Abstract
Background: Some lysosomal storage disorders (LSDs), including Muccopolysaccharidosis type 1 (MPSI), are associated with characteristic facies. Methods such as three-dimensional (3D) facial scanning and geometric morphometric techniques can potentially generate detailed objective descriptions of these facial phenotypes. This approach can facilitate discriminating the inherent overlap in facial phenotypes within these disease spectra, and the non-invasive monitoring of disease progression and treatment.
Methods: 3D facial images of three MPS I-affected individuals and 400 reference subjects (aged 5–25 years) were obtained using a 3dMD camera (Atlanta, Georgia). Images were fitted with an anthropometric mask, comprising a set of spatially dense quasi-landmarks. A statistical face-space was constructed from the reference image set and the MPS I-affected individuals were compared to this face-space utilising an emerging methodology known as dysmorphometrics. This facilitated simultaneous identification of harmonic and discordant facial regions. A relative significant discordance (RSD) score quantified proportional facial discordance for a given individual, whilst a root-mean-squared-error (RMSE) score measured the degree of facial discordance providing a severity measure.
Results: A consistent facial pattern, with differential severities, primarily affecting the frontal, nasal, infraorbital and cheek regions, was detected in all three individuals. As expected, there was greater discordance (RMSE, RSD) with clinically severe MPS I when compared to attenuated disease.
Conclusions: Objective detection and localisation of MPS I facial characteristics was achieved, and severity scores were attributed. This spatially dense dysmorphometric facial phenotyping technique has the potential to be used for non-invasive treatment monitoring and as a discriminatory tool.
Background
Lysosomal storage disorders (LSDs) are a group of inborn metabolic disorders, associated with disrupted lysosomal function that causes widespread lysosomal accumulation of undegraded macromolecules (Aldenhoven et al. 2009). Untreated, they inevitably result in progressive multisystem disease. With the advent of disease-modifying treatments, timely diagnosis and monitoring is increasingly pivotal to improving prognosis of affected individuals including, but not limited to, some muccopolysaccharidoses (e.g. MPS I) and Fabry disease. MPS-affected individuals are described as having characteristic ‘coarse’ facies. However, the detection of LSDs based on facial features can be challenging owing to overlapping facial phenotypes (Clarke 1997), clinical inexperience and attenuated disease. Supplementary approaches to discriminate between and within disorders and monitor treatment in a definitive manner are, therefore, required.
There have been promising, but limited, attempts towards objective definition of LSD-associated facial phenotypes, and advances in imaging technology are facilitating extension of these preliminary investigations. A two-dimensional (2D) photogrammetric study by Boehringer et al. (2006) discriminated MPS III from a number of non-LSD syndromic conditions, and a proof-of-principle study by Cox-Brinkman et al. (2007) utilised three-dimensional (3D) face shape analysis to characterise the face of Fabry disease. The latter study used spatially dense 3D surface modelling and morphometric analysis to quantify differences between male and female Fabry individuals, as well as in comparison to healthy controls, with classification specificities of 85 % and 67 %, respectively (Cox-Brinkman et al. 2007).
Quantitative morphometric techniques have demonstrated great potential in detecting facial dysmorphology and variation. Hammond et al. (2007) established facial archetypes for a small number of syndromic conditions. However, this methodology did not discriminate the impact of the condition from normal facial variation, and only visualised the facial dysmorphology expressed as net population difference. It was also based on a closed classification setup, with the individual always attributed to either one of the defined populations, even when they did not belong to any of them. Statistically, this setup is equivalent to an unpaired analysis, which requires the appropriate sample number to obtain sufficient statistical power. These limitations have, in part, been recognised by Hammond et al. (2012) and they have similarly been addressed by Claes et al. (2012a) with, an alternate dysmorphometrics strategy that makes comparisons to population-based ‘averages’. This was previously applied to measure changes in facial shape as a result of surgical intervention (Claes et al. 2012b) and asymmetric abnormalities (Claes et al. 2011).
This chapter explores the use of dysmorphometrics (Claes et al. 2010, 2012a) to discriminate subtle facial characteristics of MPS I. Normal variation can be learned from a reference dataset of individuals without pathology and obtained with relative ease. However, the collection of facial data from individuals with rare diseases is more challenging. A dysmorphometric approach does not require comprehensive datasets of patient groups to generate a facial anomaly map. This facilitates investigations into facial phenotypic signatures in rare conditions. In difference to archetype analysis, an open-classification is performed, using only the normal variation from an appropriate reference dataset, which is encoded into a statistical face-space (Aeria et al. 2010; Wei et al. 2011; Claes et al. 2012a). This facilitates an individual-specific assessment and visualisation of a problem or hypothesis, through the construction of a normal-equivalent or case-specific control (Claes et al. 2010, 2012a).
This approach facilitates investigations into syndromic phenotypic signatures of rare conditions and a process to address the inherent overlap in facial phenotypes within disease spectra. It can also be applied to the non-invasive monitoring of disease progression and evaluation of therapeutic effects. From this exploratory study of three MPS I–affected individuals, we describe regional discrepancy scores that can be employed to establish discordance signatures in combination with a continuous severity index, to investigate aspects of metabolic and other rare diseases.
Methods
Ethics Approvals
Ethics approvals (PMHHEC: 1801/EP, 1443/EP and 1488/EP) were granted by the Princess Margaret Hospital for Children Ethics Committee in Perth, Australia.
Participants
The normative reference cohort (Perth face-space project) consisted of 400 healthy individuals aged 5–25 years. Subjects completed a questionnaire on relevant medical history and informed consent. Subjects with prior craniofacial surgery or a suspected syndromic condition with craniofacial manifestations were excluded.
Three participants clinically diagnosed with MPS I (severe or attenuated), and of Western European ancestry, were recruited at the 11th International Symposium on MPS and related diseases (Adelaide, SA, Australia; July 2010). Individual I was a 21-year-old female diagnosed with Hurler-Scheie syndrome (MPS IHS, OMIM #607015); Individual II was a 23-year-old female diagnosed with Scheie syndrome (MPS IS, OMIM #607016); and Individual III was a 14-year-old male diagnosed with Hurler syndrome (MPS IH, OMIM #607014).
D Image Acquisition
3D images were captured using a 3dMDFacialTM stereophotogrammetric system (3dMD Inc., Atlanta, Georgia, USA) with sub-millimetre precision (Aldridge et al. 2005). Facial shape was represented as a point cloud consisting of approximately 200,000 points defined in a 3D coordinate space.
Anthropometric Masks and Facial Mapping
An anthropometric mask (AM) (Claes et al. 2012b) consisting of a spatially dense ~10,000 quasi-landmark configuration, was non-rigidly mapped onto 3D facial scans of affected and normative reference individuals (403 3D images). This mapping process is equivalent to the indication of traditional anthropometric landmarks (Claes et al. 2011, 2012b) and establishes homology among the 3D faces, thus allowing image data from different individuals to be standardised and analysed in a spatially dense manner.
Statistical Face-Space
A statistical face-space, constructed from normative reference individuals, describes facial variations and covariances (harmonic interrelationships) present within the general population (Aeria et al. 2010; Wei et al. 2011; Claes et al. 2012a). A generalised Procrustes fit (Rohlf and Slice 1990) rotates, translates and scales the quasi-landmark configurations into the same coordinate space. Shape variation is then described by Procrustes distance residuals. Subsequently, the extent and modes of facial variation are calculated using Principal Component Analysis (PCA) to elucidate complex harmonic interrelationships found in facial form variations. This ‘normative’ statistical face-space was created to define the boundaries or statistical limits of typical facial variation found in the reference population (Claes et al. 2012a).
Dysmorphometrics and Normal Equivalents
Dysmorphometrics identifies abnormal facial morphology, as outliers in comparison to a given normative reference (Claes et al. 2012a). In this scenario, normative references are encoded within the face-space and the outliers reflect discordancy in facial form. Dysmorphometrics involves a robust superimposition of the reference face-space onto the patient’s facial scan, where each of the 10,000 quasi-landmarks is assigned a confidence value. This reflects the confidence of such a point being harmonic (value closer to 1) or discordant (= outlier, value closer to 0) against a p-value of 0.05.
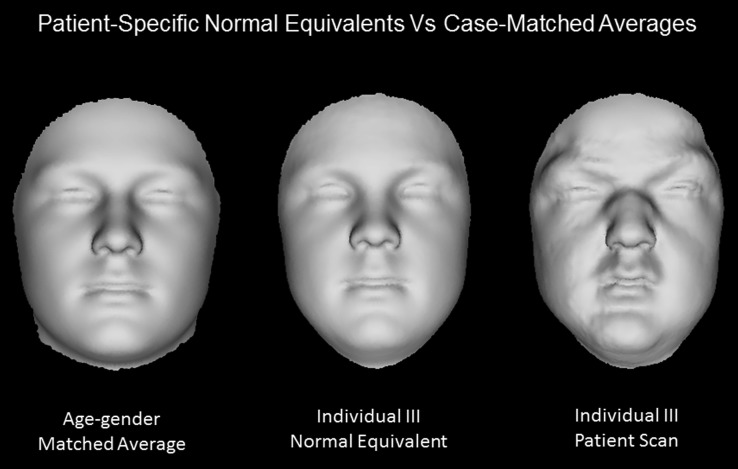
This superimposition establishes the best description of a given face only in terms of harmonious facial variation, known as the ‘normal equivalent’ (NE). It eliminates confounding variables like position and orientation differences, and considers typical within-population differences (e.g. variances induced by gender, age, BMI, ethnicity, etc.) as confounders as well. This allows for construction of case-matched controls, more specifically, generation of a patient-specific and population-based matched reference (see Fig. 1 for comparison to averaged age-gender-matched control), as long as the variation is described by the face-space. Thus, providing a more sensitive and specific analysis on the syndromic facial phenotype. We used a face-space comprised predominantly of Western European ethnic variance. Technical details can be found in (Claes et al. 2012a).
Fig. 1.
Comparison of the normal equivalent (NE) versus an average case-matched control. The NE of Individual III (middle), synthetically matched for variables (e.g. age, gender and ethnicity) according to the dysmorphometric approach, is visually a closer matched reference to the original patient scan (right), in comparison to an average 14-year-old male scan (left)
Scoring, Analysis and Visualisation of Facial Variants
When the NE is superimposed on to the patient scan, differences between corresponding landmarks of the two configurations provides the means to measure magnitude (distance) and directions (vector) of facial discordances. Distance maps were summarised by a root-mean-squared-error (RMSE) score, which takes into account both variance and possible bias, as an error in millimetres (mm). This RMSE score was applied to measure severity for the observed discordancy. Confidence maps were summarised by relative significant discordance (RSD) percentages. This localised the discrepancy and provided an overall proportion of dysmorphology for a given individual. Finally, vector maps provide directional information on the observed facial discordance. The distance, outlier and vector maps collectively provide a facial discordance signature specific to the MPS I–affected individual.
Normative Population Reference Statistics
Some discordance is to be expected in the ‘normative’ reference range as a consequence of scan/mapping artefacts and/or the reduction of total variance modelled to 98 %. Typical facial values in the reference population were established using a leave-one-out approach to compute discordance scores for the 400 healthy individuals. As a means of reference, distributions of overall RMSE and RSD scores for the ‘normative’ cohort were used to express patient values as Z-scores.
Results
Reference cohort distributions were characterised by a mean of 10.6 % RSD (1.8 % SD) and 0.91 mm RMSE (0.22 mm SD), summary statistics are provided in Table 1. These summary statistics provided the data for the calculation of Z-scores for the MPS I subjects.
Table 1.
Overall NE assessment and Z-scores for the three MPS I individuals in relation to summary reference statistics
| Dysmorphology (RSD in %) | Relative severity (RMSE in mm) | Z-RSD (%) | Z-RMSE (mm) | |
|---|---|---|---|---|
| Reference (mean) | 10.6 | 0.91 | - | - |
| Reference (SD) | 1.8 | 0.22 | - | - |
| Individual I (MPS IHS) | 9.08 | 0.95 | −0.84 | +0.18 |
| Individual II (MPS IS) | 10.31 | 1.19 | −0.16 | +1.27 |
| Individual III (MPS IH) | 12.84 | 1.46 | +1.24 | +2.50 |
% Dysmorphology scores were based on RSD values of the outlier map, which quantified the extent of the discordance and depicted the ‘affected area’. The RMSE (mean + SD) score quantified the overall degree of discordance in millimetre and provided a measure of severity of the ‘affected area’. Z-scores described the extent of difference to reference distributions (reference range). MPS IHS (Hurler-Scheie Syndrome); MPS IS (Scheie Syndrome); and MPS IH (Hurler Syndrome)
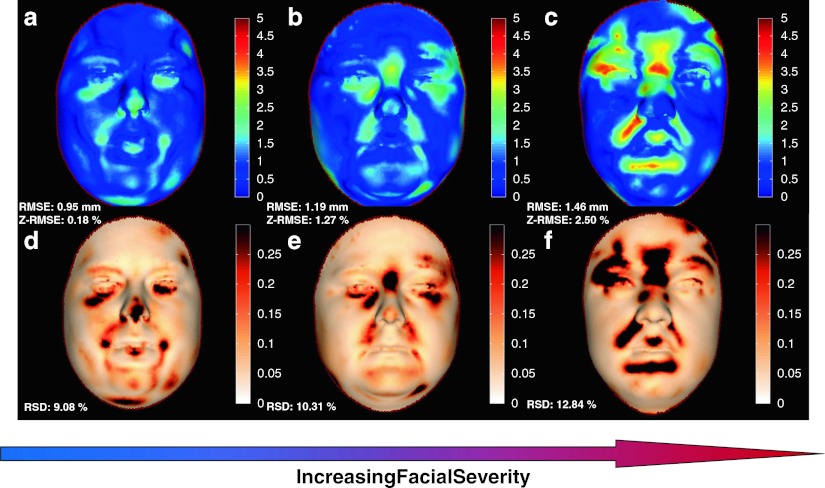
Discordance signatures (i.e. outlier and distance maps) for the three MPS I individuals are depicted in Fig. 2. A consistent facial discordance with varying severity was observed for all three MPS I individuals (Fig. 2; Table 1). Individual I exhibited facial variants in the infraorbital region, nasal tip and nasio/mento-labial sulci (Fig. 2a, d). A similar discordance pattern was observed in Individual II with an additional variant in the frontal region (Fig. 2b, e). All facial variants detected in Individuals I and II were also observed in Individual III, with the exception of the nasal tip (Fig. 2c, f). Among all discordant facial regions observed in Individuals I, II and III, two individuals were found to be discordant at the naso/mentolabial sulci, as well as the frontal, nasal and infraorbital regions.
Fig. 2.
Discordance severity in MPS I clinical subtypes. Dysmorphometric facial assessment depicting the distance and outlier map components of each MPS I–affected individual’s discordance signature. The distance maps (top row) of Individuals I, II and III, respectively (a, b and c), illustrates in millimetre the magnitude of the facial discordance observed where regions of discordance above a threshold of 3 mm (0 on the scale) are visualised using a colour bar. The corresponding images d, e and f (bottom row) are outlier maps that depict statistically significant areas of facial discordance. Distance map and scale bar in 0–5 mm (0 blue, 5 mm red), while outlier map and scale bar in 0–0.3 (0 white, 0.3 black)
Sorting the cases on RMSE and RSD values provides a severity differential, with Individual I presenting discordance scores of 0.95 mm RMSE and 9.08 % RSD, these were elevated in Individual II (RMSE 1.19 mm; RSD 10.31 %), Individual III had the most severe discordance scores of 1.46 mm RMSE and 12.84 % RSD (Table 1). The facial dysmorphology for both Individual I and II was subtle with discordance scores that were within the reference range (Z-RSD: –0.84 % and Z-RSD: –0.16 % respectively). The severity of the observed facial discordance was likely to be mediated, as all patients were treated (e.g. ERT/BMT), indicating the sensitivity of the technique to detect subtle differences.
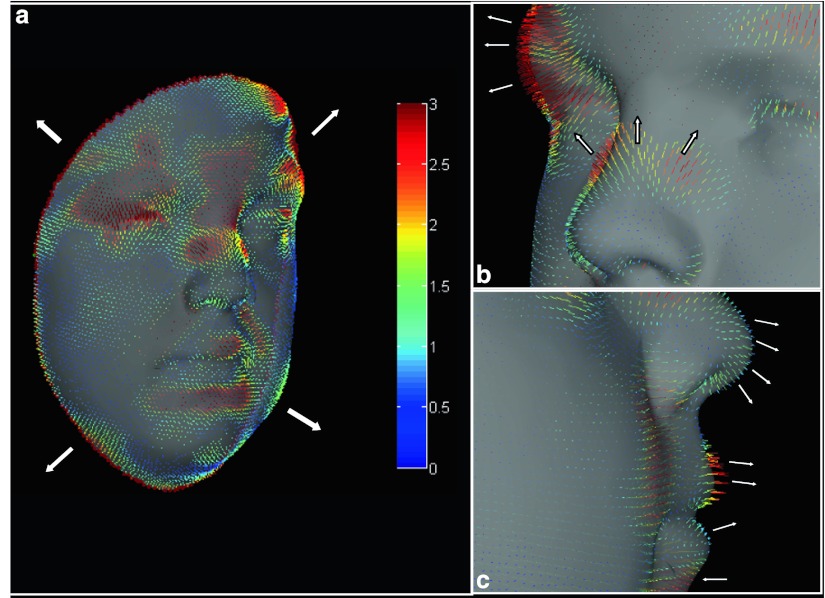
The vector map provided information on magnitude and direction of the facial discordance. In Individual III, the vector map revealed an overall ‘ballooning effect’ (Sandubray et al. 2012) of the facial tissues; a close up of this in profile view showed specific vector displacements at the brow ridges, glabella/frontal region, infraorbital region, upper lip and both nasiolabial and mentolabial sulci (Fig. 3). Expansive displacements were observed at the brow ridges and upper lip, different to the glabella, nasolabial and mentolabial sulci, and infraorbital regions that were depressed.
Fig. 3.
Individual III MPS I–affected vector map. Dysmorphometric vector map of Individual III depicting vectors associated with substrate accumulation resulting in a ‘ballooning’ effect (a) of facial features and tethering in the nasiolabial folds. A close up view (b) and a profile view (c) are also shown, illustrating specific vector movements at the brow ridges, nasal bridge/tip, upper lip and infraorbital, nasiolabial and mentolabial regions
Discussion
Lysosomal Storage Disorders (LSDs) can manifest a broad phenotypic spectrum. MPS I–affected individuals have been classified to have Hurler, Hurler-Scheie or Scheie syndrome. Previously this classification was thought to reflect separate entities; however, they are now recognised as part of a continuum and affected individuals can be classed on a spectrum of severe or attenuated MPS I (Muenzer 2004). We objectively quantified a facial phenotype of three individuals affected with MPS I and sorted them on a severity scale using summary discordance values (Fig. 2). This severity scale reflected established criteria (severe - attenuated) and found a consistent pattern of facial discordance indicative of a specific signature.
This signature was detectable in Individuals I and II, with attenuated forms, despite discordance scores that fell within the limits of the normative reference. It is not unexpected that phenotypic facial signatures, particularly of attenuated MPS I, can exist within normative ranges. However, a consistent pattern of facial discordance was found, indicating that detection of subtle phenotypes was achievable. This signature was similarly seen in Individual III who was diagnosed with a more severe form of MPS I. He presented with the greatest facial variation, as reflected in the dysmorphometry scores that were outside the reference range (Z-RSD; 1.24; and Z-RSME; 2.50).
A pattern of accumulation of extracellular substrates in facial compartments may be delineated by tethering of the submuscular aponeurotic system (SMAS) (Thaller et al. 1990). The pattern of facial variation was consistent with this proposition, manifesting a flattened frontal region in relation to prominently raised brow ridges, depressed infraorbital regions, and a prominent nasal tip in relation to a flattened nasal bridge and expansive alae nasi. The hypothesised tethering was, in particular, evident in the depressed nasolabial and mentolabial sulci; this is in accordance with the overall facial ballooning (Figure 3A), or ‘puffiness’, characteristic of this condition (Sandubray et al. 2012).
The potential of dysmorphometrics to provide an objective facial discordance signature and attribute severity on a continuous scale was demonstrated in a small cohort of MPS I individuals. Facial histograms provide data of the overall pattern of dysmorphology as well as patterns most frequently encountered, facilitating detection of subtle presentations. This is made possible by using NE controls that effectively remove confounding normal facial variations associated with gender, age and population affinity (Claes et al. 2012a). This approach, when combined with a large normative dataset, can potentially address sample size limitations inherent in the study of rare disorders. Additionally, in contrast to other morphometric analyses (Hammond et al. 2005; Shaweesh et al. 2006), it allows for open classifications and individual-specific assessments, which facilitates a progression from descriptive dysmorphology to quantitative dysmorphometrics. Our findings supported the possibility that using these techniques on an expanded cohort of individuals, a consistent pattern of facial variation may be revealed in both severe and attenuated MPS I. Given the rarity of these conditions, further investigations will optimally be approached by multicenter collaborations.
In theory, dysmorphometrics allows the use of a mixed control group (e.g. in gender, age and ethnicity). The face-space is a global shape model of covariance requiring all facial areas and features to be consistent, and in harmony, with each other for construction of the NE. It is therefore impossible for the face-space to randomly combine facial features from different subgroups that do not match. The reference face-space is inclusive in nature, enabling the same face-space to generate different age-, sex- and ethnicity-matched controls for answering distinct biological questions, as long as the variation is within the face-space. If an affected individual has facial features that show some degree of overlap with a subgroup of individuals of another gender or ethnicity, it is possible to make comparisons to a subspace constructed from subjects of the overlapping gender/ethnicity. However, this may only be required when the overlap extends to the majority of the face (e.g. > 40 % of the facial area). Limits of the applicability of subspace reference will require further testing.
There are considerable difficulties in objectively monitoring LSD treatment and establishing optimal dosages, particularly for presymptomatic patients in the absence of overt clinical features or suitable biomarkers (Fuller et al. 2004; Langford-Smith et al. 2010). Given the finding of apparent gradation of facial phenotype, we propose further study on the potential utility of 3D facial analysis for non-invasive disease monitoring and assessment of treatment response.
The development of new therapeutics (Wraith et al. 2005; Sifuentes et al. 2007; Munoz-Rojas et al. 2008; Bijarnia et al. 2009; Clarke et al. 2009; Cox-Brinkman et al. 2010; Lachmann 2010; Langford-Smith et al. 2010; Schiffmann 2010) provides an impetus for timely LSD diagnosis, as the efficacies of these treatments are dependent on initiating therapy prior to the development of irreversible complications (Meikle et al. 2004; Meikle 2007). The challenge of achieving a definitive LSD diagnosis is complicated by overlapping phenotypic spectra (Meikle et al. 2004; Meikle 2007). Investigations have, therefore, been developed to allow simultaneous analysis for a number of these related conditions (e.g. urine metabolic screens and enzyme analysis panels) (Nielsen et al. 2010). However, with current biochemical investigations, false negatives may occur, particularly with attenuated subtypes, (Meikle et al. 2004; Meikle 2007) hence alternative approaches deserve investigation. We suggest that detection of subtle facial features can be objectively quantified and may facilitate MPS screening and diagnosis.
Development of effective LSD therapies is hindered by disease rarity (Muro 2010), and some current therapeutic strategies remain suboptimal and costly. Insights from specific LSD registries have significantly contributed to knowledge of disease spectra and natural history, but incomplete data on long-term outcomes and cost-effectiveness has raised concerns of the utility of this approach (Hollak et al. 2011). These factors argue for studies with multicentre coordination that cross boundaries of individual therapies. 3D facial analysis lends itself to such studies as hardware costs are modest, there is minimal consumable cost, data can be attained non-invasively from multiple sites, and centralised analysis for existing registries is possible (Pastores et al. 2007). Lifelong treatments for some LSDs necessitates longitudinal outcome-monitoring regimes, hence the need for innovative monitoring strategies that are cost effective and preferably non-invasive. Additionally, the following factors support facial analysis as particularly suitable for very young individuals: (1) it poses no health risk, (2) capture time is fast in comparison to other medical imaging and (3) if required, image capture can be easily repeated until a suitable image is obtained.
3D facial analysis may be useful for investigating disease biology to identify factors underlying phenotypic expression and to trace causal links between genotypes, environmental factors and phenotypes. Genotype-phenotype correlations in individuals with MPS I are poorly understood and are only partly related to the frequency of private IDUA gene mutations (Terlato and Cox 2003). Therefore, analysis of spatially dense facial phenotypes may provide a novel avenue for resolving these genotype-phenotype relationships.
Phenomics, defined as the acquisition of high-dimensional phenotypic data that inherently spans multiple levels of an organism, has been suggested for exploring pathogenesis (Houle et al. 2010). When coupled with other scientific methods, 3D facial analysis has yielded insights into rare disease biology (Tobin et al. 2008). According to Houle et al. (2010), the three key elements that foster phenomic developments are technological development, statistical and analytical capabilities, and integration incentives. Our approach satisfies the first two criteria and the rarity of LSDs provides an impetus for the last. We argue that quantitative phenotyping approaches, such as dysmorphometrics, (Claes et al. 2012a) can augment the repertoire of scientific protocols traditionally applied to disease studies (Houle et al. 2010; Klingenberg 2002). Ultimately, the unique combination of the multisystem nature of LSDs, disorder-specific treatments and high-resolution multidimensional LSD facial data may provide a platform for new insights into facial and systems biology.
Conclusions
This exploratory study using dysmorphometrics supported the validity of non-invasive 3D quantification of facial profiles of individuals with MPS I. Accordingly, objective high-resolution determination of patterns of facial variants may facilitate delineation of the MPS I disease spectrum, including in those with subtle facial phenotypes. This variance-based approach, which is uniquely suitable to multicentre applications, provides the means to quantify LSD facial dysmorphology as a holistic entity. Discrete phenotypes hereby defined can support further investigations including correlations with other LSD-related endpoints, such as disorder-related complications, which may facilitate treatment monitoring. Furthermore, when combined with molecular approaches, it may allow for novel explorations of pathogenic processes in other metabolic and genetic syndromic conditions.
Acknowledgements
The authors would like to thank all participants for their permission to use their images, everyone involved with the 11th International Symposium on MPS and Related Diseases in Adelaide, David Oliver and his colleagues from The Australian MPS and related diseases and Genzyme Australia. This work is supported by the Princess Margaret Hospital (PMH) Foundation in Perth, Western Australia, and has been approved by the PMH Ethics Committee (PMHHEC: 1801/EP, 1443/EP and 1488/EP). Genzyme provided an unrestricted educational grant and had no role in the interpretation or analysis of the data or in the decision to submit the manuscript for publication.
Abbreviations
- 2D
Two-dimensional
- 3D
Three-dimensional
- AM
Anthropometric mask
- BMT
Bone marrow transplantation
- EMA
European medicines agency
- ERT
Enzyme replacement therapy
- IDUA
α-L-iduronidase
- LSDs
Lysosomal storage disorders
- MPS I
Muccopolysaccharidosis type I
- MPS IH
Muccopolysaccharidosis type I, Hurler Syndrome
- MPS IHS
Muccopolysaccharidosis type I, Hurler-Scheie Syndrome
- MPS IS
Muccopolysaccharidosis type I, Scheie Syndrome
- NE
Normal equivalent
- PCA
Principal component analysis
- PCs
Principal components
- RMSE
Root-mean-squared-error
- RSD
Relative significant discordance
- SD
Standard deviation
- SMAS
Submuscular aponeurotic system
Authors’ Information
GB (MBBS, DCH, FRACP, PhD) Clinical Geneticist at Genetic Services of Western Australia (GSWA), Senior Clinical Lecturer at the School of Paediatrics and Child Health (SPACH), University of Western Australia. MW (MSc), Senior Research Scientist, Cranio-Maxillo-Facial Unit, Princess Margaret Hospital for Children (PMH), developer of facial assessment tools investigating normal and disordered facial growth patterns. PC (PhD), Computer Science Engineer and research expert on cranio-maxillofacial morphometrics, Catholic University of Leuven, Belgium. Winthrop Professor PLS (MBBS, MRCP, FRACP, MD) Head of SPACH and Consultant Physician at the Department of Respiratory Medicine, PMH. JG (AM, MB ChB, MD, FRACP) Clinical Professor at SPACH and Director of Genetic Services and the Familial Cancer Program of WA. SK (BSc Hons), PhD student at SPACH with a background in anatomy and genetics.
Synopsis
Objective 3D facial phenotyping using dysmorphometrics provides prospective avenues for non-invasive treatment monitoring of metabolic conditions.
Authors’ Contributions
SK wrote the manuscript with major input and revisions from all other authors. MW and GB were involved in drafting of the manuscript, while PC, JG and PLS provided valuable critical feedback. PC developed the fundamentals behind the anthropometric mask, the statistical face-space, dysmorphometrics, and the normal equivalent, with conceptual input from MW. PC also provided the facial mapping of the data and constructed the normative facial model of covariance. Finally, he ran the leave-one-out analysis to establish normative reference discordancy statistics. GB and JG provided clinical insight into investigated syndromes, rallied support from the MPS Society and fostered international collaborations. All authors read and approved the final manuscript.
Guarantor
As guarantor for this article, GB accepts full responsibility for this work and conduct of this study, has had access to the data, and controls the decision to publish.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Competing interests: None declared
References
- Aeria G, Claes P, Vandermeulen D, Clement JG. Targeting specific facial variation for different identification tasks. Forensic Sci Int. 2010;201:118–124. doi: 10.1016/j.forsciint.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Aldenhoven M, Sakkers RJB, Boelens J, de Koning TJ, Wulffraat NM. Musculoskeletal manifestations of lysosomal storage disorders. Ann Rheum Dis. 2009;68:1659–1665. doi: 10.1136/ard.2008.095315. [DOI] [PubMed] [Google Scholar]
- Aldridge K, Boyadjiev SA, Capone GT, DeLeon VB, Richtsmeier JT. Precision and error of three-dimensional phenotypic measures acquired from 3dMD photogrammetric images. Am J Medical Genetics Part A. 2005;138A:247–253. doi: 10.1002/ajmg.a.30959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijarnia S, Shaw P, Vimpani A, et al. Combined enzyme replacement and haematopoietic stem cell transplantation in Hurler syndrome. J Paediatr Child Health. 2009;45:469–472. doi: 10.1111/j.1440-1754.2009.01537.x. [DOI] [PubMed] [Google Scholar]
- Boehringer S, Vollmar T, Tasse C, et al. Syndrome identification based on 2D analysis software. Eur J Hum Genet. 2006;14:1082–1089. doi: 10.1038/sj.ejhg.5201673. [DOI] [PubMed] [Google Scholar]
- Claes P, Walters M, Clements J. Novel approaches in 3-dimensional facial profiling to establish facial aesthetic objectives in the treatment of facial dysmorphologies. Annalls of The Royal Australasian College of Dental Surgeons. 2010;20:56–58. [PubMed] [Google Scholar]
- Claes P, Walters M, Vandermeulen D, Clement JG. Spatially-dense 3D facial asymmetry assessment in both typical and disordered growth. J Anat. 2011;219:444–455. doi: 10.1111/j.1469-7580.2011.01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes P, Daniel K, Walters M, Clement JG, Vandermeulen D, Suetens P. Dysmorphometrics: The modelling of morphological anomalies. Theor Biol Med Model. 2012;9(5):1–39. doi: 10.1186/1742-4682-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes P, Walters M, Clement JG. Improved facial outcome assessment using a 3D anthropometric mask. Int J Oral Maxillofac Surg. 2012;41:324–330. doi: 10.1016/j.ijom.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Clarke LA. Clinical diagnosis of lysosomal storage diseases. In: Applegarth DA, Dimmick JE, Hall JG, editors. Organelle diseases clinical features. Diagnosis, pathogenesis and management. London: Chapman and Hall Medicine; 1997. [Google Scholar]
- Clarke LA, Wraith JE, Beck M, et al. Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics. 2009;123:229–240. doi: 10.1542/peds.2007-3847. [DOI] [PubMed] [Google Scholar]
- Cox-Brinkman J, Vedder A, Hollak C, et al. Three-dimensional face shape in Fabry disease. Eur J Hum Genet. 2007;15:535–542. doi: 10.1038/sj.ejhg.5201798. [DOI] [PubMed] [Google Scholar]
- Cox-Brinkman J, van den Bergh Weerman MA, Wijburg FA, et al. Ultrastructural analysis of dermal fibroblasts in mucopolysaccharidosis type I: Effects of enzyme replacement therapy and hematopoietic cell transplantation. Ultrastruct Pathol. 2010;34:126–132. doi: 10.3109/01913121003648485. [DOI] [PubMed] [Google Scholar]
- Fuller M, Rozaklis T, Ramsay SL, Hopwood JJ, Meikle PJ. Disease-specific markers for the mucopolysaccharidoses. Pediatr Res. 2004;56:733–738. doi: 10.1203/01.PDR.0000141987.69757.DD. [DOI] [PubMed] [Google Scholar]
- Hammond P. The use of 3D face shape modelling in dysmorphology. Arch Dis Child. 2007;92:1120–1126. doi: 10.1136/adc.2006.103507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P, Hutton TJ, Allanson JE, et al. Discriminating power of localized three-dimensional facial morphology. Am J Hum Genet. 2005;77:999–1010. doi: 10.1086/498396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P, Hannes F, Suttie M, et al. Fine-grained facial phenotype-genotype analysis in Wolf-Hirschhorn syndrome. Eur J Hum Genet. 2012;20:33–40. doi: 10.1038/ejhg.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollak CE, Aerts JM, Ayme S, Manuel J. Limitations of drug registries to evaluate orphan medicinal products for the treatment of lysosomal storage disorders. Orphanet J Rare Dis. 2011;6:16. doi: 10.1186/1750-1172-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle D, Govindaraju DR, Omholt S. Phenomics: the next challenge. Nat Rev Genet. 2010;11:855–866. doi: 10.1038/nrg2897. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. Morphometrics and the role of the phenotype in studies of the evolution of developmental mechanisms. Gene. 2002;287:3–10. doi: 10.1016/S0378-1119(01)00867-8. [DOI] [PubMed] [Google Scholar]
- Lachmann R. Treatments for lysosomal storage disorders. Biochem Soc Trans. 2010;38:1465–1468. doi: 10.1042/BST0381465. [DOI] [PubMed] [Google Scholar]
- Langford-Smith K, Arasaradnam M, Wraith JE, Wynn R, Bigger BW. Evaluation of heparin cofactor II-thrombin complex as a biomarker on blood spots from mucopolysaccharidosis I, IIIA and IIIB mice. Mol Genet Metab. 2010;99:269–274. doi: 10.1016/j.ymgme.2009.10.175. [DOI] [PubMed] [Google Scholar]
- Meikle MC. Remodeling the dentofacial skeleton: the biological basis of orthodontics and dentofacial orthopedics. J Dent Res. 2007;86:12–24. doi: 10.1177/154405910708600103. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Fietz MJ, Hopwood JJ. Diagnosis of lysosomal storage disorders: current techniques and future directions. Expert Rev Mol Diagn. 2004;4:677–691. doi: 10.1586/14737159.4.5.677. [DOI] [PubMed] [Google Scholar]
- Muenzer J. The mucopolysaccharidoses: a heterogeneous group of disorders with variable pediatric presentations. J Pediatr. 2004;144:S27–34. doi: 10.1016/j.jpeds.2004.01.052. [DOI] [PubMed] [Google Scholar]
- Munoz-Rojas M-V, Vieira T, Costa R, et al. Intrathecal enzyme replacement therapy in a patient with mucopolysaccharidosis type I and symptomatic spinal cord compression. Am J Med Genet A. 2008;146A:2538–2544. doi: 10.1002/ajmg.a.32294. [DOI] [PubMed] [Google Scholar]
- Muro S. New biotechnological and nanomedicine strategies for treatment of lysosomal storage disorders. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:189–204. doi: 10.1002/wnan.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TC, Rozek T, Hopwood JJ, Fuller M. Determination of urinary oligosaccharides by high-performance liquid chromatography/electrospray ionization-tandem mass spectrometry: application to Hunter syndrome. Anal Biochem. 2010;402:113–120. doi: 10.1016/j.ab.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Pastores GM, Arn P, Beck M, et al. The MPS I registry: design, methodology, and early findings of a global disease registry for monitoring patients with mucopolysaccharidosis type I. Mol Genet Metab. 2007;91:37–47. doi: 10.1016/j.ymgme.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ, Slice D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Zool. 1990;39:40–59. doi: 10.2307/2992207. [DOI] [Google Scholar]
- Sandubray JM, Van Den Berghe G, Walter JH. Inborn metabolic diseases: diagnosis and treatment. Germany: Springer; 2012. [Google Scholar]
- Schiffmann R. Therapeutic approaches for neuronopathic lysosomal storage disorders. J Inherit Metab Dis. 2010;33:373–379. doi: 10.1007/s10545-010-9047-0. [DOI] [PubMed] [Google Scholar]
- Shaweesh AI, Clement JG, Thomas CD, Bankier A. Construction and use of facial archetypes in anthropology and syndrome diagnosis. Forensic Sci Int. 2006;159(Suppl 1):S175–185. doi: 10.1016/j.forsciint.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Sifuentes M, Doroshow R, Hoft R, et al. A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years. Mol Genet Metab. 2007;90:171–180. doi: 10.1016/j.ymgme.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Terlato NJ, Cox GF. Can mucopolysaccharidosis type I disease severity be predicted based on a patient’s genotype? A comprehensive review of the literature. Genet Med. 2003;5:286–294. doi: 10.1097/01.GIM.0000078027.83236.49. [DOI] [PubMed] [Google Scholar]
- Thaller SR, Kim S, Patterson H, Wildman M, Daniller A. The submuscular aponeurotic system (SMAS): a histologic and comparative anatomy evaluation. Plast Reconstr Surg. 1990;86:690–696. doi: 10.1097/00006534-199010000-00012. [DOI] [PubMed] [Google Scholar]
- Tobin JL, Di Franco M, Eichers E, et al. Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung’s disease in Bardet-Biedl syndrome. Proc Natl Acad Sci USA. 2008;105:6714–6719. doi: 10.1073/pnas.0707057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R, Claes P, Walters M, Wholley C, Clement JG. Augmentation of linear facial anthropometrics through modern morphometrics: a facial convexity example. Aust Dent J. 2011;56:141–147. doi: 10.1111/j.1834-7819.2011.01315.x. [DOI] [PubMed] [Google Scholar]
- Wraith EJ, Hopwood JJ, Fuller M, Meikle PJ, Brooks DA. Laronidase treatment of mucopolysaccharidosis I. BioDrugs. 2005;19:1–7. doi: 10.2165/00063030-200519010-00001. [DOI] [PubMed] [Google Scholar]