Abstract
Among the long-term complications of Classic Galactosaemia (Gal) is premature ovarian insufficiency (POI) in female patients with subtle abnormalities of reproductive function also reported in male patients. Leptin is a circulating hormone which reflects body energy stores and which affects the neuroendocrine reproductive axis and pubertal development.
We measured serum leptin in 28 children (10 girls, 18 boys; mean age 7.6 years, range 0.5–17.9 years) and in 22 adults (10 females, 12 males; mean age 23.9 years, range 18–37 years) with Gal on a strict galactose-restricted diet in comparison with control data.
Leptin levels (expressed as SDS for gender and pubertal stage) were lower in Gal children than controls (mean leptin-SDS = –0.71 for girls, p < 0.05, –0.97 for boys compared with SDS = 0 for controls, p < 0.05). In an age-related analysis, leptin levels did not correlate with age in children with Gal for both sexes as it did for matched controls.
As expected, females had higher leptin levels than males in either group. In adults with Gal, leptin concentrations were within normal limits for both sexes when adjusted for gender and BMI. There was a linear relationship between log-leptin and BMI in children with Gal and in controls. For Gal women, log-leptin was also associated with BMI. However, for Gal men, and hence for the entire group of adult Gal patients, this association between log-leptin and BMI was not detectable. Our findings suggest that leptin dysregulation may play a role in fertility issues in individuals with Gal from an early age.
Introduction
Classic Galactosaemia (Gal; OMIM 230400) is an autosomal recessive disorder which is characterised by a deficiency of galactose-1-phosphate uridyltransferase (GALT). Mutation p.Q188R in the GALT gene is the most common mutation in patients with Gal. Despite newborn screening and early treatment with a strict galactose-restricted diet, a high percentage of patients experience long-term complications such as speech deficits, tremor, cognitive and/or behavioural impairment, and osteopenia in both sexes as well as primary or premature ovarian insufficiency (POI) in females (Hughes et al. 2009; Fridovich-Keil et al. 2011). POI is a spectrum disorder of ovarian insufficiency which may range from subfertility to primary amenorrhoea or demonstrate a fluctuating course of ovarian dysfunction. POI can be detected by measuring follicle-stimulating hormone (FSH), luteinising hormone (LH), oestradiol and anti-Mullerian hormone (AMH) in blood of females with Gal, but given the inactivity of the hypothalamic-pituitary-ovarian (HPO) axis in childhood, these levels may be not sensitive enough to identify ovarian dysfunction in a presymptomatic state.
Although it is well known that females with Gal have a high risk of hypergonadotropic hypooestrogenic ovarian dysfunction along with infertility, the underlying mechanisms for POI in galactosaemic patients remain unknown (Rubio-Gozalbo et al. 2010). They may comprise, for example, toxic effects of accumulated galactose and its metabolites, disturbances in glycoprotein and glycolipid synthesis, altered cell signalling pathways and dysregulated gene expression (Coman et al. 2010; Coss et al. 2012a). FSH levels are often found to be elevated in females with Gal but biologic activity of gonadotropins was described as essentially normal (Kaufman et al. 1981; Berry 2008). Along these lines, FSH isoform patterns did not differ between patients with Gal and postmenopausal controls (Gubbels et al. 2011a). Data on ovarian histology in galactosaemic females is scarce, but hypoplastic or streak gonads with decreased numbers of primordial follicles along with reduced or absent intermediate and Graafian follicles were found, similar to other genetic diseases which directly affect the ovary (Rubio-Gozalbo et al. 2010; Berry 2008). Hormone replacement therapy (HRT) with oestrogen and progesterone as appropriate along with clinical monitoring are reasonable interventions at the current time. However, spontaneous pregnancies may occur in women with Gal, and a predicting factor for the possibility to conceive is spontaneous menarche (Gubbels et al. 2008).
Males with Gal spontaneously reach puberty but onset of puberty may be delayed (Rubio-Gozalbo et al. 2010). Recently Gubbels and colleagues have reported a higher-than-predicted prevalence of cryptorchidism in Gal males with subtle decreases in testosterone, inhibin B, semen volume and sperm count which might indicate mild defects in Sertoli and Leydig cell function (Gubbels et al. 2011b).
Among the endocrine and metabolic key molecules which directly modulate the HPO axis is leptin. This is a 146 amino acid non-glycosylated protein which is secreted predominantly by adipocytes but which is also expressed in other tissues such as stomach, kidney and liver (Meissner et al. 2005). Leptin is involved in body weight regulation and provides information on body energy stores (Knerr et al. 2006). Leptin is involved in regulation of food intake and energy expenditure at the hypothalamic level as well as in reproductive maturation and fertility. It is capable of increasing oestrogen production through the stimulation of aromatase expression and activity in luteinized granulosa cells and adipocytes (Catalano et al. 2003). The leptin receptor is expressed in the brain and several other tissues such as liver, stomach, kidney and immune cells (Denver et al. 2011; Xu et al. 2012). Leptin acts on multiple brain regions including the brain stem, hypothalamus, hippocampus and ventral tegmental area by activating the cytokine type 1 leptin receptor which is coupled to the JAK2 signalling mechanism (Trinko et al. 2011). As we have reported dysregulation of multiple central cell signalling pathways in Gal (Coman et al. 2010), we proposed to study circulating leptin levels in children and adults with Gal.
Patients and Methods
Patients
Our study was approved by the Ethics Committee of Children’s University Hospital, Dublin. Informed consent was obtained prior to enrolment. We recruited 28 children and 22 adult Irish Gal patients, including 10 pairs of siblings, who were being treated at the National Centre for Inherited Metabolic Disorders, Dublin, and the Metabolic Unit at The Royal Belfast Hospital for Sick Children. Our paediatric cohort comprised of 10 girls (age 0.6–17.9 years, mean 7.7 years.) and 18 boys (age 0.5–16.7 years, mean 7.6 years). All children with Gal were diagnosed on newborn screening (day 5 of life) or selective screening (day 1) in the case of a positive family history for Gal and immediately commenced on a lactose-free diet. The adult study cohort comprised 10 females (age 19–37 years, mean 25.4 years) and 12 males (age 18–28 years, mean 22.4 years). The mean age at diagnosis in our adult patient group was 12 days (median 7 days, range day 1–day 45 of life). Mean age at menarche in female adults was 14.1 years. (range 12–16 years) and 15.3 for adolescent girls (range 13–17 years); only four female patients had had spontaneous puberty and menarche. At the age the blood sample was taken, all women except one and all but one adolescent girl were on HRT. Adequate intake of calcium and vitamin D was also recommended along with physical exercise. All patients were on a strict galactose-restricted diet for life. No female patient in this study group had given birth and no male patient had fathered a child at the time of the study.
In the entire Gal cohort, 46 patients (92%) were homozygous for the GALT mutation p.Q188R; two siblings were compound heterozygous for p.Q188R/p.R333W and p.Q188R/p.K285N, respectively; and two patients were not genotyped. Clinical data and serum samples were compiled during routine outpatient visits. Weight and height were measured, and BMI calculated as body weight (kg) divided by height (m) squared.
Leptin Assay
Serum leptin was measured using a human leptin immunoassay. A microtitre plate-based DELFIA assay was applied, and antibodies and standards were purchased from R&D Systems (R&D Systems Europe, Abingdon UK). The intra- and inter-assay CVs (%) were between 3.9 % and 7.1 %. The lower limit of detection was 0.1 ng/ml. Samples were analysed in duplicate.
Control Data
The control data for our paediatric cohort was obtained by recruiting apparently healthy age- and sex-matched control children and adolescents (age range 1–17.0 years). The control data for adults were obtained from 1,670 healthy young adults under 45 years of age and stratified according to gender and BMI. We obtained data from 340 men and 558 women with a BMI less than 25 and 456 men and 316 women with a BMI of 25–29.9 kg/m2. These volunteers have been recruited for ongoing population-based studies in the UK after gaining ethical approval in 2004. Exclusion criteria for our control subjects were chronic diseases such as obesity, diabetes or cancer, and details are published elsewhere (Kilpeläinen et al. 2011).
Statistical Analyses
Accounting for the logarithmic distribution of leptin levels, leptin standard deviation (SDS) according to gender, BMI and pubertal stage was calculated using a standard equation based on published normative data (Blum et al. 1997). In adults, a base-10 logarithm transformation of serum leptin concentrations was used to ensure normal distribution of data. Statistical analyses were performed using Prism 5 Software (GraphPad, La Jolla, CA, USA), including Pearson correlation, linear regression analysis, and Student’s t-test. A significance level of p < 0.05 was chosen for all comparisons.
Results
In our cohort, we found lower leptin levels and leptin-SDS in children with Gal than in controls (mean leptin-SDS = –0.71 for girls, –0.97 for boys compared with SDS = 0 for controls, p < 0.05 for each analysis, Figs. 1, 2). In general, girls had higher leptin levels than boys (4.4 ± 4.1 for Gal girls vs. 0.9 ± 0.5 for Gal boys, p < 0.01, and 8.1 ± 3.2 for control girls vs. 2.1 ± 1.4 for control boys, p < 0.05, Fig. 1).
Fig. 1.
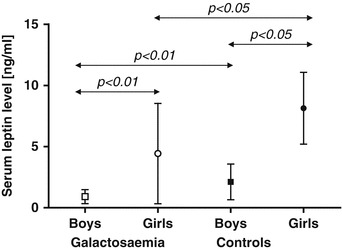
Serum leptin levels for children with Gal and age- and gender-matched controls are presented as mean values ± SD
Fig. 2.
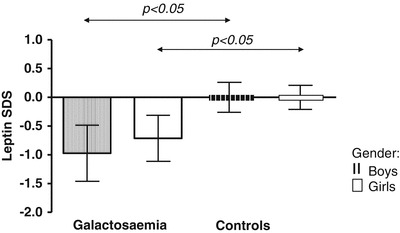
Leptin standard deviation scores (SDS) for gender and pubertal stage are given for children with Gal and age- and gender-matched controls as mean values ± SD
In an age-related analysis, serum leptin levels did not correlate with age in the entire paediatric Gal group (r = 0.0598, n.s.) or for both sexes (r = 0.1326, n.s., for girls with Gal, r = –0.0877, n.s., for boys with Gal).
In detail, we also found no significant correlation between leptin and age when data of prepubertal boys with Gal was tested separately to exclude effects of rising testosterone levels (r = –0.4690, n.s.). Conversely, leptin was found positively correlated to age in our paediatric control group (r = 0.6546, p < 0.01). In detail, leptin correlated positively with age in our female paediatric controls (r = 0.8188, p < 0.01). For our male paediatric controls, we found a correlation between leptin and age only in the subgroup of prepubertal boys (r = 0.6655, p < 0.05). We found higher leptin levels along with higher BMI in women compared to girls with Gal (p < 0.001), and lower leptin levels despite higher BMI in men compared to boys with Gal (p < 0.01). Essentially, serum leptin concentrations were within normal limits for women and men with Gal when adjusted for gender and BMI (Fig. 3). As expected, leptin and log-leptin concentrations in male adults were lower than in female adults in the entire cohort (p < 0.0001 for Gal patients, p < 0.00001 for control subjects, for both comparisons) and also in the subgroup of individuals with normal body weight (BMI 19–24.9 kg/m2, p < 0.001 for Gal patients and p < 0.00001 for controls, respectively, Fig. 3).
Fig. 3.
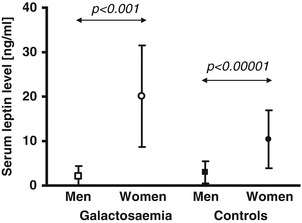
Illustrative data set (mean values ± SD) for serum leptin concentrations for men and women of normal weight with Gal as well as controls
A linear regression model was then fitted to determine the relation between log-leptin and BMI for each gender and cohort. We found a significant linear relationship between log-leptin and BMI in children with Gal (r 2 = 0.9942, p < 0.0001 for girls, r 2 = 0.9939, p < 0.0001 for boys and r 2 = 0.9888, p < 0.0001 for the entire group of children with Gal) and also for our control subjects (r 2 = 0.8791, p < 0.01 for girls, r 2 = 0.8287, p < 0.01 for boys, and r 2 = 0.8681, p < 0.001 for the entire paediatric control group, Fig. 4a). Among female adults with Gal, BMI was strongly associated with log-leptin values (r 2 = 0.4793, p < 0.05). This was also found in healthy controls of both sexes (r 2 = 0.4700, p <0.00001 for females, and r 2 = 0.3800, p < 0.00001 for males, Fig. 4b). However, in male adults with Gal and, therefore, in the entire group of Gal patients, this association between log-leptin and BMI was no longer detectable (r 2 = 0.1360, p = 0.2382, n.s. and r 2 = 0.0070, p = 0.7104, n.s., respectively, Fig. 4b).
Fig. 4.
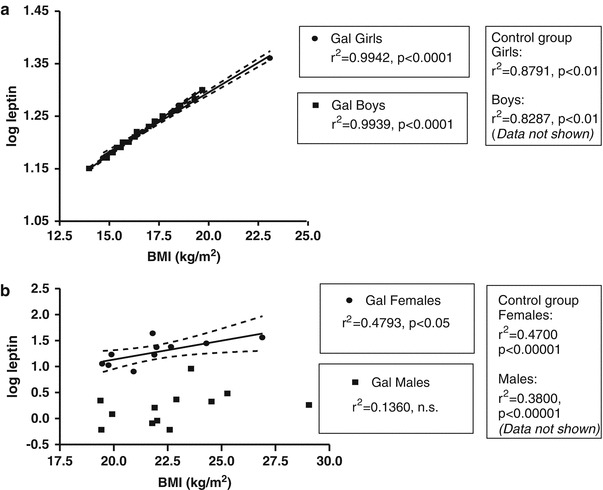
Linear regression analysis between log-leptin concentration and BMI in children (a) and adults (b) with Gal. Linear regression r 2 and p values for control subjects are also given but data is not shown in this diagram
Discussion
Our data demonstrate that children with Gal had lower serum leptin levels than controls, expressed as SDS for gender and pubertal stage. In an age-related analysis, serum leptin levels did not correlate with age in our paediatric Gal group as it did in our control group and peak leptin levels were lower in children with Gal than in controls. As expected, females had higher leptin levels than males in either group. Although leptin concentrations were essentially within normal limits for women and men with Gal when adjusted for gender and BMI, there was a lack of the physiological association between BMI and log-leptin in males and, therefore, also in the entire adult Gal cohort. In females with Gal on HRT, this strong association between BMI and log-leptin was preserved.
Fertility issues in female patients with Gal are complex and multifactorial. Possible mechanisms which may underlie POI in females with Gal may comprise (1) increased oocyte loss, e.g. due to direct toxic effects of galactose and its metabolites or due to hypoglycosylation; (2) altered dynamics of follicle development, e.g. due to dysregulated balance of apoptosis and survival pathways in the folliculogenesis; (3) perturbated gene expression pattern along with metabolic dyshomeostasis; and (4) altered follicle maturation due to reduced biopotency of nurturing factors such as FSH or others (Coman et al. 2010; Rubio-Gozalbo et al. 2010; Fridovich-Keil et al. 2011; Coss et al. 2012a). We have described earlier that, consistent with the international experience, 91.2% of females with Gal suffer from hypergonadotropic hypogonadism along with pubertal delay and fertility issues (Coss et al. 2012b).
Leptin is a key molecule for pubertal development in many mammalian species. It has been proposed that leptin may exercise immediate action in the function of the reproductive glands and leptin receptors have been identified in ovaries and testes (Goumenou et al. 2003). Essentially, leptin levels increase in early pubertal stages but to a higher extent in girls than in boys due to an increase in testosterone in the latter (Garcia-Mayor et al. 1997). These differences and age-dependence of leptin concentrations become especially evident after appropriate logarithmic transformation (Blum et al. 1997). Essentially, there is a significant correlation of leptin, or log-transformed values of leptin, and BMI in healthy males and females of a wide age range (5–77 years) (Isidori et al. 2000; Gómez et al. 2003; Falorni et al. 1997). Contrary to what is expected, there was a lack of a correlation between log-leptin and BMI in adult males with Gal and in our entire adult Gal cohort. Along these lines, one might explain the differences between our paediatric and adult Gal cohort, at least partly, by the chronic nature of this disorder. Ninety percent of women in our study have been treated with HRT at the time the sample was taken which might contribute to subsequent normal leptin levels in these individuals. Therefore, our findings do not rule out partial leptin deficiency in galactosaemic women who do not use HRT, or an individual susceptibility to low leptin levels that may act as a ‘second hit’. It has been shown, for example, that leptin serum levels declined in women who underwent bilateral ovariectomy for benign reasons and that treatment with oestrogen and progesterone prevented a decrease in leptin concentrations (Messinis et al. 2000).
The essential role of leptin in fertility is highlighted by the fact that mice lacking leptin (ob/ob mice) or leptin receptor (db/db mice) are typically infertile as they fail to enter puberty (Donato et al. 2011). Leptin deficiency in mice is associated with impaired folliculogenesis and increased follicular atresia (Hamm et al. 2004), findings reminiscent of those seen in ovarian tissues obtained from females with Gal. Female offspring of pregnant rats placed on a high galactose diet show altered germ cell migration, increased rate of follicular atresia, attenuated FSH bioactivity and a higher galactose-incorporation of serum proteins compared with control animals (Banerjee et al. 2012). Among the follicular survival factors are gonadotropins and integrin (Hussein 2005). Isolated galactose-treated rat granulosa cells demonstrated increased expression of p53, a protein which can facilitate apoptosis and cell cycle arrest. In principle, apoptosis takes place predominantly in oocytes during foetal life and in granulosa cells of secondary and antral follicles during adult life, and there are subsequent mechanisms which eventually lead to apoptosis in these cells (Hussein 2005). It is unclear at present whether females with Gal experience disturbed development or a progressive loss of ovarian tissue or, most likely, both, as the ovarian tissue in humans is vulnerable throughout life (Fridovich-Keil et al. 2011).
An association between galactose absorption and leptin has been demonstrated; leptin inhibits galactose uptake in the small intestine (Lostao et al. 1998) by acting on the Na(+)/glucose cotransporter (Barrenetxe et al. 2004). We have shown that glycoprotein processing defects persist in treated Gal patients which could potentially affect many cell signalling mechanisms to include leptin-mediated cell signalling (Coss et al. 2012a). Essentially, patients with Gal consume considerable amounts of soy products from birth onwards. These soy products contain isoflavones (phytooestrogens) which can function as oestrogen agonists or selective oestrogen receptor modulators. Infants fed with soy formula have higher concentrations of phytooestrogens in plasma and urine than breastfed babies; however, it cannot be concluded that these soy isoflavones are biologically active (Vandenplas et al. 2011). Along these lines, human studies did not detect any clinical significance of the amounts of nutritional phytooestrogens in infants fed with soy formula, but animal studies with phytooestrogens provide conflicting data, including an association between the intake of a related compound, coumestrol, and decreased fertility (Vandenplas et al. 2011). In a group of 40 children with Gal who had decreased body weight and height, body fat mass (FM) has been shown to be correlated with soy intake (Panis et al. 2005). In this group of patients, body composition was abnormal, possibly due to their metabolic defect, decreased levels of IGF-I and/or soy nutrition as speculated by the authors (Panis et al. 2005). However, daily isoflavone intake (up to 130 mg/day) had essentially no effect on leptin levels in healthy premenopausal and postmenopausal women (Phipps et al. 2001). Oestrogen increases leptin production, but it has been demonstrated that even high levels of isoflavone consumption do not alter leptin concentrations in females studied over 3 months (Phipps et al. 2001).
However, data on galactosaemic individuals are lacking and difficult to obtain given a long-term ‘lactose-free’ diet and high soy intake. It thus remains speculative whether a lower FM and higher intake of phytooestrogens may ultimately lead to pseudo-normal leptin levels.
The relative increase of leptin with BMI during puberty is comparable in both sexes but the absolute values at a given BMI are lower in boys in late puberty (Blum et al. 1997). However, leptin is positively correlated with BMI in healthy boys (El-Eshmawy et al. 2010). Early puberty might be a particular vulnerable period in individuals at risk of decreased reproductive capacity. Along these lines, reduced leptin levels were found in adolescent boys with constitutional delay of growth and puberty (El-Eshmawy et al. 2010). Additionally, decreased leptin and its impact on the reproductive axis may help to explain delayed pubertal development in adolescent boys with Gal and perhaps also cryptorchidism in some cases (Gubbels et al. 2011b).
Recent results from our group, as well as others, have demonstrated abnormal N-glycan processing and assembly in individuals with Gal (Coss et al. 2012a; Coman et al. 2010). This might affect G-protein-coupled receptors, including the insulin-like peptide relaxin receptors LGR7 and LGR8 which have roles in reproductive tissue remodelling. Hypothalamic KiSS-1 neurons, which express leptin receptors, execute a prominent role in the neuroendocrine and metabolic control of fertility, including regulation of the gonadotropic axis and its full activation at puberty (Tena-Sempere 2006). We speculate that hypoleptinaemia may, therefore, contribute to a delay in pubertal maturation and dysregulation of reproductive function in individuals with Gal at a neuroendocrine level from an early age. In addition to gonadal function loss, e.g. POI in women with Gal, hypoleptinaemia may be an aggravating factor in individuals with Gal at risk for reproductive dysfunction. Moreover, gonadal function loss may further decrease circulating leptin levels independently from age and BMI (Benetti-Pinto et al. 2010).
In summary, we present data which suggests that leptin dysregulation may play a role in delayed pubertal maturation and fertility issues from an early age in Gal patients. Further studies are now needed, including the application to animal models, to study the impact of altered leptin levels in Gal.
Acknowledgement
We would like to thank all our patients who volunteered for the study. We wish to thank our colleagues Dr. Ellen Crushell and Dr. Ahmad A. Monavari for their excellent collaboration. We gratefully acknowledge Dr. Jian’an Luan for his excellent statistical advice. Leptin assays were performed by the NIHR Cambridge Biomedical Research Centre, Core Biochemical Assay Laboratory. CHFFH, Children’s University Hospital and Shire Industries are thanked for financial assistance to perform these studies.
Abbreviations
- AMH
Anti-Mullerian hormone
- BMI
Body mass index
- FSH
Follicle-stimulating hormone
- Gal
(Classic) Galactosaemia
- GALT
Galactose-1-phosphate uridyltransferase
- HPO
Hypothalamic-pituitary-ovarian
- HRT
Hormone replacement therapy
- LH
Luteinising hormone
- n.s.
Not significant
- SD
Standard deviation
Synopsis
Leptin is an interesting key molecule for reproductive development and fertility and its dysregulation might play a role in individuals with Gal.
Footnotes
Competing interests: None declared.
References
- Banerjee S, Chakraborty P, Saha P, Bandyopadhyay SA, Banerjee S, Kabir SN. Ovotoxic effects of galactose involve attenuation of follicle-stimulating hormone bioactivity and up-regulation of granulosa cell p53 expression. PLoS One. 2012;7(2):e30709. doi: 10.1371/journal.pone.0030709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrenetxe J, Sainz N, Barber A, Lostao MP. Involvement of PKC and PKA in the inhibitory effect of leptin on intestinal galactose absorption. Biochem Biophys Res Commun. 2004;7(317):717–721. doi: 10.1016/j.bbrc.2004.03.106. [DOI] [PubMed] [Google Scholar]
- Berry GT. Galactosemia and amenorrhea in the adolescent. Ann N Y Acad Sci. 2008;1135:112–117. doi: 10.1196/annals.1429.038. [DOI] [PubMed] [Google Scholar]
- Benetti-Pinto CL, Castro N, Grassiotto Oda R, Garmes HM. Leptin and adiponectin blood levels in women with premature ovarian failure and age- and weight-matched women with normal menstrual cycles. Menopause. 2010;17:174–177. doi: 10.1097/gme.0b013e3181b00dad. [DOI] [PubMed] [Google Scholar]
- Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Müller J, Skakkebaek NE, Heiman ML, Birkett M, Attanasio AM, Kiess W, Rascher W. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab. 1997;82:2904–2910. doi: 10.1210/jc.82.9.2904. [DOI] [PubMed] [Google Scholar]
- Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, Andò S. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem. 2003;278:28668–28676. doi: 10.1074/jbc.M301695200. [DOI] [PubMed] [Google Scholar]
- Coman DJ, Murray DW, Byrne JC, Rudd PM, Bagaglia PM, Doran PD, Treacy EP. Galactosemia, a single gene disorder with epigenetic consequences. Pediatr Res. 2010;67:286–292. doi: 10.1203/PDR.0b013e3181cbd542. [DOI] [PubMed] [Google Scholar]
- Coss KP, Byrne JC, Coman DJ, Adamczyk B, Abrahams JL, Saldova R, Brown AY, Walsh O, Hendroff U, Carolan C, Rudd PM, Treacy EP. IgG N-glycans as potential biomarkers for determining galactose tolerance in classical Galactosaemia. Mol Genet Metab. 2012;105:212–220. doi: 10.1016/j.ymgme.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Coss KP, Doran PP, Owoeye C, Codd MB, Hamid N, Mayne PD, Crushell E, Knerr I, Monavari AA, Treacy EP (2012b) Classical Galactosaemia in Ireland: incidence, complications and outcomes of treatment. J Inherit Metab Dis. 2012 Jul 3. [Epub ahead of print] [DOI] [PubMed]
- Denver RJ, Bonnett RM, Boorse GC. Evolution of leptin structure and function. Neuroendocrinoloy. 2011;94:21–38. doi: 10.1159/000328435. [DOI] [PubMed] [Google Scholar]
- Donato J, Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S, Jr, Coppari R, Zigman JM, Elmquist JK, Elias CF. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Eshmawy MM, Abdel Aal IA, El Hawary AK. Association of ghrelin and leptin with reproductive hormones in constitutional delay of growth and puberty. Reprod Biol Endocrinol. 2010;8:153. doi: 10.1186/1477-7827-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falorni A, Bini V, Molinari D, Papi F, Celi F, Di Stefano G, Berioli MG, Bacosi ML, Contessa G. Leptin serum levels in normal weight and obese children and adolescents: relationship with age, sex, pubertal development, body mass index and insulin. Int J Obes Relat Metab Disord. 1997;21:881–890. doi: 10.1038/sj.ijo.0800485. [DOI] [PubMed] [Google Scholar]
- Fridovich-Keil JL, Gubbels CS, Spencer JB, Sanders RD, Land JA, Rubio-Gozalbo E. Ovarian function in girls and women with GALT-deficiency galactosemia. J Inherit Metab Dis. 2011;34:357–366. doi: 10.1007/s10545-010-9221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mayor RV, Andrade MA, Rios M, Lage M, Dieguez C, Casanueva FF. Serum leptin levels in normal children: relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. J Clin Endocrinol Metab. 1997;82:2849–2855. doi: 10.1210/jc.82.9.2849. [DOI] [PubMed] [Google Scholar]
- Gómez JM, Maravall FJ, Gómez N, Navarro MA, Casamitjana R, Soler J. Interactions between serum leptin, the insulin-like growth factor-I system, and sex, age, anthropometric and body composition variables in a healthy population randomly selected. Clin Endocrinol. 2003;58:213–219. doi: 10.1046/j.1365-2265.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Goumenou AG, Matalliotakis IM, Koumantakis GE, Panidis DK. The role of leptin in fertility. Eur J Obstet Gynecol Reprod Biol. 2003;106:118–124. doi: 10.1016/S0301-2115(02)00359-7. [DOI] [PubMed] [Google Scholar]
- Gubbels CS, Thomas CM, Wodzig WK, Olthaar AJ, Jaeken J, Sweep FC, Rubio-Gozalbo ME. FSH isoform pattern in classic galactosemia. J Inherit Metab Dis. 2011;34:387–390. doi: 10.1007/s10545-010-9180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels CS, Welt CR, Dumoulin J, Robben SG, Gordon CM, Dunselman G, Rubio-Gozalbo ME, Berry GT (2011b) The male reproductive system in classic Galactosemia: cryptorchidism and low semen volume. J Inherit Metab Dis 34 (Suppl 3). S177 [DOI] [PubMed]
- Gubbels CS, Land JA, Rubio-Gozalbo ME. Fertility and impact of pregnancies on the mother and child in classic galactosemia. Obstet Gynecol Surv. 2008;63:334–343. doi: 10.1097/OGX.0b013e31816ff6c5. [DOI] [PubMed] [Google Scholar]
- Hamm ML, Bhat GK, Thompson WE, Mann DR. Folliculogenesis is impaired and granulosa cell apoptosis is increased in leptin-deficient mice. Biol Reprod. 2004;71:66–72. doi: 10.1095/biolreprod.104.027292. [DOI] [PubMed] [Google Scholar]
- Hughes J, Ryan S, Lambert D, Geoghegan O, Clark A, Rogers Y, Hendroff U, Monavari A, Twomey E, Treacy EP. Outcomes of siblings with classical galactosemia. J Pediatr. 2009;154:721–726. doi: 10.1016/j.jpeds.2008.11.052. [DOI] [PubMed] [Google Scholar]
- Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update. 2005;11:162–177. doi: 10.1093/humupd/dmi001. [DOI] [PubMed] [Google Scholar]
- Isidori AM, Strollo F, Morè M, Caprio M, Aversa A, Moretti C, Frajese G, Riondino G, Fabbri A. Leptin and aging: correlation with endocrine changes in male and female healthy adult populations of different body weights. J Clin Endocrinol Metab. 2000;85:1954–1962. doi: 10.1210/jc.85.5.1954. [DOI] [PubMed] [Google Scholar]
- Kaufman FR, Kogut MD, Donnell GN, Goebelsmann U, March C, Koch R. Hypergonadotropic hypogonadism in female patients with galactosemia. N Engl J Med. 1981;304:994–998. doi: 10.1056/NEJM198104233041702. [DOI] [PubMed] [Google Scholar]
- Kilpeläinen TO, den Hoed M, Ong KK, Grøntved A, Brage S; Early Growth Genetics Consortium, Jameson K, Cooper C, Khaw KT, Ekelund U, Wareham NJ, Loos RJ (2011) Obesity-susceptibility loci have a limited influence on birth weight: a meta-analysis of up to 28,219 individuals. Am J Clin Nutr 93:851–860 [DOI] [PubMed]
- Knerr I, Herzog D, Rauh M, Rascher W, Horbach T. Leptin and ghrelin expression in adipose tissues and serum levels in gastric banding patients. Eur J Clin Invest. 2006;36:389–394. doi: 10.1111/j.1365-2362.2006.01642.x. [DOI] [PubMed] [Google Scholar]
- Lostao MP, Urdaneta E, Martínez-Ansó E, Barber A, Martínez JA. Presence and leptin effect on sugar absorption. FEBS Lett. 1998;423:302–306. doi: 10.1016/S0014-5793(98)00110-0. [DOI] [PubMed] [Google Scholar]
- Messinis IE, Kariotis I, Milingos S, Kollios G, Seferiadis K. Treatment of normal women with oestradiol plus progesterone prevents the decrease of leptin concentrations induced by ovariectomy. Hum Reprod. 2000;15:2383–2387. doi: 10.1093/humrep/15.11.2383. [DOI] [PubMed] [Google Scholar]
- Meissner U, Hänisch C, Ostreicher I, Knerr I, Hofbauer KH, Blum WF, Allabauer I, Rascher W, Dötsch J. Differential regulation of leptin synthesis in rats during short-term hypoxia and short-term carbon monoxide inhalation. Endocrinology. 2005;146:215–220. doi: 10.1210/en.2004-0782. [DOI] [PubMed] [Google Scholar]
- Panis B, Forget PP, Nieman FH, van Kroonenburgh MJ, Rubio-Gozalbo ME. Body composition in children with galactosaemia. J Inherit Metab Dis. 2005;28:931–937. doi: 10.1007/s10545-005-0189-4. [DOI] [PubMed] [Google Scholar]
- Phipps WR, Wangen KE, Duncan AM, Merz-Demlow BE, Xu X, Kurzer MS. Lack of effect of isoflavonic phytoestrogen intake on leptin concentrations in premenopausal and postmenopausal women. Fertil Steril. 2001;75:1059–1064. doi: 10.1016/S0015-0282(01)01777-0. [DOI] [PubMed] [Google Scholar]
- Rubio-Gozalbo ME, Gubbels CS, Bakker JA, Menheere PP, Wodzig WK, Land JA. Gonadal function in male and female patients with classic galactosemia. Hum Reprod Update. 2010;16:177–188. doi: 10.1093/humupd/dmp038. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M. KiSS-1 and reproduction: focus on its role in the metabolic regulation of fertility. Neuroendocrinology. 2006;83:275–281. doi: 10.1159/000095549. [DOI] [PubMed] [Google Scholar]
- Trinko R, Gan G, Gao XB, Sears RM, Guarnieri DJ, DiLeone RJ. Erk1/2 mediates leptin receptor signaling in the ventral tegmental area. PLoS One. 2011;6(11):e27180. doi: 10.1371/journal.pone.0027180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenplas Y, De Greef E, Devreker T, Hauser B. Soy infant formula: is it that bad? Acta Paediatr. 2011;100:162–166. doi: 10.1111/j.1651-2227.2010.02021.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, O'Brien WG, 3rd, Lee CC, Myers MG, Jr, Tong Q. Role of GABA release from leptin receptor-expressing neurons in body weight regulation. Endocrinology. 2012;153:2223–2233. doi: 10.1210/en.2011-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]


