Abstract
Classical citrullinemia type I (CTLN1) is an autosomal recessive disorder encoded by the ASS1 gene, which codes for argininosuccinate synthetase (ASS), the rate-limiting enzyme in the urea cycle. Previously, we identified the mutation p.G390R in patients with CTLN1 in the San Luis Province of Argentina. Here, we report the results of p.G390R analysis in a larger number of probands, relatives of involved families and additionally, a population study to identify carriers. Altogether, we analyzed 420 alleles, belonging to 12 probands, 26 relatives, and 172 healthy volunteers. All the probands were homozygous for the mutation, and 21 of 26 relatives were carriers. The occurrence of the disease in descendants of couples at risk was 57% showing a preferential transmission of the mutant allele compared to the normal allele. The carrier frequency in the general San Luis Province population was 4.1%, suggesting the incidence of CTLN1 to be 1:2,427, which is approximately 20 times higher than for the general population. This work suggests that there should be an increased awareness of preconceptual screening of CTNL1 among individuals/couples who are at risk in the San Luis Province in order to better inform them of their reproductive options.
Cascade/family and population molecular screening for carrier identification were performed in an Argentinean province with high incidence of CTLN1, a first step to preconceptional screening.
Introduction
Citrullinemia type I (CTLN1, MIM# 215700), first described by McMurray et al. (1962), is a metabolic genetic disease with autosomal recessive inheritance. CTLN1 causes an accumulation of citrulline in the body fluids, due to a deficiency of argininosuccinate synthetase (ASS, EC 6.3.4.5), which is the third enzyme of the urea cycle and catalyzes the formation of argininosuccinate from citrulline and aspartate. The classical variant is associated with a neonatal/infantile onset and typically leads to hyperammoniemia and early death if untreated (Brusilow and Horwich 2001).
The ASS enzyme is encoded by the ASS1 gene (RefSeq accession number NM_000050.4), which is located in chromosome 9q34.1. At least 87 mutations have been described in this gene. Specifically, the substitution p.G390R (c.1168G>A) is one of the most common mutation described in cases of CTLN1 in different ethnic groups. This mutation has been exclusively associated, in homozygous condition, with an early/severe phenotype (Engel et al. 2009).
Previously, we reported the first identification of several patients affected by CTLN1 in a limited geographic area of Argentina (San Luis Province). The molecular ASS1 gene analysis in probands and relatives of involved families (cascade screening) showed the same mutation: p.G390R (Laróvere et al. 2009). Here, we widen our previous study in relation to the number of probands and also describe results from cascade screening. In addition, we determined the prevalence of the p.G390R mutation testing carriers of CTLN1 in 172 unaffected individuals in San Luis Province. The aim of this work was to identify carriers in the risk area in order to informing individuals and couples at risk about their reproductive options; in addition, the data could be used to support improved surveillance and postnatal management.
Materials and Methods
Subjects
Patients
Twelve newborns (3 males, 9 females) of 7 apparently unrelated families, with the biochemical/molecular diagnosis of CTLN1, neonatal form. All the cases presented the severe phenotype including hyperammoniemia, metabolic coma, and early death. The molecular analysis of 8 of them has been reported previously (Laróvere et al. 2009).
Cascade Screening
Twenty-six close, unaffected relatives of the seven families.
Population Study
One hundred and seventy-two unrelated healthy volunteer individuals of Villa Mercedes City (estimated population: 96,700 individuals), San Luis Province. The studied region was selected from the hometown of the most affected children.
Genetic analysis was performed after informed consent was obtained. This study was authorized by the Research Committee of Hospital de Niños de Córdoba.
DNA Diagnosis
The p.G390R mutation was investigated by PCR and restriction enzyme assay. Exon 15 of ASS1 gene and the flanking intron–exon junction were amplified and digested with MspI according to the method described previously (Laróvere et al. 2009).
Results and Discussion
The analysis of the G390R mutation in the ASS1 gene revealed that the probands (n = 12; alleles = 24) were homozygous for the mutation. Cascade/family testing showed that 21 of 26 individuals were carriers (Fig. 1). The nine couples where both parents were carriers had a total of 21 births, 12 of them homozygous for G390R mutation, which leads to the occurrence of the disease in descendants to be 57%. This frequency doubles the percentage of expected affected pregnancies (25%) according to Mendel’s first law for an autosomal recessive disease. These results suggest the occurrence of preferential transmission of the mutant allele compared to the normal allele. Kleijer et al. (2006) suggested that this phenomenon could be explained by a protective role of ASS deficiency in mutant sperm cells against the possible detrimental or apoptotic effect of nitric oxide produced normally from arginine by nitric oxide synthase.
Fig. 1.
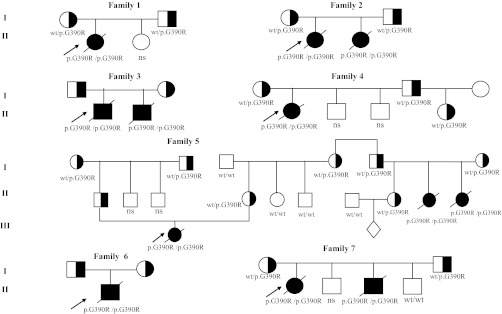
Genetic pedigree of the studied families with CTLN1. Probands are indicated by arrows; ns not studied subject; modified from Laróvere et al. (2009)
The results of the population study indicate that of the 172 individuals from Villa Mercedes City, 7 were heterozygous for the p.G390R mutation. Thus, the carrier frequency is approximately 1/25 inhabitants of Villa Mercedes City, which suggests a population incidence of CTLN1 to be 1 affected individual per 2,427 births. This figure compares to the worldwide incidence of CTLN1 of 1 in 57,000 births (Brusilow and Horwich 2001), which is approximately 20 times lower.
The Basis for a Preconception Screening
The analyzed community of the Argentinean Province of San Luis appears to be an appropriate subject for a genetic study, because it had allegedly been an “isolated” population; although sociological and anthropological data to reinforce this assumption have not been carried out yet.
This high apparent carrier frequency in our study group suggests that preconception carrier screening could be used to inform parents of reproductive outcomes and to allow better postnatal management. In this context, the work of Kaback and O’Brien (1973) is instructive as they undertook the first voluntary community-based attempt at mass screening of an Ashkenazi Jewish population for Tay–Sachs disease. Three decades later, the incidence of this disease had been reduced by at least 90% (Kaback 2000).
In addition to the above success in reducing incidence by a screening program, one of the recommendations of the Human Genome Variation Society (http://www.hgvs.org) encourages “…ethnicity and country specific mutation databases to deliver the most efficient health care” and so would improve geographic and ethnic-specific health care (Appelbe et al. 2007). Moreover, Godard et al. (2003) established that “carrier screening at antenatal clinics is easy to organize: the risk of being a carrier is of current interests and the partner already exists. …Of all the types of genetic screening, preconception carrier screening is the preferred way to go.”
Due to the high carrier frequency of this mutation in this population, we recommend preconception carrier screening for the common mutation G390R in ASS1 at least in San Luis Province. Hopefully, this intervention will decrease the incidence of CTLN1 in this high-risk population, as preconception carrier detection has done in other risk populations (Kaback 2000; ACOG 2009).
Acknowledgements
The authors gratefully acknowledge Prof. Dr. Antonio Blanco and Dr. Marshall Summar for the critical readings of the manuscript. We also thank the patients’ families and the volunteers from Villa Mercedes for their cooperation and the technical assistance of María Fernanda Santi. This study was supported partially by grants from SECYT (No. 214/10) and FONCYT (PICT 05, No. 5-34226) and Innovative Medicines Company, Argentina.
Footnotes
Competing interests: None declared
References
- ACOG Committee on Genetics ACOG Committee Opinion No. 442: preconception and prenatal carrier screening for genetic diseases in individuals of Eastern European Jewish descent. Obstet Gynecol. 2009;114:950–953. doi: 10.1097/AOG.0b013e3181bd12f4. [DOI] [PubMed] [Google Scholar]
- Appelbe W, Auerbach AD, Becker K, et al. Recommendations of the 2006 Human Variome Project meeting. Nat Genet. 2007;39(4):433–436. doi: 10.1038/ng2024. [DOI] [PubMed] [Google Scholar]
- Brusilow SW, Horwich AL. Urea cycle enzymes. In: Scriver C, Beaudet A, Valle D, Sly W, editors. Metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 1909–1963. [Google Scholar]
- Engel K, Höhne W, Häberle J. Mutations and polymorphisms in the human argininosuccinate synthetase (ASS1) gene. Hum Mut. 2009;30(3):300–307. doi: 10.1002/humu.20847. [DOI] [PubMed] [Google Scholar]
- Godard B, ten Kate L, Evers-Kiebooms G, Aymé S. Population genetic screening programmes: principles, techniques, practices, and policies. Eur J Hum Genet. 2003;11:S49–S87. doi: 10.1038/sj.ejhg.5201113. [DOI] [PubMed] [Google Scholar]
- Kaback M, O’Brien J. Tay–Sachs: prototype for prevention of genetic disease. In: McKusic V, Claiborne R, editors. Medical genetics. New York: HP Publishing; 1973. pp. 253–262. [Google Scholar]
- Kaback MM. Population-based genetics screening for reproductive counseling: the Tay–Sachs experience. Eur J Pers. 2000;159:S192–S195. doi: 10.1007/pl00014401. [DOI] [PubMed] [Google Scholar]
- Kleijer WJ, Garritsen VH, van der Sterre MLT, Berning C, Häberle J, Huijmans JGM. Prenatal diagnosis of citrullinemia and argininosuccinic aciduria: evidence for a transmission ratio distortion in citrullinemia. Prenat Diagn. 2006;26(3):242–247. doi: 10.1002/pd.1390. [DOI] [PubMed] [Google Scholar]
- Laróvere LE, Angaroni CJ, Antonozzi SL, Shimohama M, de Kremer RD. Citrullinemia type I, classical variant. Identification of ASS-p.G390R (c.1168G>A) mutation in families of a limited geographic area of Argentina: a possible population cluster. Clin Biochem. 2009;42(10/11):1166–1168. doi: 10.1016/j.clinbiochem.2009.03.024. [DOI] [PubMed] [Google Scholar]
- McMurray WC, Mohyuddin F, Rossiter RJ, et al. Citrullinuria, a new aminoaciduria associated with mental retardation. Lancet. 1962;1:138. doi: 10.1016/S0140-6736(62)91135-2. [DOI] [Google Scholar]


