Introduction
The aim of our study was to analyze the ultrasound characteristics of carotid plaques in an outpatient population and to determine their implications for planning the ultrasound follow-up.
Materials and methods: We studied 747 consecutive outpatients (397 [53%] of whom were women) who underwent color Doppler sonography of the carotid arteries. Most of the patients presented multiple cardiovascular risk factors or were being followed-up for carotid artery stenosis.
Results: Stenosis ranging from 1% to 69% was observed at the level of the right internal carotid arteries (ICA) in 419 (56.1%) of the 747 patients and in the left ICA in 408 of 747 (54.5%). One hundred twenty-four (29.5%) of the 419 RICA plaques and 77 (18.8%) of the 408 LICA plaques were classified as type 1 or type 2 according to the modified Gray-Weale classification.
Conclusions: Type 1 and type 2 plaques, which are referred to as “vulnerable plaques,” were found in 160 (21.4%) of the 747 patients we examined. These patients should be subjected to closer ultrasound follow-up, even if they have only moderate carotid artery stenosis.
Keywords: Color-Doppler ultrasound (CDUS), Carotid arteries (CA), Carotid plaques, Echolucent plaques, Intima-media thickness (IMT)
Sommario
Introduzione
L’obiettivo del nostro lavoro è stato quello di analizzare le caratteristiche ecografiche delle placche carotidee di una popolazione di pazienti ambulatoriali e proporre le indicazioni per la programmazione dei follow-up ecografici.
Materiali e metodi: Il campione era costituita da 747 pazienti consecutivi che hanno eseguito un esame eco-color Doppler dei tronchi sovraortici, 397 femmine (53%) e 350 maschi (47%). La popolazione era costituita da pazienti in prevalenza affetti da molteplici fattori di rischio cardiovascolare oppure pazienti in follow-up per stenosi carotidee.
Risultati: I dati più significativi relativi alle arterie carotidi interne (ICA) segnalavano che per l’ICA destra, n = 747, il 56,1% delle arterie (n = 419) presentava una stenosi compresa tra l′1% e il 69% mentre per ciò che riguarda l’ICA sinistra, n = 747, il 54,5% delle arterie (n = 408) segnalavano stenosi comprese tra l’1% e il 69%. Le placche carotidee sono state suddivise in base alla classificazione di Gray-Weale modificata e per ciò che riguarda le stenosi comprese tra l’1% e il 69%, per l’ ICA destra (n = 419), le placche di tipo 1 e tipo 2 sono risultate complessivamente n = 124 (29.5%), mentre per l’ICA sinistra (n = 408) le placche di tipo 1 e tipo 2 sono risultate n = 77 (18,8%).
Conclusioni: Le placche di tipo 1 e tipo 2 sono le cosiddette “placche vulnerabili”; nella nostra popolazione 160 pazienti, corrispondenti al 21,4% dei pazienti totali (n = 747), hanno presentato queste caratteristiche. Questi sono i pazienti che, anche se con stenosi moderata, dovrebbero essere privilegiati per la programmazione dei follow-up ecografici.
Introduction
The objective of this study was to analyze the ultrasonographic (US) characteristics of the carotid arteries in an outpatient population and propose indications for the planning of US follow-up based on data in the literature. The study focused on type 1 and type 2 carotid plaques (modified Gray-Weale [G-W] classification) [1,2] because they are ones most frequently involved in cardiovascular events (CVE) during short-to-intermediate-term follow-up.
Materials and methods
In 2010 (1 January – 31 December) color Doppler ultrasound examinations (CDUS) of the CA were performed on a total of 747 patients in the Clinical and Vascular Sonography outpatient clinic. All examinations were performed by the same operator using a General Electric Logiq E 9 scanner (General Electric Company, Milwaukee, Wisconsin, USA) equipped with a 9L linear multifrequency transducer. The B-mode settings were adjusted to optimize the quality of the gray-scale images, and the pulse repetition frequency (PRF) used with color Doppler flow imaging was adjusted according to the flow velocity.
A carotid artery plaque was defined as a localized protrusion of the vessel wall, which 1) extended into the lumen ≥ 1.5 mm, or 2) had a thickness exceeding the intima-media thickness (IMT) of the adjacent portion of the vessel wall by >50%. The characteristics of the plaques were described in accordance with the modified Gray-Weale classification [1,2], and plaque morphology was defined in terms of its echogenicity. Stenoses involving the internal carotid artery (ICA) were described in accordance with the Consensus Panel Gray-Scale and Doppler Ultrasound Criteria for Diagnosis reported by Grant et al. [3].
The patients were referred to our outpatient clinic because they had multiple cardiovascular risk factors or for follow-up of known carotid stenosis, previous cardiovascular events (myocardial infarction [MI]), or previous cerebrovascular events (stroke or transient ischemic attacks [TIAs]). The study population included 397 women (mean age: 70.8 years [SD 9.6]) and 350 men (mean age: 69.6 years [SD 9.8]). Two hundred sixty-four (35%) of the patients had type 2 diabetes mellitus (DM2), which had been diagnosed within the previous 10 years in 124 cases (47%) and more than 10 years earlier in the other 140 (53%). Five hundred sixteen (69%) patients had hypertension (256 women, 260 men), and 64% of the patients (n = 475) had dyslipidemia (mixed types in most cases). One hundred forty-nine (20%) of the patients had already experienced at least one episode of CVE (stroke, TIA, or MI).
Results
Well over half (69.4%: n = 518; 257 women, 261 men) of the 747 patients examined in this study were found to have some degree of ICA stenosis. No stenosis was observed in the remaining 30.6% (n = 229; 139 women, 90 men). Considered separately, the right and left ICAs (RICA and LICA, respectively) were found to be stenosis-free in 289 (38.7%) and 293 (39.2%) of the patients, respectively. As shown in Table 1, the RICAs and LICAs were divided into four groups based on the degree of stenosis they presented: stenosis < 50%, stenosis of 50–69%, stenosis ≥ 70%, and near-occlusion. We also recorded the number of complete carotid occlusions (Table 1). We excluded from our analysis the ICAs that had already been revascularized (via endarterectomy or stenting); those containing plaques with marked surface irregularity and features suggestive of ulceration; and those that were kinked or coiled (total number of patients excluded: 54). The sonographic characteristics of the plaques were described using the modified G-W classification [1,2]: type 1 – uniformly anechoic or hypoechoic (Fig. 1); type 2 – predominantly (>50%) hypoechoic (Fig. 2); type 3 – predominantly (>50%) hyperechoic (Fig. 3); type 4 – uniformly hyperechoic (Fig. 4); and type 5 – uniformly echogenic with posterior shadowing (the so-called calcified plaque) (Fig. 5). The distribution of modified G-W plaque types associated with RICA and LICA stenosis of 1%–69% is shown in Table 2.
Table 1.
Right and left internal carotid artery stenoses observed in the study population.
| Stenosis | RICAa (no. – %) | LICAb (no. – %) |
|---|---|---|
| <50% | 371 (49.7%) | 368 (49.2%) |
| 50–69% | 48 (6.4%) | 40 (5.0%) |
| ≥70% | 4 (0.5%) | 7 (1.0%) |
| Near-occlusion | 4 (0.5%) | 2 (0.2%) |
| Occlusion | 8 (1.0%) | 6 (0.8%) |
RICA: Right internal carotid artery.
LICA: Left internal carotid artery.
Figure 1.
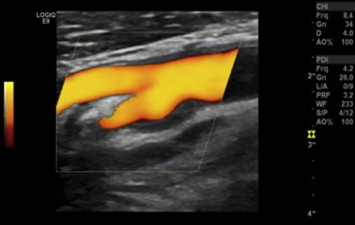
Gray-Weale classification: type 1 carotid plaque. Longitudinal US scan with power Doppler imaging shows a plaque at the level of the right carotid bifurcation that extends to the origin of the ICA.
Figure 2.
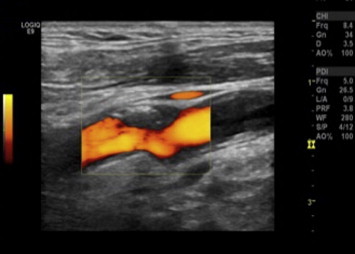
Gray-Weale classification: type 2 carotid plaque. Longitudinal US scan with power Doppler imaging shows a plaque in the left carotid at the level of the anterior wall of the bifurcation. It extends to the origin of the ICA.
Figure 3.
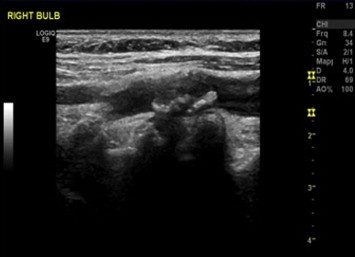
Gray-Weale classification: type 3 carotid plaque. Longitudinal B-mode US scan of the right carotid shows a plaque at the level of the bifurcation that extends to the origin of the ICA.
Figure 4.
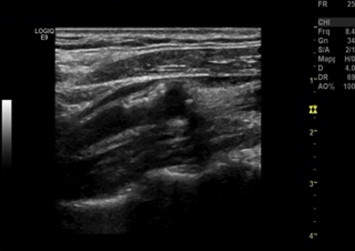
Gray-Weale classification: type 4 carotid plaque. Longitudinal B-mode scan shows a plaque in the anterior wall of the origin of the right ICA.
Figure 5.
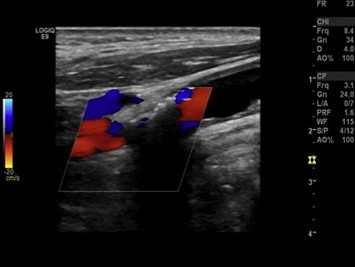
Gray-Weale classification: type 5 carotid plaque. Longitudinal US san with color Doppler imaging shows a plaque on the anterior wall of the origin of the right ICA.
Table 2.
Modified Gray-Weale plaque types associated with stenosis of 1%–69%.
| Mod. G-W classificationa | RICAb stenosis 1%–69% | LICAc stenosi 1%–69% |
|---|---|---|
| Type 1 | 46 (10.9%) | 28 (7.0%) |
| Type 2 | 78 (18.4%) | 49 (12.2%) |
| Type 3 | 145 (34.3%) | 138 (34.3%) |
| Type 4 | 99 (23.4%) | 127 (31.6%) |
| Type 5 | 55 (13.0%) | 60 (14.9%) |
| Total no. plaques | 423 | 402 |
Mod. G-W Classification: Modified Gray-Weale classification.
RICA: Right internal carotid artery.
LICA: Left internal carotid artery.
The 419 patients with RICA stenoses ranging from 1% to 69% included 238 (56.8%) with DM2, 332 (79.2%) with hypertension, and 326 (77.8%) who had already had a CVE.
A total of 201 ICAs presented stenosis of 1%–69% and type 1 or type 2 plaques (the main focus of our study). In 124 (29.3%) cases (males: 51.6%), the stenosis involved the RICA and in 19.2% (n = 77; males, 50.6%) the LICA was affected.
In terms of patients (rather than arteries), 160 (21.4%) of the 747 presented stenosis of 1%–69% and type 1 or 2 plaques. These 160 patients included 83 (51.4%) with unilateral RICA stenosis, 36 (21.4%) with unilateral LICA stenosis, and 41 (25.6%) with bilateral stenosis.
Of the 229 patients who presented no evidence of stenosis or plaques in either ICA, 139 (60.7%) had a plaque at the level of the right carotid bifurcation, and 64 (46%) of these plaques were type 1 or type 2. As for the left carotid bifurcation, 143 (62.4%) of the patients had plaques at this level, and 53 (37.1%) had type 1 or type 2 characteristics. When there were no plaques in the ICA, the plaque involvement we observed was limited almost exclusively to the carotid bifurcation, and other segments of the carotids were virtually plaque free.
Discussion
The early 1990s represented a turning point in the diagnosis and treatment of ICA stenosis. At this point, symptomatic patients with ICA stenosis ≥ 70% were identified as those who needed surgical treatment, and the degree of stenosis became the focal point of physicians' attention. The sonographic appearance of the plaques themselves assumed secondary importance. As a result, in most clinical settings, follow-up frequency for patients with atherosclerotic carotid artery disease is determined by the degrees of stenosis, and little attention is given to plaque features.
The first prospective study to demonstrate a link between stroke and the echogenicity of carotid plaques was conducted by Johnson et al. [4]. Three years of follow-up data on 297 symptomatic patients showed that TIA or strokes had occurred in 51% of those whose plaques had been hypo or anechoic at baseline, as opposed to only 4.4% whose plaques had appeared hyperechoic. One of the most interesting studies was a multicenter initiative organized by the European Carotid Plaque Study Group. Initially published in July 1995 and republished in September of 2011 [5,6] this study included 270 patients scheduled for carotid endarterectomy in 9 different facilities in Europe. The main findings were as follows: 1) carotid plaque echogenicity on B-mode imaging was inversely correlated with the “soft” material contained in the plaque (p = 0.005), and plaque hyperechogenicity was directly correlated with the presence of calcifications (p < 0.0001); 2) the most recent symptoms of CVD were reported by patients with the “softest” plaques. Mathiesen et al. [7] reported that in patients with type 1 or type 2 plaques but no carotid stenosis, the relative risk (RR) for cerebrovascular events was 13 (95% CI 4.5–37.4) versus only 3.7 (95% CI 0.7–18.2) in those with type 3 or type 4 plaques. Reiter et al. [8] followed 574 patients for a mean of 3.2 years to determine whether those with hypoechoic or anechoic plaques were at risk for major adverse cardiovascular events (MACEs). Carotid plaque echogenicity was assessed at baseline and every 6–9 months (mean 7.5) thereafter with dedicated software for gray-scale median (GSM) analysis. The presence of a hypoechoic plaque predicted a MACE with a hazard ratio (HR) di 1.71 (95% CI 1.09–2.66) when patients in the lowest quartile were compared with those in the highest quartile. On the whole, a total of 269 MACEs were observed in 177 (31%) of the patients studied (21 MIs, 48 coronary artery angioplasty procedures, 17 coronary artery by-pass procedures, 34 strokes, 69 angioplasty procedures involving peripheral vessels, 13 surgical procedures involving peripheral vessels, 5 amputations for critical limb ischemia, and 89 deaths).
Carotid artery plaque morphology and IMT are expressions of different biological aspects of atherosclerosis with different implications in terms of vascular disease [9]. The IMT is an important predictor of cardiovascular disease, but it displays closer correlation with left ventricular hypertrophy than with atherosclerotic coronary artery disease. Compared with the IMT, carotid plaque morphology and surface area are both better predictors of stroke, myocardial infarction, and cardiovascular death [10–12]. A systematic review of the literature on cardiovascular risk stratification of asymptomatic patients [13] examined numerous studies (n) that had assessed flow-mediated vessel dilatation (FMD, n = 2), carotid IMT (n = 12), carotid plaques (Plaque, n = 6), and coronary artery calcification (CAC, n = 9). Twenty-five studies were selected for the final analysis. The authors concluded that increases in the IMT or the presence of carotid plaques provide better estimates of the level of risk than CACs in patients with low to intermediate cardiovascular risks. In a recently published study [14], the carotid IMT plus the presence/absence of carotid plaques proved to be a better marker of CVD than either of these parameters alone. Naturally, the assessment of other markers (besides the classical indices regarding management and outcomes) in asymptomatic patients has cost-benefit implications that have to be considered.
In our study, 518 (69.4%) of the 747 patients (males 261[50.4%], females 257 [49.6%]) presented ICA stenosis, and no stenosis was found in 229 (30.6%) of the patients (males 90 [39.3%], females 139 [60.7%]). The 91 patients with ICA stenosis of >50% represented 12.2% of our cohort. This is a considerably higher proportion than that (3.6%) reported by Weerd et al. [15], but they examined a sample of the general population, whereas our patients all had CVD or were at risk for CVD. Data from the studies discussed above clearly show the importance of identifying patients with type 1 or type 2 carotid artery plaques, which are associated with an increased risk of CVD. In our study, 160 (21.4%) of the patients had ICA stenosis of 1–69% with type 1 or 2 plaques. In 83 (51.9%) of these individuals the stenosis involved the RICA, in 36 (22.5%) it was confined to the LICA, and in 41 (25.6%) it was bilateral. An interesting aspect that could have important implications for the follow-up of these patients is the progression rates of these lesions [16]. We know that the thickness of a carotid plaque increases 2.4 times faster than the IMT (0.0147 mm–0.0176 mm per year), but the latter increase is below the detection threshold of sonography (approximately 0.3 mm). For this reason, measurement of plaque thickness is more sensitive than IMT measurements [9].
Hirt [17] conducted a retrospective analysis of 1469 patients from the deferred endarterectomy arm of the Asymptomatic Carotid Surgery Trial. He found that the rate of progression of carotid stenosis should be considered a marker of the risk of future homolateral neurological events. But in all probability, the symptoms caused by atherosclerotic plaques are not based solely on hemodynamic mechanisms related to reduction of the carotid lumen and secondary diminution of perfusion: they are also related to thromboembolic mechanisms [18]. Consequently, plaques that are not associated with significant carotid stenosis or that appear to be small on sonography can also be sources of high risk, particularly if the plaque shows signs of inflammation (i.e., macrophage infiltrates), rupture of the fibrous cap, a high lipid content, or intraplaque hemorrhage, as shown by the prospective study of Takaya et al. [19], the first group to demonstrate the association between carotid plaques and cerebrovascular events using magnetic resonance imaging (MRI). Numerous reports indicate that medical therapy—in particular, aggressive statin therapy—plays a very important role in the progression of carotid stenosis and the stability of the plaques [20–22].
In light of these studies and their clinical correlations, the follow-up of patients with ICA stenosis should be based not only on the degree of stenosis (as it is in most clinical settings) but also on the sonographic morphology of the carotid plaques. For this reason, our study (Table 3) included cases of ICA stenosis of <50% and those with ICA stenosis ranging from 50% to 69%, as well as plaque type classified according to the modified G-W system. This analysis identified four groups of patients—class A, class B, class C, and class D—whose follow-ups (duration in months, initial follow-up visit, subsequent follow-up visit if the sonographic picture was stable) were planned with different temporal characteristics. In particular, for class A and class B patients the interval between the initial and subsequent follow-up visits was shorter than it was for patients in class C or D, who were considered at lower risk. In our opinion, this type of approach is more consistent with current clinical knowledge, and it should ensure more appropriate control of patients with high-risk plaques.
Table 3.
Follow-up schedules for four patient classes defined by sonographic plaque type (Gray-Weale) and percentage of ICA stenosis.
| G-W modifieda – Stenose ICAb % | F–U iniziale | F–U successivo |
|---|---|---|
| Class A: Tipo 1 – Tipo 2, stenosi < 50% | 6 months | 12 months |
| Class B: Tipo 1 – Tipo 2, stenosi 50%–69% | 3 months | 6 months |
| Class C: Tipo 3 – Tipo 4 – Tipo 5, stenosi < 50% | 12 months | 18 months |
| Class D: Tipo 3 – Tipo 4 – Tipo 5, stenosi 50%–69% | 6 months | 12 months |
Modified Gray-Weale classification.
ICA: internal carotid artery.
One of the limitations that must be considered in our study is related to differentiation of the five types of plaque envisioned by the G-W system, which involves subjective judgments, especially for distinguishing between types 2 and 3. The use of software for analyzing plaque echogenicity could substantially facilitate this task although it is poorly suited for routine clinical use. Identification of type 1 plaques, which are uniformly anechoic or hypoechoic, might also represent a limitation of this study. In the latter case, proper settings (B-mode signal, gray-scale level, color signal, PRF) should be diagnostically useful. A final limitation is related to the rigidly defined temporal characteristics of the follow-up. It could be overcome by adapting the follow-up to the overall cardiovascular risk status of the patient being examined.
Conclusions
Atherosclerotic disease of the carotid arteries plays a role in 10%–25% of all ischemic cerebrovascular clinical manifestations such as TIA and stroke. However, in a study of middle-aged subjects in the general population, this type of disease, measured in terms of the presence/absence of carotid plaques, their number, and the total area of plaque involvement by carotid plaques, has proved to be an independent predictor of long-term reductions in cognitive performance [23].
CDUS long ago replaced digitalized angiography for the diagnosis of carotid stenosis in part because it provides better definition of plaque morphology [20,24]. Currently available diagnostic imaging modalities include CDUS [5–10,20,24], contrast-enhanced CDUS [25,26], 3-dimensional sonography [27], computed tomography [20], MRI [28], dynamic contrast-enhanced MRI [20,29], ultrasmall superparamagnetic iron oxide-enhanced MRI [30], contrast-enhanced MRA [20,31], and positron emission tomography (PET)/CT with fluorodeoxyglucose [32] although for the time being some of these are used only in research settings.
The carotid plaques described as anechoic or “echolucent” or “predominantly echolucent”—those that correspond to types 1 and 2 in the G-W classification—are unstable plaques that can become symptomatic, regardless of whether or not they are associated with stenosis. These plaques require greater attention during carotid artery CDUS, especially in asymptomatic patients with cardiovascular risk factors. For this reason, it is time to re-think our strategies for managing these cases, shifting our emphasis from the vulnerable plaque to the vulnerable patient, not only in terms of treatment but also in the phases of sonographic diagnosis and follow-up [16,33].
Conflict of interests
The authors have no conflict of interest to disclose.
Footnotes
Award for the best communication presented at the 23rd SIUMB Congress.
Appendix A. Supplementary material
The following is the Supplementary material related to this article:
References
- 1.Gray-Weale A.C., Graham J.C., Burnett J.R., Byrne K., Lusby R.J. Carotid artery atheroma: comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg. 1988;29:676–681. [PubMed] [Google Scholar]
- 2.Geroulakos G., Ramaswami G., Nicolaides A., James K., Labropoulos N., Belcaro G. Characterization of symptomatic and asymptomatic carotid plaques using high resolution real-time ultrasonography. Br J Surg. 1993;80:1274–1277. doi: 10.1002/bjs.1800801016. [DOI] [PubMed] [Google Scholar]
- 3.Grant E.G., Benson C.B., Moneta G.L., Alexandrov A.V., Baker J.D., Bluth E.I. Carotid artery stenosis: gray-scale and Doppler US diagnosis-society of radiologists in ultrasound consensus conference. Radiology. 2003 Nov;229(2):340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 4.Johnson J.M., Kennelly M.M., Descare D., Morgan S., Sparrow A. Natural history of asymptomatic carotid plaque. Arch Surg. 1985;120:1010–1012. doi: 10.1001/archsurg.1985.01390330022004. [DOI] [PubMed] [Google Scholar]
- 5.European Carotid Plaque Study Group Reprinted article “ carotid artery plaque composition-relationship to clinical presentation and ultrasound B-mode imaging”. Eur J Vasc Endovasc Surg. 2011 Sep;42(Suppl. 1):S32–S38. doi: 10.1016/j.ejvs.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Golledge J. Commentary on: reprinted article “carotid artery plaque composition-relationship to clinical presentation and ultrasound B-mode imaging”. Eur J Vasc Endovasc Surg. 2011 Sep;42:S39–S40. doi: 10.1016/j.ejvs.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 7.Mathiesen E.B., BØnaa K.H., Joakimsen O. Echolucent plaque are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the TromsØ study. Circulation. 2001 May;103(17):2171–2175. doi: 10.1161/01.cir.103.17.2171. [DOI] [PubMed] [Google Scholar]
- 8.Reiter M., Effenberger I., Sabeti S., Mlekusch W., Schlager O., Dick P. Increasing carotid plaque echolucency is predictive of cardiovascular events in high-risk patients. Radiology. 2008 Sep;248(3):1050–1055. doi: 10.1148/radiol.2483071817. [DOI] [PubMed] [Google Scholar]
- 9.Spence J.D. The role of lipoprotein(a) in the formation of arterial plaques, stenoses and occlusions. Can J Cardiol. 2010 Mar;26(Suppl. A):37A–40A. doi: 10.1016/s0828-282x(10)71060-6. [DOI] [PubMed] [Google Scholar]
- 10.Prati P., Tosetto A., Casaroli M., Bignamini A., Canciani L., Bornstein N. Carotid plaque morphology improves stroke risk prediction: usefulness of a new ultrasonographic score. Cerebrovasc Dis. 2011;31:300–304. doi: 10.1159/000320852. [DOI] [PubMed] [Google Scholar]
- 11.Mathiesen E.B., Johnsen S.H., Wilsgaard T., BØnaa K.H., LØchen M.L., NjØlstad I. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the TromsØ study. Stroke. 2011 Apr;42(4):972–978. doi: 10.1161/STROKEAHA.110.589754. [DOI] [PubMed] [Google Scholar]
- 12.Inaba Y., Chen J.A., Bergmann S.R. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012 Jan;220(1):128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Peters S.A., den Ruijter H.M., Bots M.L., Moons K.G. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart. 2012 Feb;98(3):177–184. doi: 10.1136/heartjnl-2011-300747. [DOI] [PubMed] [Google Scholar]
- 14.Xie W., Liang L., Zhao L., Shi P., Yang Y., Xie G. Combination of carotid intima-media thickness and plaque for better predicting risk of ischaemic cardiovascular events. Heart. 2011 Aug;97(16):1326–1331. doi: 10.1136/hrt.2011.223032. [DOI] [PubMed] [Google Scholar]
- 15.de Weerd M., Greving J.P., Hedblad B., Lorenz M.W., Mathiesen E.B., O'Leary D.H. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke. 2010;41:1294–1297. doi: 10.1161/STROKEAHA.110.581058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naylor A.R. Time to rethink management strategies in asymptomatic carotid artery disease. Nat Rev Cardiol. 2011 Oct 11;9(2):116–124. doi: 10.1038/nrcardio.2011.151. [DOI] [PubMed] [Google Scholar]
- 17.Hirt L.S. Progression rate and ipsilateral neurological events in asymptomatic carotid stenosis. Stroke. 2011 Jul 28 doi: 10.1161/STROKEAHA.111.613711. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Moustafa R.R., Izquierdo-Garcia D., Jones P.S., Graves M.J., Fryer T.D., Gillard J.H. Watershed infarcts in transient ischemic attack/minor stroke with > or = 50% carotid stenosis: hemodynamic or embolic? Stroke. 2010 Jul;41(7):1410–1416. doi: 10.1161/STROKEAHA.110.580415. [DOI] [PubMed] [Google Scholar]
- 19.Takaya N., Yuan C., Chu B., Saam T., Underhill H., Caj J. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI-initial results. Stroke. 2006 Mar;37(3):818–823. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 20.Degnan A.J., Young V.E., Gillard J.H. Advances in noninvasive imaging for evaluating clinical risk and guiding therapy in carotid atherosclerosis. Expert Rev Cardiovasc Ther. 2012;10(1):37–53. doi: 10.1586/erc.11.168. [DOI] [PubMed] [Google Scholar]
- 21.Spence J.D., Coates V., Li H., Tamayo A., Muňoz C., Hackam D.G. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol. 2010 Feb;67(2):180–186. doi: 10.1001/archneurol.2009.289. [DOI] [PubMed] [Google Scholar]
- 22.Abbott A.L. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke. 2009 Oct;40(10):e573–e583. doi: 10.1161/STROKEAHA.109.556068. [DOI] [PubMed] [Google Scholar]
- 23.Arntzen K.A., Schirmer H., Johnsen S.H., Wilsgaard T., Mathiesen E.B. Carotid atherosclerosis predicts lower cognitive test results: a 7 year follow-up study of 4,371 stroke-free subjects – the TromsØ study. Cerebrovasc Dis. 2012;33:159–165. doi: 10.1159/000334182. [DOI] [PubMed] [Google Scholar]
- 24.Vancraeynest D., Pasquet A., Roelants V., Gerber B.L., Vanoverschelde J.L. Imaging the vulnerable plaque. J Am Coll Cardiol. 2011;57:1961–1979. doi: 10.1016/j.jacc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Faggioli G.L., Pini R., Mauro R., Pasquinelli G., Fittipaldi S., Freyrie A. Identification of carotid “vulnerable plaque” by contrast-enhanced ultrasonography: correlation with plaque histology, symptoms and cerebral computed tomography. Eur J Vasc Endovasc Surg. 2011;41:238–248. doi: 10.1016/j.ejvs.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Partovi S., Loebe M., Aschwanden M., Baldi T., Jäger K.A., Feinstein S.B. Contrast-enhanced ultrasound for assessing carotid atherosclerotic plaque lesions. AJR Am J Roentgenol. 2012;198:W13–W19. doi: 10.2214/AJR.11.7312. [DOI] [PubMed] [Google Scholar]
- 27.Makris G.C., Lavida A., Griffin M., Geroulakos G., Nicolaides A.N. Three-dimensional ultrasound imaging for the evaluation of carotid atherosclerosis. Atherosclerosis. 2011 Dec;219(2):377–383. doi: 10.1016/j.atherosclerosis.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Underhill H.R., Yuan C. Carotid MRI: a tool for monitoring individual response to cardiovascular therapy? Expert Rev Cardiovasc Ther. 2011 Jan;9(1):63–80. doi: 10.1586/erc.10.172. [DOI] [PubMed] [Google Scholar]
- 29.Dong L., Kerwin W.S., Chen H., Chu B., Underhill H.R., Neradilek M.B. Carotid artery atherosclerosis: effect of intensive lipid therapy on the vasa vasorum-evaluation by using dynamic contrast-enhanced MR imaging. Radiology. 2011 Jul;260(1):224–231. doi: 10.1148/radiol.11101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang T.Y., Muller K.H., Graves M.J., Li Z.Y., Walsh S.R., Young V. Iron oxide particles for atheroma imaging. Arterioscler Thromb Vasc Biol. 2009 Jul;29(7):1001–1008. doi: 10.1161/ATVBAHA.108.165514. [DOI] [PubMed] [Google Scholar]
- 31.Papini G.D., Di Leo G., Tritella S., Nano G., Cotticelli B., Clemente C. Evaluation of inflammatory status of atherosclerotic carotid plaque before thromboendarterectomy using delayed contrast-enhanced subtracted images after magnetic resonance angiography. Eur J Radiol. 2011 Dec;80(3):e373–e380. doi: 10.1016/j.ejrad.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 32.Kwee R.M., Truijman M.T., Mess W.H., Teule G.J., ter Berg J.W., Franke C.L. Potential of integrated [F18] fluorodeoxyglucose positron-emission tomography/CT in identifying vulnerable carotid plaques. AJNR Am J Neuroradiol. 2011 May;32(5):950–954. doi: 10.3174/ajnr.A2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.2011ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Writing committee members: Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. Circulation. 2011;124:000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


