Abstract
The lack of direct bonding between the surface of an implant and the mineralized bony tissue is among the main causes of aseptic loosening in titanium-based implants. Surface etching and ceramic coatings have led to improved osteointegration, but their clinical performance is still limited either by partial bonding or by coating delamination. In this work, a solid-phase synthesis method has been optimized to produce poly(ε-lysine) dendrons, the outermost branching generation of which is functionalized by phosphoserine (PS), a known catalyst of the biomineralization process. The dendrons were deposited onto etched titanium oxide surfaces as a near-to-monolayer film able to induce the formation of a homogeneous calcium phosphate phase in a simulated body fluid over 3 days. The dendron films also stimulated MG63 and SAOS-2 osteoblast-like cells to proliferate at a rate significantly higher than etched titanium, with SAOS-2 also showing a higher degree of differentiation over 14 days. PS-tethered dendron films were not affected by various sterilization methods and UV treatment appeared to improve the cell substrate potential of these films, thus suggesting their potential as a surface functionalization method for bone implants.
Keywords: biomineralization, dendrimer, surface modification, peptide, osteoblast
1. Introduction
The clinical performance of most biomedical implants is linked to the ability of their surface to integrate with the surrounding tissue. Tissue integration has been pursued by materials scientists through surface modification techniques. In the case of implants for dental and orthopaedic applications, metal surfaces have been treated by etching techniques or by coating with ceramic or polymeric materials. These treatments aim at enabling the implant surface to promote the adhesion, proliferation and differentiation of the relevant tissue cell types and their ability to produce a new extracellular matrix [1]. Indeed, in the case of bone implants, integration is also dependent on the ability of the surface to establish a covalent bonding with the mineralized extracellular matrix of the repairing tissue [2]. For example, etching and other types of surface treatments can provide the implant with a nanotopography, improving cell adhesion, and with functional groups catalysing biomineralization [3,4]. Although these treatments have led to a significant improvement in osteointegration, their clinical performance is still limited by the implant destabilization caused by either partial metal/bone bonding or coating delamination [4,5].
As an alternative, methods have been developed to functionalize the surface of biomedical implants with molecular films able to expose specific functional groups, encouraging cell adhesion and the deposition of a mineral phase [5–7].
Phosphoserine (PS) is considered as the most effective functional group in inducing biomineralization in living tissues [8]. When integrated in the structure of proteins and cell membrane phospholipids (i.e. phosphatidylserine), this phosphated amino acid is able to catalyse the formation of apatite crystals. The key role played by the phosphatidylserine-bearing matrix vesicles in bone formation is a typical example of the PS biomineralization potential. Recently, phosphatidylserine-based coatings that are able to induce implant surface mineralization in vitro and osteointegration in vivo have been developed [9–11]. Although effective in inducing osteointegration, the deposition of these phospholipid-based coatings onto biomaterial surfaces is not easy to control, thus leading to relatively thick and unstable, soft coatings [11].
Dendrimers are highly ordered three-dimensional, hyperbranched polymers forming nanostructures with tuneable physico-chemical properties [12]. Dendrimers can be obtained from different types of monomeric molecules that share the ability to develop into branching macromolecules. Dendrimers have been produced from synthetic molecules (e.g. polyamido amine) and from amino acids (e.g. polylysine) and carbohydrates [12–14]. There are two main methods to synthesize dendrimers [12,15]: (i) divergent synthesis where a core molecule with multiple reactive sites is used to form chemical bonding with a reactant and where the formed complex is later reacted with a molecule capable of generating another branching point and (ii) convergent synthesis where fragments of dendrimers are added to the core molecules and thus assembled. When the synthesis is performed in liquid phase, although the shape and symmetry of the dendrimer depends on the physico-chemical properties of the molecules used for its synthesis, the polymer branching generally leads to a spherical structure [16]. Conversely, when the synthesis is performed in solid phase, the branching polymer develops a dome-like (semi sphere) or tree-like structure, the dendron. By both methods, it is possible to obtain dendrimers (or dendrons) with several branching levels (generations, Gn). It has been reported that dendrimers of up to 10 branching generations can be synthesized [17]. However, molecular imperfections caused by steric hindrance during the synthesis are likely to occur with macromolecules exceeding four generations of branching. For this reason, in the present work, G3 PS-tethered dendrons were chosen to increase PS exposure and nano-roughness without incurring any molecular defects.
From a biotechnological viewpoint, both dendrimers and dendrons offer a unique opportunity to expose both functionalities favouring bio-interactions and a nanostructure controlling nanotopography. Indeed, dendrons have been designed to increase the affinity of specific bioligands to cell receptors by functionalizing the outermost branching of the dendrimer with the targeted bioligand [18].
In this work, a solid-phase synthesis method is used to generate poly(ε-lysine) (PL) dendrons, the last branching generation of which (G3) is functionalized by PS. This new class of dendrons (PL-PS) was used to deposit a near-to-monolayer film on titanium-based substrates used in the manufacture of dental and orthopaedic implants. The optimization of the surface functionalization as well as its ability to promote surface biomineralization and cell adhesion and proliferation was evaluated in comparison with titanium surfaces etched by conventional methods.
2. Material and methods
2.1. Poly(ε-lysine)–phosphoserine dendron synthesis and characterization
All reagents used were of analytical grade. PL-PS dendron assembly was performed by means of a solid-phase synthesis on commercial TentaGel NH2 resin (0.5 g; Iris Biotech, Germany). A Rink amide linker (Iris Biotech) was attached to the resin to allow the later cleavage of the synthesized dendron. PL-PS dendron synthesis was initiated by grafting a core amino acid (e.g. glycine) to the linker. Three branching generations were then obtained by sequentially adding Fmoc-Lys(Fmoc)-OH molecules and by terminating the synthesis with Fmoc-Ser(PO(OBzl)OH)-OH. The coupling agent O-(1H-6-chlorobenzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate was used in each synthetic step to activate the amino acid carboxylic group, while exposure of the amino group was obtained by cleavage of the Fmoc protecting group with 20% (v/v) piperidine in dimethylformamide. After deprotection of the PS group, PL-PS dendrons were cleaved from the resin and isolated by a standard preparative high-performance liquid chromatography (HPLC) procedure. The PL-PS dendron peak fraction was collected and characterized by standard analytical HPLC and micrOTOF mass spectroscopy (MS; Bruker, UK). The final product was freeze-dried and its yield of reaction assessed by a gravimetric method. To assess the stability of the dendrons after sterilization procedures, MS was also performed on freeze-dried PL-PS dendrons previously sterilized by either UV or gamma rays according to regulatory standards. The synthesis reproducibility was assessed by the preparation of three scaled-up batches (over 60 mg per batch).
2.2. Metal implant functionalization
Freeze-dried PL-PS powder was re-suspended in ethanol/water (75 : 25 v/v) solutions at 2.3 mg ml−1. Medical grade 5 titanium alloy (Ti6Al4V, code 6421) discs were supplied by Eurocoating and were etched in 5 M NaOH for 24 h at 60°C in a water bath with shaking at 100 r.p.m. The etched discs underwent either dip coating or several steps of spray coating with the G3 PL-PS solutions. The optimized spray-coating procedure was performed by a commercial spray system (Fisher Scientific, UK) at a distance of 15 cm from the target. Spray pulses of 1 s were applied. The discs were washed three times in deionized water and subsequently dehydrated at room temperature under sterile conditions. Finally, the discs were sterilized by either UV or gamma irradiation following standard industrial procedures. Coating stability was also evaluated by repeated washing steps in either distilled water or a surfactant solution (7X-PF; MP Biomedicals LLC, UK) under sonication for 30 min. The release of PL-PS dendrons in solution was tested by the Bradford protein assay (Biorad, UK). Six discs per coating procedure were prepared to assess the reproducibility of the various methods. The surface morphology of the coatings and their stability during washing procedures were assessed by scanning electron microscopy (SEM) at 5 keV at different magnifications and compared with the surface morphology of the NaOH-etched discs.
2.3. Assessment of the biomineralization potential
The coating mineralization potential was tested by incubation in Kokubo's simulated body fluid (SBF), at 37°C for 72 h [19]. Ti6Al4V surfaces before and after PL-PS dendron functionalization as well as after their incubation in SBF were thoroughly washed in deionized water, freeze-dried, mounted on SEM stubs and coated with palladium by means of a standardized procedure. The samples were analysed by SEM at 5 keV at different magnifications and their elemental composition assessed by electron diffraction by elemental dispersive X-ray (EDX) analysis. Alternatively, uncoated and coated disc surfaces before and after incubation in SBF were stained by alizarin red according to an established procedure and the samples visually inspected for the development of red staining.
2.4. Assessment of the cell substrate properties
The ability of the PL-PS films to support osteoblast adhesion and proliferation was tested on two cell lines, the osteosarcoma MG-63 and the SAOS-2 osteoblast-like cells. Cell adhesion tests were performed by incubating MG63 cells at a density of 3 × 103 cells/disc in 10% (v/v) foetal calf serum-enriched Dulbecco's modified Eagle medium. The cells were allowed to adhere for 3 h, at 37°C, under a 5 per cent CO2/95 per cent air flow, in static conditions. After three washes in phosphate-buffered saline, pH 7.2, cell morphology was studied by staining of the samples with 10% (w/v) rhodamine–phalloidin followed by epi-fluorescence, using an Olympus microscope equipped with a Nikon camera. The experiments were performed in triplicate on both uncoated (NaOH etched) and PL-PS-coated discs.
MG-63 cells were also used for a preliminary proliferation study on UV-sterilized PL-PS-coated discs. The cells were seeded at 5 × 103 cells/disc and proliferation stopped at 24, 48 and 72 h. Samples were washed and rapidly stained with Hoechst–propidium iodide and their number counted by epi-fluorescence microscopy. Later, a complete set of cell proliferation experiments was repeated on gamma-irradiated uncoated and coated surfaces by using SAOS-2 cells at the same cell density in McCoy's 5A medium. As a comparison with the previous experiments, this analysis was also performed on PL-PS-functionalized surfaces that were sterilized by either UV or gamma rays. All the experiments were performed in triplicate for each type of surface treatment and readings were taken at 30× magnification from three different fields on each disc. Cell proliferation was expressed as the mean±s.d. of the number of viable cells/field.
SAOS-2 osteoblasts were also used to assess the ability of the substrates to support cell differentiation. The cells were seeded at 5 × 105 cells/disc and allowed to proliferate for 7, 14 and 21 days. After proliferation, the samples underwent a lysis step by repeated freeze–thawing. The lysis supernatants were separated and frozen at –70°C until tested for alkaline phosphatase (ALP) activity and total protein content. Both ALP activity and total protein content were established by standardized methods [17]. Data were obtained in duplicate from each sample. Six discs per type were analysed for each time point. Data were expressed as the mean±s.d. of the ALP-specific activity (arb. unit/mg protein).
All data were statistically analysed by ANOVA (Tukey's test) using Minitab software (v. 15).
3. Results
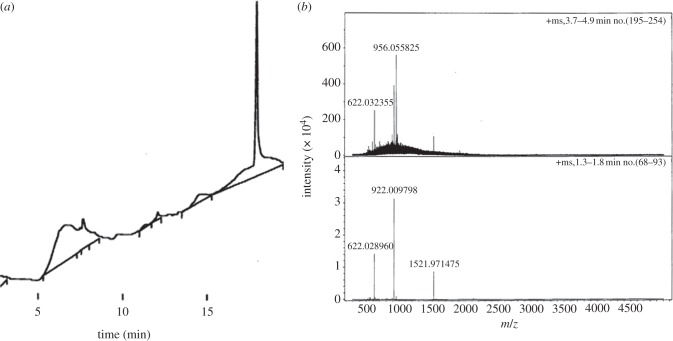
HPLC and MS showed the successful synthesis of G3 PL-PS dendrons, confirming the theoretical molecular weight of 4611 (figure 1a,b). The peaks shown in figure 1b represent the fragments (M – CO2H)3+ at 1521.9 m/z, (G3PL – CHN)2+ at 956 m/z, M5+ at 922 m/z and (G3PL + 7PS)5+ at 622 m/z. The fragments at 1521.9 and 922 m/z represent the complete synthesis of the dendron (G3 PL-PS, which is represented as M). Only one peak was observed after analytical HPLC and the MS spectra of the PL-PS dendrons after their sterilization by either UV or gamma-irradiation showed no detectable mass change (data not shown). These data combined with the gravimetric analysis showed that PL-PS dendrons could be synthesized at a scale higher than 60 mg per batch, with a degree of purity higher than 95 per cent, as determined by HPLC (figure 1a).
Figure 1.
Physico-chemical characterization of G3 PL-PS dendrons. (a) HPLC and (b) MS. Fragments shown represent (M – CO2H)3+ at 1521.9 m/z, (G3PL – CHN)2+ at 956 m/z, M5+ at 922 m/z and (G3PL+7PS)5+ at 622 m/z.
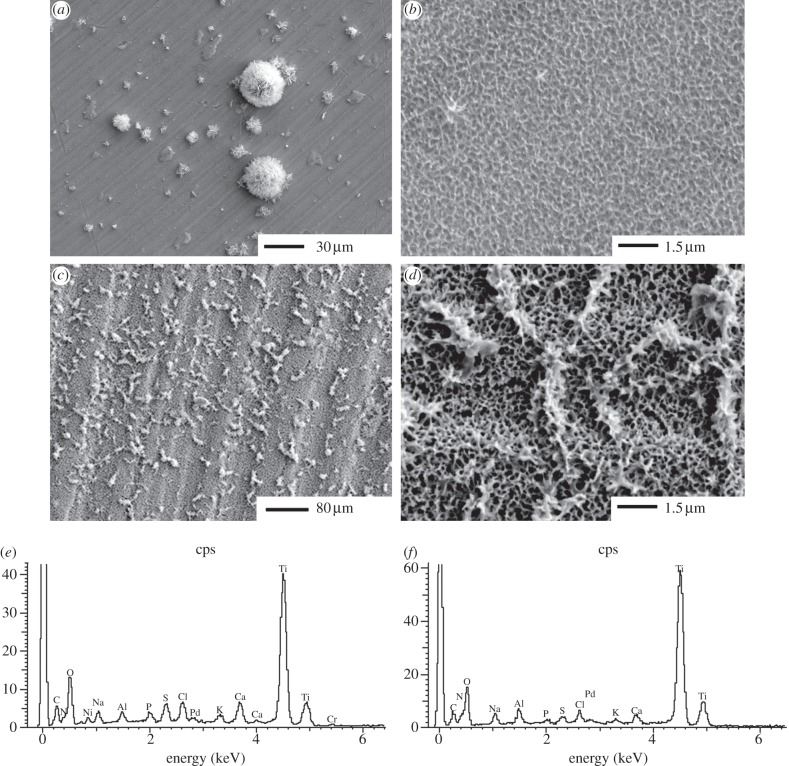
Dip coating in PL-PS solution under different conditions resulted in films of different thickness with a relatively low degree of reproducibility (data not shown). Conversely, the optimized spray-coating procedure, consisting of three spraying steps at a distance of 15 cm for 1 s, led to the reproducible deposition of a thin PL-PS film (figure 2). When compared with the control etched surfaces (figure 2a), spray coating of PL-PS on Ti6Al4V discs showed a nano-structured surface with a scale of texturization that was relatively finer than the etched materials (figure 2b). The presence of the coating after extensive surface washing with distilled water suggested a relatively strong binding of PL-PS dendrons onto Ti6Al4V. Indeed, only a relatively prolonged washing regime in detergent led to release of dendrons in the supernatants.
Figure 2.
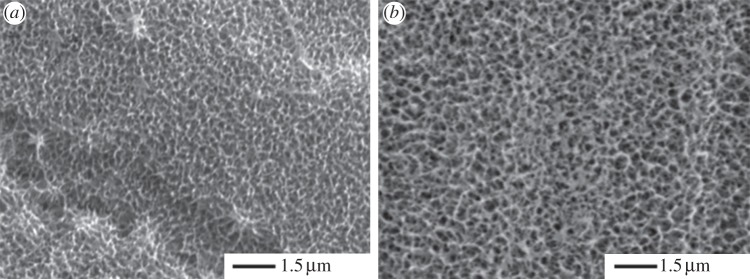
Scanning electron microscopy of (a) unmodified etched TiO2 surfaces and (b) G3 PL-PS-functionalized TiO2 surfaces.
Incubation of Ti6Al4V surfaces in SBF was detectable mineralization after 72 h (figure 3a,b), while PL-PS-coated Ti6Al4V induced the deposition of a calcium/phosphate-based mineral phase homogeneously encased in the dendrimeric film and larger crystal formations emerging from the film mesh (figure 3c,d). EDX confirmed the presence of calcium and phosphate on the functionalized surfaces (figure 3e,f). The two ions were positively identified in the SBF-treated PL-PS films. However, the thin nature of the film was close to the EDX detection limit, thus producing only relatively small signals. Alizarin red staining showed no detectable biomineralization on the uncoated discs after 72 h, but a positive staining was consistently observed in the case of the PL-PS film. The irradiation of the film with either UV or gamma rays did not affect its biomineralization potential (data not shown).
Figure 3.
Surface analysis of TiO2 surfaces. SEM of (a and b) unmodified etched TiO2 surfaces, (c and d) PL-PS-functionalized TiO2 surfaces after 72 h incubation in SBF. Electron diffraction by X-ray analysis of nucleated sites (e) on unmodified etched surface and (f) PL-PS-functionalized TiO2 surfaces.
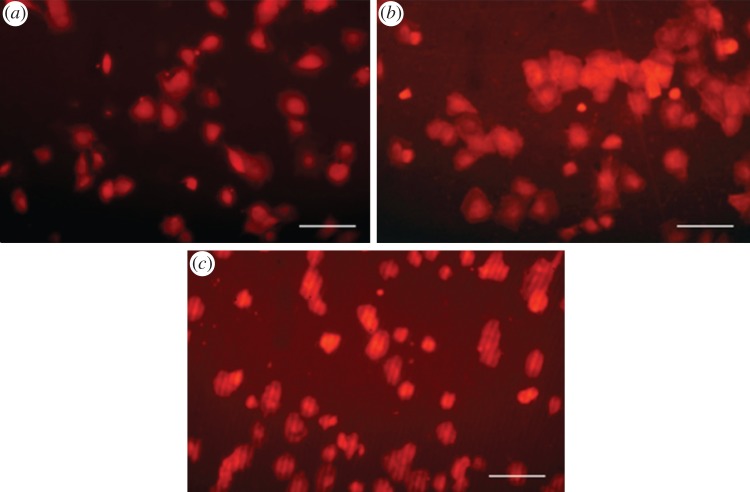
The cell adhesion study with a short incubation time clearly showed the ability of the PL-PS film to support the spreading of osteoblast-like cells (figure 4a–c). Although undergoing satisfactory spreading, cells adhering on control etched titanium surfaces tended to form clusters rather than consistently colonizing the implant surface (figure 4a). When adhering on PL-PS-coated discs, the cell spreading led to an organization of the cytoskeleton that followed the surface texture profile (figure 4b). The cells were also homogeneously distributed across the disc surface and no significant sign of clustering was observed. The degree of spreading and distribution across the surface was similar to that observed in the case of the tissue culture plate samples (figure 4c).
Figure 4.
MG-63 osteoblast-like cell adhesion after 3 h incubation on (a) tissue culture plastic, (b) unmodified etched TiO2 and (c) PL-PS-functionalized TiO2. Size bar represents 100 µm.
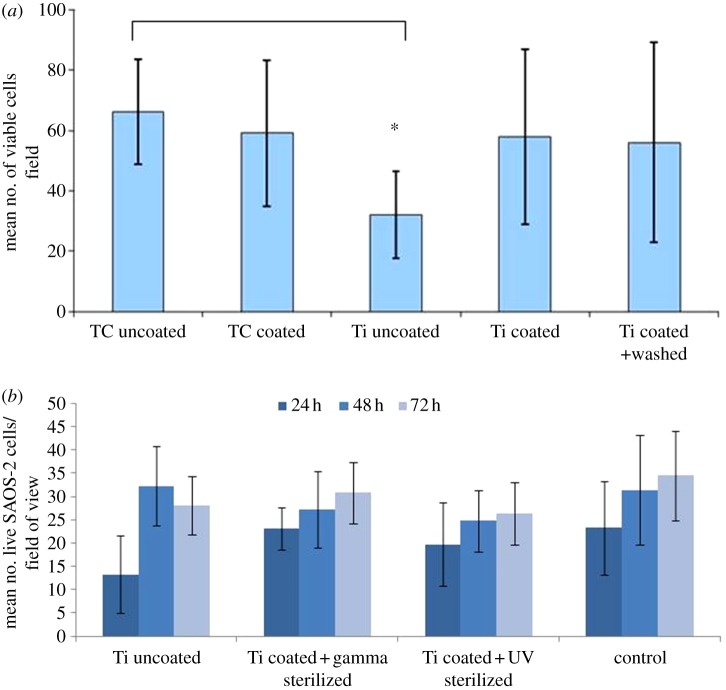
Cell proliferation with both MG-63 and SAOS-2 osteoblast-like cells clearly showed that the presence of the PS-PL films on the titanium surface significantly increased the ability of the metal disc to accelerate cell proliferation (figure 5a,b). In the case of both the MG-63 and the SAOS-2 cells, higher numbers of viable cells were observed at 24 h, while SAOS-2 cells adhering on gamma-irradiated titanium recovered their proliferation at longer incubation times. The cell number found on PL-PS films at the different experimental times was comparable to that of the control tissue culture plate.
Figure 5.
Osteoblast-like cell proliferation on unmodified etched TiO2 and PL-PS-functionalized TiO2 surfaces. (a) MG-63 proliferation on UV-sterilized samples (TC uncoated, tissue culture plastic; TC coated, tissue culture plastic coated with G3 PL-PS; Ti uncoated, TiO2; Ti coated, TiO2 coated with G3 PL-PS; Ti coated and washed, TiO2 coated with G3 PL-PS and washed); (b) SAOS-2 proliferation on UV and gamma-irradiated samples. Tissue culture plastic plates were used as the control. Data were statistically analysed by ANOVA (Tukey's test) and values were considered statistically significant at p ≤ 0.05. Asterisk indicates significantly different samples.
The differentiation of SAOS-2 cells on UV- and gamma-irradiated surfaces over 21 days clearly demonstrated that the peak of ALP-specific activity at day 14 was higher for cells adhering on UV-irradiated PL-PS films than for those adhering on uncoated gamma-irradiated titanium and gamma-irradiated PS-PL films, the latter still showing levels of ALP enzymatic activity similar to the control (figure 6).
Figure 6.
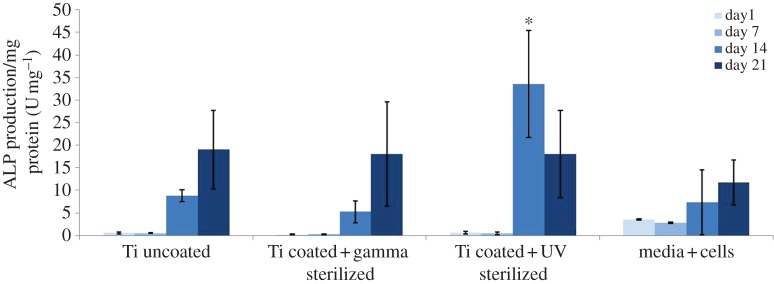
SAOS-2 osteoblast-like cell differentiation on unmodified etched TiO2 and PL-PS-functionalized TiO2 surfaces. Tissue culture plastic plates were used as the control. Data were statistically analysed by ANOVA (Tukey's test) and values were considered statistically significant at p ≤ 0.05. Asterisk indicates significantly different samples.
4. Discussion
Aseptic mobilization of metal implants and their consequent failure are largely determined by the lack of a stable bond between the implant surface and the surrounding bone mineralized extracellular matrix [20]. Surface engineering has so far focused on producing macroporous surfaces that favour the interlocking of the bone with the implant [21]. This macroporosity has been obtained by plasma spraying of either titanium beads or ceramic coatings on the metal surface [22,23]. Plasma spraying of metal beads as well as surface treatments including surface etching are to produce a surface topography with a degree of roughness at the microscale level. This surface texturization has been shown to lead to a clear improvement in cell adhesion in vitro [24,25]. However, in vivo studies have demonstrated that these surface treatments are still not adequate to ensure complete bonding of the mineralized bone extracellular matrix to the implant surface [26,27]. Rather, a micrometre-scale gap of soft tissue forms that prevents intimate bonding between the metal surface and the implant.
The use of ceramic coatings has successfully led to complete bonding between the implant surface and the surrounding bony tissue [28]. However, it is known that the use of plasma spray leads to the formation of a relatively thick coating that lacks sufficient bonding stability with the underlying metal substrate. As a result, upon integration with the surrounding bone, ceramic-coated metal implants tend to undergo delamination and, consequently, mobilization [29,30].
It has been envisaged that the oxide layer that spontaneously forms on the surface of titanium during its exposure to the environment can be exploited for thin and stable osteointegrative films. In a recent approach, anodic spark deposition has been used to obtain films of approximately 2 µm thickness encased in the titanium oxide layer and exposing a surface morphology with a degree of roughness in the cell-recognition range [31,32]. Although representing a significant step forward towards the stabilization of ceramic coatings, the control of the anodic spark deposition process and the micrometre-scale thickness of the coating may still not ensure batch-to-batch reproducibility and adequate stability under mechanical stress. For these reasons, it has been envisaged that thin molecular layers could be adopted that have the necessary characteristics to facilitate direct bonding of the repairing bone to the metal surface [33,34]. Following this approach, previous work has attempted to optimize the deposition of thin films of calcium-binding phospholipids able to accelerate the formation of a mineral phase upon contact with body fluids. Coatings of phosphatidylserine have been applied to titanium surfaces and have been shown to improve the biomineralization and cell substrate potential of metal surfaces in vitro [9,35]. However, the deposition of these phosphatidylserine-based coatings is difficult to control, leading to relatively thick coatings with a relatively slow absorption rate in vivo [11].
The present work aimed at optimizing osteointegrative films of macromolecular thickness able to expose a high surface density of the most potent natural biomineralization agent, PS. The activation of the titanium surface by chemical etching allowed the formation of a stable bond between the titanium oxide layer and the dendron. It is envisaged that such a thin and tightly bound film could be relatively more stable under surgical procedures and biomechanical stresses, thus reducing the risk of aseptic loosening.
Unlike the usual pattern of material biomineralization where hydroxyapatite crystal nuclei randomly and sparsely form across the surface after relatively long periods of incubation in SBF [36], the PL-PS film is homogeneously and rapidly encased in a calcium phosphate-based mineral phase. Previously the use of poly(ethylene imine) dendrons has been shown to induce nucleation of calcium phosphate onto the surface of NaOH-treated titanium upon incubation in SBF [37]. Regardless of their limited clinical significance, biomineralization experiments in SBF were performed to assess whether the grafting of the PS to the G3 PL dendron had changed its calcium phosphate crystal nucleation potential. The relatively rapid biomineralization of the titanium surface when functionalized with the PL-PS film suggests that the grafted PS is accessible to the calcium and phosphate ions of the SBF. These results suggested that the reaction between the titanium oxide layer activated by the chemical etching and the dendron did not significantly involve the PS moiety. Moreover, the experiments in SBF provide insights into the molecular basis of the PS biomineralization potential. The chemistry adopted in this study to graft the PS to the G3 PL allowed the peptidic bonding of PS to the exposed PL primary amino groups through the PS carboxylic group. Such a covalent bond left exposed the phosphorus groups that, in natural phosphatidylserine, act as bridges between PS and the phospholipid aliphatic chains. It has been suggested that the crystal nucleation potential of the PS resides in the relatively high calcium-binding capacity of the molecule rather than in a high binding affinity [38]. A high calcium-binding affinity would subtract the calcium ion to the phosphate, thus limiting the clustering of the two counterions and the crystal nucleus formation. In the case of the PS, the relatively high binding capacity leads to the retention of calcium ions in proximity to the PS zwitterionic molecule, thus enhancing the ion's ability to interact with the phosphate ions. According to this proposed mechanism of action, the data collected in the present study demonstrate that the availability of free carboxylic groups is not necessary to preserve the PS calcium-binding capacity and, as a consequence, its crystal nucleation potential.
While enhancing the stable bonding of the implant surface with the mineralized bone extracellular matrix, the presence of a mineralized PL-PS film was thought to improve the substrate properties of the implant towards osteoblasts. The data in the present study show that, regardless of the type of cell line used, the presence of the film improves osteoblast adhesion and proliferation. More importantly, once colonized by osteoblasts, this film appears to stimulate their differentiation at a level significantly higher than that of the unmodified surfaces. Therefore, it can be speculated that the rate of bone formation around PL-PS-functionalized implants in vivo may be significantly higher than non-modified implants. The combination of a relatively fast biomineralization process and cell activity suggests that this type of surface may reduce the risks of formation of a non-mineralized layer at the implant–tissue interface. Studies of differentiation of primary murine bone marrow stem cells on the PS-tethered dendron films have clearly shown the ability of these substrates to induce cell differentiation into mature osteoblasts [39]. Real-time PCR showed a significant increase in the expression of markers such as ALP and osteocalcin.
In view of its possible clinical use, the osteointegrative potential of the PL-PS film was also tested after sterilization with conventional irradiation methods. In particular, the effect of UV and gamma radiation was tested on the freeze-dried PL-PS dendrons prior to their application as films on titanium surfaces as well as on the films deposited on titanium surfaces. Although MS analysis showed no significant change in the molecular weight profile of the dendrons, and SBF experiments showed no alteration of the biomineralization potential of the film, cell studies showed a different response of the osteoblasts to the films sterilized by different methods. It appears that sterilization by UV irradiation was the most suitable approach. This method appeared to increase the cell proliferation rate in the first 24 h of incubation and led to a significantly high level of cell differentiation, with the ALP-specific activity reaching its highest value after 14 days in culture. However, it has to be outlined that, even after the gamma-irradiation treatment, the sterile PL-PS film offered substrate properties that encouraged levels of cell proliferation and differentiation similar to those of the control tissue culture plate.
5. Conclusion
PL-PS dendrons are a reproducible and scalable technological platform to produce thin macromolecular films on titanium oxide surfaces that provide the advantage of increasing the density of functional groups key to the integration of an implant with the surrounding tissue. When tethered with PS molecules, dendrons can be deposited as a film able to induce the formation of a thin and homogeneous mineral phase encased in its mesh. The mesh also exposes to adhering osteoblast cells a nano-texture that favours their adhesion and that stimulates their short-term proliferation and differentiation. When combined, the properties of PL-PS films delineate a novel route towards the development of new fully osteointegrative metal implants.
Acknowledgements
This work has been supported by the Biosintering project granted by the Provincia Autonoma di Trento, Trento, Italy.
References
- 1.Mendonça G, Mendonça DBS, Aragão FJL, Cooper LF. 2008. Advancing dental implant surface technology: from micron to nanotopography. Biomaterials 29, 3822–3835 10.1016/j.biomaterials.2008.05.012 (doi:10.1016/j.biomaterials.2008.05.012) [DOI] [PubMed] [Google Scholar]
- 2.Cao W, Hench LL. 1996. Bioactive materials. Ceram. Int. 22, 493–507 10.1016/0272-8842(95)00126-3 (doi:10.1016/0272-8842(95)00126-3) [DOI] [Google Scholar]
- 3.Orsini G, Assenza B, Scarano A, Piattelli M, Piattelli A. 2000. Surface analysis of machined versus sandblasted and acid-etched titanium implants. Int. J. Oral Maxillofac. Implants 15, 779–84 [PubMed] [Google Scholar]
- 4.Habibovic P, Barrere F, van Blitterswijk CA, DeGroot K, Layrolle P. 2002. Biomimetic hydroxyapatite coating on metal implants. J. Am. Ceram. Soc. 85, 517–522 10.1111/j.1151-2916.2002.tb00126.x (doi:10.1111/j.1151-2916.2002.tb00126.x) [DOI] [Google Scholar]
- 5.Wen J, Leng Y, Chen J, Zhang C. 2000. Chemical gradient in plasma-sprayed HA coatings. Biomaterials 21, 1339–1343 10.1016/S0142-9612(99)00273-2 (doi:10.1016/S0142-9612(99)00273-2) [DOI] [PubMed] [Google Scholar]
- 6.Gajjeraman S, He G, Narayanan K, George A. 2008. Biological assemblies provide novel templates for the synthesis of hierarchical structures and facilitate cell adhesion. Adv. Funct. Mater. 218, 3972–3990 10.1002/adfm.200801215 (doi:10.1002/adfm.200801215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durrieu MC, Pallu S, Guillemot F, Bareille R, Amedee J, Baquey Ch, Labrugere C, Dard M. 2004. Grafting RGD containing peptides onto hydroxyapatite to promote osteoblastic cell adhesion. J. Mater. Sci. Mater. Med. 15, 779–786 10.1023/B:JMSM.0000032818.09569.d9 (doi:10.1023/B:JMSM.0000032818.09569.d9) [DOI] [PubMed] [Google Scholar]
- 8.Reinstorf A, Ruhnow M, Gelinsky M, Pompe W, Hempel U, Wenzel K-W, Simon P. 2004. Phosphoserine: a convenient compound for modification of calcium phosphate bone cement collagen composites. J. Mater. Sci. Mater. Med. 15, 451–455 10.1023/B:JMSM.0000021119.14870.3d (doi:10.1023/B:JMSM.0000021119.14870.3d) [DOI] [PubMed] [Google Scholar]
- 9.Santin M, Rhys-Williams W, O'Reilly J, Davies MC, Shakesheff K, Love WG, Lloyd AW, Denyer SP. 2006. Calcium-binding phospholipids as a coating material for implant osteointegration. J. R. Soc. Interface 3, 277–281 10.1098/rsif.2005.0088 (doi:10.1098/rsif.2005.0088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosetti M, Santin M, Lloyd AW, Denyer SP, Sabbatini M, Cannas M. 2007. Cell behaviour on phospholipid-coated surfaces. J. Mater. Sci. Mater. Med. 18, 611–617 10.1007/s10856-007-2309-1 (doi:10.1007/s10856-007-2309-1) [DOI] [PubMed] [Google Scholar]
- 11.Merolli A, et al. 2006. In vivo assessment of the osteointegrative potential of phosphorylserine-based coatings. J. Mater. Sci. Mater. Med. 17, 789–794 10.1007/s10856-006-9836-z (doi:10.1007/s10856-006-9836-z) [DOI] [PubMed] [Google Scholar]
- 12.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. 1985. A new class of polymers: starburst-dendritic macromolecules. Polym. J. (Tokyo) 17, 117–132 [Google Scholar]
- 13.Denkewalter RG, Kolc J, Lukasavage WJ. 1979 Preparation of lysine based macromolecular highly branched homogeneous compound. US Patent 4360646.
- 14.Roy R, Zanini D, Meunier SJ, Romanowska A. 1993. Solid-phase synthesis of dendritic sialoside inhibitors of influenza A virus haemagglutinin. J. Chem. Soc. Chem. Commun. 24, 1869–1872 10.1039/C39930001869 (doi:10.1039/C39930001869) [DOI] [Google Scholar]
- 15.Hawker CJ, Frechet JMJ. 1990. A new convergent approach to monodisperse dendritic macromolecules. J. Chem. Soc. Chem. Commun. 15, 1010–1013 10.1039/C39900001010 (doi:10.1039/C39900001010) [DOI] [Google Scholar]
- 16.Newcome GR, He E, Moorefield CN. 1999. Suprasupermolecules with novel properties: metallodendrimers. Chem. Rev. 99, 1689–1746 [DOI] [PubMed] [Google Scholar]
- 17.Tomalia DA, Naylor AM, Goddard WA. 1990. Starburst dendrimers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem. Int. Ed. Engl. 29, 138–175 10.1002/anie.199001381 (doi:10.1002/anie.199001381) [DOI] [Google Scholar]
- 18.Lloyd AW, Olivier GWJ, Standen G, Santin M, Meikle ST. Biomaterial with functionalised surfaces. PCT/GB2007/050741. [Google Scholar]
- 19.Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. 1990. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 24, 721–734 10.1002/jbm.820240607 (doi:10.1002/jbm.820240607) [DOI] [PubMed] [Google Scholar]
- 20.Davies JE. 2007. Bone bonding at natural and biomaterials surfaces. Biomaterials 28, 5058–5067 10.1016/j.biomaterials.2007.07.049 (doi:10.1016/j.biomaterials.2007.07.049) [DOI] [PubMed] [Google Scholar]
- 21.Mendes VC, Moineddin R, Davies JE. 2007. The effect of discrete calcium phosphate nanocrystals on bone-bonding to titanium surfaces. Biomaterials 28, 4748–4755 10.1016/j.biomaterials.2007.07.020 (doi:10.1016/j.biomaterials.2007.07.020) [DOI] [PubMed] [Google Scholar]
- 22.Ong JL, Carnes DL, Bessho K. 2004. Evaluation of titanium plasma-sprayed and plasma-sprayed hydroxyapatite implants in vivo. Biomaterials 25, 4601–4606 10.1016/j.biomaterials.2003.11.053 (doi:10.1016/j.biomaterials.2003.11.053) [DOI] [PubMed] [Google Scholar]
- 23.Aparicio C, Rodriguez D, Gil FJ. 2011. Variation of roughness and adhesion strength of deposited apatite layers on titanium dental implants. Mater. Sci. Eng. C 31, 320–324 10.1016/j.msec.2010.09.018 (doi:10.1016/j.msec.2010.09.018) [DOI] [Google Scholar]
- 24.Sandrini E, Morris C, Chiesa R, Cigada A, Santin M. 2005. In vitro assessment of the osteointegrative potential of a multiphase anodic spark deposition coating for orthopaedic and dental implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 73B, 392–399 10.1002/jbm.b.30241 (doi:10.1002/jbm.b.30241) [DOI] [PubMed] [Google Scholar]
- 25.Anselme K, Noël B, Hardouin P. 1999. Human osteoblast adhesion on titanium alloy, stainless steel, glass and plastic substrates with same surface topography. J. Mater. Sci. Mater. Med. 10, 815–819 10.1023/A:1008992109670 (doi:10.1023/A:1008992109670) [DOI] [PubMed] [Google Scholar]
- 26.Roccuzzo M, Bunino M, Prioglio F, Bianchi SD. 2001. Early loading of sandblasted and acid-etched (SLA) implants: a prospective split-mouth comparative study. Clin. Oral Implants Res. 12, 572–578 10.1034/j.1600-0501.2001.120604.x (doi:10.1034/j.1600-0501.2001.120604.x) [DOI] [PubMed] [Google Scholar]
- 27.Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. 1991. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 25, 889–902 10.1002/jbm.820250708 (doi:10.1002/jbm.820250708) [DOI] [PubMed] [Google Scholar]
- 28.de Groot K, Geesink RGT, Klein CPAT, Serekian P. 1987. Plasma sprayed coatings of hydroxylapatite. J. Biomed. Mater. Res. 21, 1375–1381 10.1002/jbm.820211203 (doi:10.1002/jbm.820211203) [DOI] [PubMed] [Google Scholar]
- 29.Oonishi H, Yamamoto M, Ishimaru H, Tsuji E, Kushitani S, Aono M, Ukon Y. 1989. The effect of hydroxyapatite coating on bone growth into porous titanium alloy implants. J. Bone Joint Surg. Br. 71B, 213–216 [DOI] [PubMed] [Google Scholar]
- 30.Collier JP, Surprenant VA, Mayor MB, Wrona M, Jensen RE, Surprenant HP. 1993. Loss of hydroxyapatite coating on retrieved, total hip components. J. Arthroplasty 8, 389–393 10.1016/s0883-5403(06)80037-9 (doi:10.1016/s0883-5403(06)80037-9) [DOI] [PubMed] [Google Scholar]
- 31.Sul YT, Johansson C, Wennerberg A, Cho LR, Chang BS, Albrektsson T. 2005. Optimum surface properties of oxidized implants for reinforcement of osseointegration: surface chemistry, oxide thickness, porosity, roughness, and crystal structure. Int. J. Oral Maxillofac. Implants 20, 349–359 [PubMed] [Google Scholar]
- 32.Yang B, Uchida M, Kim HM, Zhang X, Kokubo T. 2004. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 25, 1003–1010 10.1016/S0142-9612(03)00626-4 (doi:10.1016/S0142-9612(03)00626-4) [DOI] [PubMed] [Google Scholar]
- 33.Heijink A, Schwartz J, Zobitz ME, Crowder KN, Lutz GE, Sibonga JD. 2008. Self-assembled monolayer films of phosphonates for bonding RGD to titanium. Clin. Orthop. Rel. Res. 466, 977–984 10.1007/s11999-008-0117-7 (doi:10.1007/s11999-008-0117-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dey T, Roy P, Fabry B, Schmuki P. 2011. Anodic mesoporous TiO2 layer on Ti for enhanced formation of biomimetic hydroxyapatite. Acta Biomater. 7, 1873–1879 10.1016/j.actbio.2010.11.011 (doi:10.1016/j.actbio.2010.11.011) [DOI] [PubMed] [Google Scholar]
- 35.Bosetti M, Lloyd AW, Santin M, Denyer SP, Cannas M. 2005. Effects of phosphatidylserine coatings on titanium on inflammatory cells and cell-induced mineralization in vitro. Biomaterials 26, 7572–7578 10.1016/j.biomaterials.2005.05.033 (doi:10.1016/j.biomaterials.2005.05.033) [DOI] [PubMed] [Google Scholar]
- 36.Liu P, Smits J, Ayers DC, Song J. 2011. Surface mineralization of Ti6Al4V substrates with calcium apatites for the retention and local delivery of recombinant human bone morphogenetic protein-2. Acta Biomater. 7, 3488–3495 10.1016/j.actbio.2011.05.025 (doi:10.1016/j.actbio.2011.05.025) [DOI] [PubMed] [Google Scholar]
- 37.Tsiourvas D, Tsetsekou A, Arkas M, Diplas S, Mastrogianni E. 2011. Covalent attachment of a bioactive hyperbranched polymeric layer to titanium surface for the biomimetic growth of calcium phosphates. J. Mater. Sci. Mater. Med. 22, 85–96 10.1007/s10856-010-4181-7 (doi:10.1007/s10856-010-4181-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann S. 1988. Molecular recognition in biomineralization. Nature 332, 119–124 10.1038/332119a0 (doi:10.1038/332119a0) [DOI] [Google Scholar]
- 39.Galli C, Piemontese M, Meikle ST, Santin M, Macaluso GM, Passeri G. In press Biomimetic coating with phosphoserine-tethered poly(epsilon lysine) dendrons on titanium surfaces enhances Wnt and osteoblastic differentiation. Clin. Oral Implant Res. [DOI] [PubMed] [Google Scholar]