further increased this amount during follow-up. Under BH4 therapy during follow-up, the total protein intake was similar to that of classic treatment (P = 0.292). However, the patients now consumed 142 % of the recommended protein as intact protein from natural foods and only 12 % as synthetic protein from AAM.
Under classical treatment BH4-sensitive patients showed a carbohydrate intake below the current recommendations (93 ± 22 %; DACH 2000), which even further decreased during the other study periods (during follow-up 70 ± 18 %, P = 0.032). The mean fat intake was stable over the course of the study, but lower than the recommendations (81 ± 25 % throughout the study).
Micronutrient Intake of BH4-Sensitive Patients
Figure 3 shows the supply of selected micronutrients of BH4-sensitive patients under BH4 therapy (study period 4) as well as under classical treatment. In addition the data are compared to data from aged-matched healthy German children (Mensink et al. 2007).
Fig. 3.
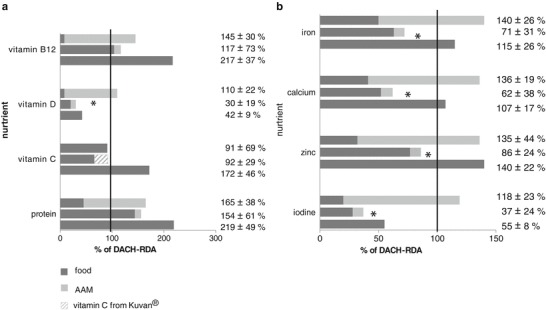
(a–b) Protein and micronutrient supply of BH4-sensitive patients (n = 7) under Phe restricted diet (upper bar) and under BH4 therapy during follow-up (middle bar) compared to age-matched healthy German children (lower bar) as percent of DACH-RDA. Shown are the shares of food and amino acid mixture (AAM) of the total nutrient supply. The vitamin C content of Kuvan® was included into the calculation. Under the Phe restricted diet all these patients took an amino acid mixture while under BH4 therapy only two patients took some (* significant differences in total micronutrient supply in BH 4 -sensitive patients under Phe restricted diet compared to BH 4 therapy: P vitamin D = 0.016; P iron = 0.002; P calcium = 0.017; P iodine = 0.005, P zinc = 0.046)
Under the Phe restricted diet (study period 1) overall micronutrient intake except for vitamin C was adequate. The highest percentage of vitamins and minerals derived from AAM and only a small amount from natural food. During study periods 2 and 3, mean total supply of vitamin D, vitamin B12, iron, calcium, iodine and zinc remained stable and no significant differences occurred compared to study period 1 as the patients still consumed the original dose of AAM.
In patients who reduced or stopped AAM intake during follow-up (study period 4), the micronutrient supply dropped markedly below the current recommendations (DACH 2000) and deteriorated compared to the classic treatment. The differences were significant for vitamin D, iron, calcium, iodine and zinc. Considering the vitamin C content of Kuvan® (5 mg vitamin C per 100 mg sapropterin dihydrochloride), total vitamin C supply did not differ between study periods 1 and 4 (P = 1.0). However, due to a reduced fruit and vegetable intake, BH4-sensitive patients consumed a lower amount of vitamin C from natural foods compared to classical dietary treatment.
Comparing the results to data from age-matched healthy German children (Mensink et al. 2007) several differences could be noted (Fig. 3). In general, healthy German children aged 6 to 17 years almost met the recommendations for all investigated micronutrients except for vitamin D and iodine. Under classical dietary treatment, the BH4-sensitive PKU patients showed a higher mean intake of vitamin D, iron, calcium and iodine, but a lower mean intake of vitamin C and vitamin B12. Under BH4 treatment the supply of almost all micronutrients proved to be markedly lower compared to the healthy German children.
Discussion
The study presented here investigated metabolic control and eating habits, including food and nutrient supply, of BH4-sensitive PKU patients. BH4-sensitive patients could increase their Phe tolerance by 100 % or more while dried blood Phe concentrations remained within the therapeutic range. In comparison to the classical dietary treatment the patients consumed more protein rich natural foods. In six of the BH4-sensitive patients the AAM administration could be stopped. Two of the BH4-sensitive patients further needed some AAM. While mean total protein intake did not change compared to the classical dietary treatment, the mean intake of carbohydrate, energy and also of some micronutrients declined and dropped below the current recommendations (DACH 2000).
Under the new therapeutic regime, the BH4-sensitive patients could increase their Phe tolerance and consumed more protein rich foods. In contrast, the markedly declined fruit and also the slightly reduced vegetable intake must be considered as critical, since they are important sources of vitamins, minerals, trace elements, phytochemicals and dietary fibre. Several factors may explain this phenomenon. Due to the increased consumption of protein rich food, patients may feel satisfied earlier, reducing their desire for more food. Furthermore, the classical Phe restricted diet quite likely does not reflect the true food preferences of our patients.
In general, factors influencing food choice are very complex, including socio-economic components as well as psychological aspects (Zabinski et al. 2006; Elfhag et al. 2008). Moreover, parents´ eating habits are an important factor influencing their children’s choice of healthy or less healthy foods (Kremers et al. 2003; De Bourdeaudhuij et al. 2008).
Interestingly, our patients did not realise their markedly decreased fruit consumption. They were only aware of and enjoyed the possibility to consume protein rich foods like meat or dairy products. Over a longer term these patients are at risk to adopt some of the bad eating habits of healthy German children (Mensink et al. 2007). The study period has certainly been too short to completely develop a new dietary regime, especially with regard to seasonal differences in food choice. Extending the study would have permitted the patients to slowly accustom to a liberalized nutrition. However, this requires a high adherence to the study protocol by the patients and it is associated with higher costs. Reevaluating the nutrient intake at least 6 months after introduction of BH4 treatment and identifying the new correct Phe tolerance would be advisable.
The changed food choice of BH4-sensitive patients resulted in changes of macro- and micronutrient supply. Under classical dietary treatment the patients` mean nutrient intake was still adequate except for fat, total energy and vitamin C. Under BH4 therapy their supply of carbohydrates and most of the investigated micronutrients dropped below the recommended range (DACH 2000). The mean total protein intake, however, was sufficient and comparable to classical dietary treatment. This observation is in accordance with data from a recent study (Singh et al. 2010). Likewise, the fat supply did not change compared to classical dietary treatment, but stayed below the recommendations (DACH 2000). A low fat intake in PKU patients under Phe restricted diet has already been described (Schulz and Bremer 1995, Rohde et al. 2012). Many of the protein rich foods, which must be avoided in the PKU diet, are simultaneously sources rich in fat. This is especially true for meat, processed meat and dairy products. Although the BH4-sensitive patients increased their consumption of these foods they did not meet the recommendation for fat supply.
Under BH4 therapy the carbohydrate intake declined and dropped below the current recommendations (DACH 2000). Taken together, the BH4-sensitive patients did not meet the recommendations for the energy supply in healthy children (DACH 2000). Although none of the patients was underweight or growth retarded, a regular examination of energy – and macronutrient supply is strongly recommended, in particular when switching from classical dietary treatment to BH4 therapy with a liberalized diet.
During the Phe restricted diet (study period 1) some of the patients showed a vitamin C supply slightly below the recommended range (DACH 2000). None of them took an AAM containing vitamin C. As a consequence of reduced fruit and vegetable consumption under the BH4 treatment, the vitamin C supply of our patients further declined. Only taking into account the vitamin C content of Kuvan®, there were no differences compared to study period 1. However, a special supplementation of vitamin C seems not to be necessary for PKU patients, as the most fruit and vegetables, the main sources for vitamin C, are low in protein and could be consumed in adequate amounts. Recent studies even showed that free consumption of fruits and vegetables does not impair the metabolic control in PKU patients (MacDonald et al. 2003; Rohde et al. 2012; Zimmermann et al. 2012). Hence, independent from the therapeutic regimes (Phe restricted diet + AAM alone or in combination with BH4) it should be the objective to encourage the patients to eat more fruits as well as vegetables, rather than supplementing vitamin C.
Following the stopped or reduced AAM administration, the patients also showed an insufficient supply of some other micronutrients, in particular of vitamin D, iron, calcium, iodine and zinc. With respect to the long-term outcome of this group of patients, an insufficient micronutrient supply should be avoided. This might be the reason why some specialized centres still supply BH4-sensitive patients with a relatively high amount of AAM despite the fact that their pure protein supply from natural food is adequate (Singh et al. 2010). It should be remarked that the micronutrient content of all AAM is calculated according to a very limited Phe tolerance. A reduction of AAM supply under BH4 treatment without adapting eating habits therefore bears the risk of an insufficient micronutrient supply. On the other hand overdosing AAM in order to supply adequate amounts of micronutrients may lead to obesity. One could argue that even healthy German children do not reach the currently recommended intake of several micronutrients, especially vitamin D. However, their micronutrient intake is still above that of the investigated children with PKU. In addition vitamin D deficiency continuous to be a common problem among otherwise healthy children. Endogenous production, cleavage by sunlight and further enzymatic modification into active vitamin D3 are not satisfactory to prevent rickets. This even led to compulsory vitamin D3 supplementation of dairy products in some countries (Unuvar and Buyukgebiz 2010).
As a consequence a micronutrient supplement not containing any protein, carbohydrate or fat should be developed for these patients. Unfortunately specifically developed supplements are only available in some countries. Alternatively, the intake of an existing vitamin and mineral supplement for healthy children is recommended at least for the period until eating habits change completely towards an adequate supply from natural food.
In conclusion, the results of the study confirm that some patients benefit from a BH4 therapy. Their Phe tolerance increases markedly, allowing a relaxed diet containing more protein rich food. Furthermore, BH4-sensitive patients do no longer need special low protein products. Nevertheless, BH4 supplementation with a relaxed diet currently bears the risk of an imbalanced nutrition. Especially, with respect to nutrition related diseases the potential micronutrient deficiency needs consideration. Supplementation may become necessary. Nutritional education seems to be important for PKU patients under a BH4 therapy as the new dietetic regime contradicts almost everything they were taught before. To prevent deterioration of eating habits a close follow-up of these patients by dieticians is recommended. Long-term multicenter settings with a higher sample size are necessary to further investigate the nutrient supply under BH4 therapy and to satisfy the dietary requirements of this special group of patients in the future.
Acknowledgements
The authors would like to thank the patients and their families for their interest and participation. The help by the nurses at the hospital’s outpatient clinic is as much appreciated as that by the technicians in the department’s laboratory.
This work was partly supported by an investigator-initiated, unrestricted research grant from Merck-Serono GmbH.
Take Home Message
Although BH4 therapy is an effective and helpful additional treatment in some patients with PKU, changes in eating habits and consequently insufficient supply of micronutrients has to be judged critically.
Footnotes
Competing interests: None declared
References
- Blau N, Bélanger-Quintana A, Demirkol M, Feillet F, Giovannini M, Mac Donald A, Trefz FK, van Spronson FJ. Optimizing the use of sapropterin (BH4) in the management of phenylketonuria. Mol Genet Metab. 2009;96:158–163. doi: 10.1016/j.ymgme.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Blau N, Hennermann JB, Langenbeck U, Lichter-Konecki U. Diagnosis, classification, and genetics of phenylketonuria and tetrahydrobiopterin (BH4) deficiencies. Mol Genet Metab. 2011;104(Suppl):S2–S9. doi: 10.1016/j.ymgme.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Bélanger-Quintana A, García MJ, Castro M, Desviat LR, Pérez B, Mejía B, et al. Spanish BH4-responsive phenylalanine hydroxylase-deficient patients: evolution of seven patients on long-term treatment with tetrahydrobiopterin. Mol Genet Metab. 2005;86(Suppl 1):S61–S66. doi: 10.1016/j.ymgme.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Burgard P, Rey F, Rupp A, Abadie V, Rey J. Neuropsychologic functions of early treated patients with phenylketonuria, on and off diet: results of a cross-national and cross-sectional study. Pediatr Res. 1997;41:368–374. doi: 10.1203/00006450-199703000-00011. [DOI] [PubMed] [Google Scholar]
- Burlina A, Blau N. Effect of BH(4) supplementation on phenylalanine tolerance. J Inherit Metab Dis. 2009;32:40–45. doi: 10.1007/s10545-008-0947-1. [DOI] [PubMed] [Google Scholar]
- Burton BK, Grange DK, Milanowski A, Vockley G, Feillet F, Crombez EA, et al. The response of patients with phenylketonuria and elevated serum phenylalanine to treatment with oral sapropterin dihydrochloride (6R-tetrahydrobiopterin): a phase II, multicentre, open-label, screening study. J Inherit Metab Dis. 2007;30:700–707. doi: 10.1007/s10545-007-0605-z. [DOI] [PubMed] [Google Scholar]
- Burton BK, Bausell H, Katz R, Laduca H, Sullivan C. Sapropterin therapy increases stability of blood phenylalanine levels in patients with BH4-responsive phenylketonuria (PKU) Mol Genet Metab. 2010;101:110–114. doi: 10.1016/j.ymgme.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Ceglarek U, Müller P, Stach B, Bührdel P, Thiery J, Kiess W. Validation of the phenylalanine/tyrosine ratio determined by tandem mass spectrometry: sensitive newborn screening for phenylketonuria. Clin Chem Lab Med. 2002;40:693–697. doi: 10.1515/CCLM.2002.119. [DOI] [PubMed] [Google Scholar]
- De Bourdeaudhuij I, te Velde S, Brug J, Due P, Wind M, Sandvik C, et al. Personal, social and environmental predictors of daily fruit and vegetable intake in 11-year-old children in nine European countries. Eur J Clin Nutr. 2008;62:834–841. doi: 10.1038/sj.ejcn.1602794. [DOI] [PubMed] [Google Scholar]
- Elfhag K, Tholin S, Rasmussen F. Consumption of fruit, vegetables, sweets and soft drinks are associated with psychological dimensions of eating behaviour in parents and their 12-year-old children. Public Health Nutrition. 2008;11:914–923. doi: 10.1017/S1368980008002371. [DOI] [PubMed] [Google Scholar]
- Fiori L, Fiege B, Riva E, Giovannini M. Incidence of BH4-responsiveness in phenylalanine-hydroxylase-deficient Italian patients. Mol Genet Metab. 2005;86(Suppl 1):S67–S74. doi: 10.1016/j.ymgme.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Germany (D), Austrian (A), Swiss (CH)DACH-Reference values for the intake of nutritive substances (Referenzwerte für die Nährstoffzufuhr, DGE 2000) Deutsche Gesellschaft für Ernährung e.V. (DGE), Österreichische Gesellschaft für Ernährung (ÖGE), die Schweizerische Gesellschaft für Ernährungsforschung SGE, Schweizerische Vereinigung für Ernährung: Referenzwerte für die Nährstoffzufuhr (Germany, [DACH, 2000] 1st ed Frankfurt am Main, Umschau/ Braus 2000
- Hartmann B (2009) Bundeslebensmittelschluessel. Max-Rubner-Institut, Karlsruhe, Germany. http://www.bls.nvs2.de/index.php?id=37
- Hennermann JB, Buhrer C, Blau N, Vetter B, Monch E. Long-term treatment with tetrahydrobiopterin increases phenylalanine tolerance in children with severe phenotype of phenylketonuria. Mol Genet Metab. 2005;86(Suppl 1):S86–S90. doi: 10.1016/j.ymgme.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Kaufman S, Berlow S, SUmmer GK, Milstien S, Schulman JD, Orloff S, Spielberg S, Pueschel S. Hyperphenylalaninemia due to a deficiency of biopterin: a variant form of phenylketonuria. N Engl J Med. 1978;299:673–679. doi: 10.1056/NEJM197809282991301. [DOI] [PubMed] [Google Scholar]
- Kersting M, Alexy U. iX—Empfehlungen für die Ernährung von Kindern und Jugendlichen (German) Bonn: aid Infodienst; 2005. [Google Scholar]
- Kremers SP, Brug J, de Vries H, Engels RC. Parenting style and adolescent fruit consumption. Appetite. 2003;41:43–50. doi: 10.1016/S0195-6663(03)00038-2. [DOI] [PubMed] [Google Scholar]
- Lambruschini N, Pérez-Dueñas B, Vilaseca MA, Mas A, Artuch R, Gassió R, et al. Clinical and nutritional evaluation of phenylketonuric patients on tetrahydrobiopterin monotherapy. Mol Genet Metab. 2005;86(Suppl 1):S54–S60. doi: 10.1016/j.ymgme.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Macdonald A. Diet and compliance in phenylketonuria. Eur J Pediatr. 2000;159(Suppl 2):S136–S141. doi: 10.1007/PL00014375. [DOI] [PubMed] [Google Scholar]
- Macdonald A, Rylance G, Davis P, Asplin D, Hall SK, Booth IW. Free use of fruits and vegetables in phenylketonuria. J Inherit Metab Dis. 2003;26:327–338. doi: 10.1023/A:1025150901439. [DOI] [PubMed] [Google Scholar]
- Macdonald A, Gokmen-Ozel H, van Rijn M, Burgard P. The reality of dietary compliance in the management of phenylketonuria. J Inherit Metab Dis. 2010;33:665–670. doi: 10.1007/s10545-010-9073-y. [DOI] [PubMed] [Google Scholar]
- Mensink GBM, Heseker H, Richter A, et al. Ernährungsstudie als KiGGS-Modul (EsKiMo). Forschungsbericht. Berlin, Paderborn: RKI, UniversitätPaderborn; 2007. [Google Scholar]
- Mütze U, Roth A, Weigel JF, et al. Transition of young adults with phenylketonuria from pediatric to adult care. J Inherit Metab Dis. 2011;34(3):701–709. doi: 10.1007/s10545-011-9284-x. [DOI] [PubMed] [Google Scholar]
- Muntau AC, Roschinger W, Habich M, et al. Tetrahydrobiopterin as an alternative treatment for mild phenylketonuria. N Engl J Med. 2002;347:2122–2132. doi: 10.1056/NEJMoa021654. [DOI] [PubMed] [Google Scholar]
- Rohde C, Mütze U, Weigel J FK, Ceglarek U, Thiery J, Kiess W, Beblo S (2012) Unrestricted consumption of fruits and vegetables in phenylketonuria: no major impact on metabolic control. Eur J Clin Nutr. http://www.nature.com/ejcn/journal/vaop/ncurrent/full/ejcn2011205a.html [DOI] [PubMed]
- Schulz B, Bremer HJ. Nutrient intake and food consumtion of adolescents and young adults with phenylketonuria. Acta Paediatr. 1995;84:743–748. doi: 10.1111/j.1651-2227.1995.tb13748.x. [DOI] [PubMed] [Google Scholar]
- Scriver CR, Kaufman S, Eisensmith RC, Woo SLC. The hyperphenylalaninemias. In: Scriver RC, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular basis of inherited disease. New York: McGraw-Hill; 1998. pp. 1015–1075. [Google Scholar]
- Singh RH, Quirk ME, Douglas TD, Brauchla MC. BH(4) therapy impacts the nutrition status and intake in children with phenylketonuria: 2-year follow-up. J Inherit Metab Dis. 2010;33:689–696. doi: 10.1007/s10545-010-9224-1. [DOI] [PubMed] [Google Scholar]
- Stemerdink BA, Kalverboer AF, van der Meere JJ, et al. Behaviour and school achievement in patients with early and continuously treated phenylketonuria. J Inher Metab Dis. 2000;23:548–562. doi: 10.1023/A:1005669610722. [DOI] [PubMed] [Google Scholar]
- Trefz FK, Burton BK, Longo N, et al. Efficacy of sapropterin dihydrochloride in increasing phenylalanine tolerance in children with phenylketonuria: a phase III, randomized, double-blind, placebo-controlled study. J Pediatr. 2009;154:700–707. doi: 10.1016/j.jpeds.2008.11.040. [DOI] [PubMed] [Google Scholar]
- Unuvar T, Buyukgebiz A. Nutritional rickets and vitamin D deficiency in infants, children and adolescents. Pediatr Endocrinol Rev. 2010;7:283–291. [PubMed] [Google Scholar]
- Weglage J, Funders B, Wilken B, et al. Psychological and social findings in adolescents with phenylketonuria. Eur J Pediatr. 1992;151:522–525. doi: 10.1007/BF01957759. [DOI] [PubMed] [Google Scholar]
- Zabinski MF, Daly T, Norman GJ, Rupp JW, Calfas KJ, Sallis JF, et al. Psychosocial correlates of fruit, vegetable, and dietary fat intake among adolescent boys and girls. J Am Diet Assoc. 2006;106:814–821. doi: 10.1016/j.jada.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Ziesch B, Weigel J, Thiele A, Mütze U, Rohde C, Ceglarek U, Thiery J, Kiess W, Beblo S (2012) Tetrahydrobiopterin (BH4) in PKU: effect on dietary treatment, metabolic control and quality of life. J Inher Metab Dis. doi:10.1007/s10545-012-9458-1 [DOI] [PubMed]
- Zimmermann M, Jacobs P, Fingerhut R, Torresani T, Thöny B, Blau N, Baumgartner MR, Rohrbach M. Positive effect of a simplified diet on blood phenylalanine control in different phenylketonuria variants, characterized by newborn BH4 loading test and PAH analysis. Mol Genet Metab. 2012;106:264–268. doi: 10.1016/j.ymgme.2012.04.016. [DOI] [PubMed] [Google Scholar]


