Abstract
Avian influenza viruses (AIVs) have been implicated in all human influenza pandemics in recent history. Despite this, surprisingly little is known about the mechanisms underlying the maintenance and spread of these viruses in their natural bird reservoirs. Surveillance has identified an AIV ‘hotspot’ in shorebirds at Delaware Bay, in which prevalence is estimated to exceed other monitored sites by an order of magnitude. To better understand the factors that create an AIV hotspot, we developed and parametrized a mechanistic transmission model to study the simultaneous epizootiological impacts of multi-species transmission, seasonal breeding, host migration and mixed transmission routes. We scrutinized our model to examine the potential for an AIV hotspot to serve as a ‘gateway’ for the spread of novel viruses into North America. Our findings identify the conditions under which a novel influenza virus, if introduced into the system, could successfully invade and proliferate.
Keywords: avian influenza, multi-host mathematical model, environmental transmission, disease hotspot
1. Introduction
Avian influenza viruses (AIVs) have played a key role in human pandemics over the past century, with avian-derived gene segments identified in all pandemic influenza strains [1–4]. Although primarily an infection of birds, ‘host shifts’ of the virus from birds to humans have been documented [5], causing severe disease or death [6] in some cases. Clearly, understanding the determinants of AIV transmission in their natural reservoir—wild birds—is both important and timely [7], though several factors combine to make this challenging [8,9]:
(i) Multiple host species. AIVs have been isolated from more than 105 bird species from 26 families [9], though most competent hosts are thought to belong to the orders Anseriformes (ducks, geese and swans) and Charadriiformes (gulls, shorebirds and terns). One of the chief complicating aspects of (low pathogenicity) AIV infection in wild birds appears to be the absence of overt clinical symptoms [1], resulting in the need for extensive field sampling of individual birds in order to paint an accurate epizootiological picture in any given population [10].
(ii) Seasonal host migration. The role of multiple hosts in the system also introduces a complex spatial element owing to the idiosyncratic migratory behaviours of different species. Many bird species are, to some degree, migrants, spending a portion of each year in locations that can be thousands of miles apart. Behaviour at different locations can also vary; mallards (Anas platyrhynchos), for example, are observed to be very territorial at their breeding grounds but social at other locations [11]. Despite the potential difficulties this spatial structure generates, migration routes for many species are well documented and can provide information on the timing and location of interspecific mixing [12]. The role of migration in disease spread has come into focus lately [13], with recent work suggesting that birds with asymptomatic AIV infections could be responsible for the spread of H5N1 across countries or even continents [14]. Observations in the field—such as that of migrating wild geese in China and Tibet wintering close to their domestic counterparts [15]—support this hypothesis.
(iii) Virus diversity. AIVs demonstrate extensive genetic variation. They are classified according to two surface glycoproteins—haemagglutinin (‘H’) and neuraminidase (‘N’)—with 144 possible subtypes in total (combinations of H1–H16 and N1–N9) [16]. The duration and extent of protective immunity following infection are open questions, with experimental work confined to short-term studies [17,18].
(iv) Mixed transmission mechanisms. Finally, it is increasingly thought that AIVs boast two distinct transmission routes in waterbirds. In addition to the essentially direct faecal–oral mechanism (short time scale; susceptible and infected birds in close proximity) [1], an environmental component to transmission has been identified [19–24]. Influenza A viruses have been shown to persist in water for several months [20,21], leading to indirect transmission chains via the environment that occur over a much longer time scale than faecal–oral transmission. On this time scale, transmission could occur between species that never directly interact but instead share a location each occupies at a different time during the year [10].
These complexities converge in Delaware Bay, USA, and, together with concerted surveillance efforts at this site, offer a unique opportunity to study the epizootiology of AIVs in their natural hosts.
Delaware Bay is a site of hemispheric importance for shorebirds [25], with bird densities reaching as high as 210 birds per square metre [26]. Multiple species migrate to Delaware Bay throughout the year [27], making it a pivotal site for understanding bird ecology. In particular, Delaware Bay has previously been identified as a ‘hotspot’ for AIVs in shorebirds, with estimated average prevalence from 1998 to 2008 about 50 times greater than for all other surveillance sites worldwide [26]. This observation needs to be explained because it suggests that Delaware Bay may act as a place where novel avian viruses can amplify and subsequently spread in North America.
A factor that many consider key to the high AIV prevalence in shorebirds (in particular, in ruddy turnstones (Arenaria interpres)) in Delaware Bay is the abundance of horseshoe crabs (Limulus polyphemus) there [26]. Every year, thousands of shorebirds congregate on the beaches of Delaware Bay and feast on the horseshoe crab eggs, laid in their millions each spring [28]. The shorebirds in Delaware Bay depend almost entirely on horseshoe crab eggs to refuel them during their spring migration [26]. This complete dependence makes them vulnerable to horseshoe crab population sizes, which have been declining in recent years [29]. This dependence is an important consideration, because of the role it will play both in shorebird population sizes (declines have already been noticed in Delaware Bay [29]) and on the AIV prevalence levels in these species.
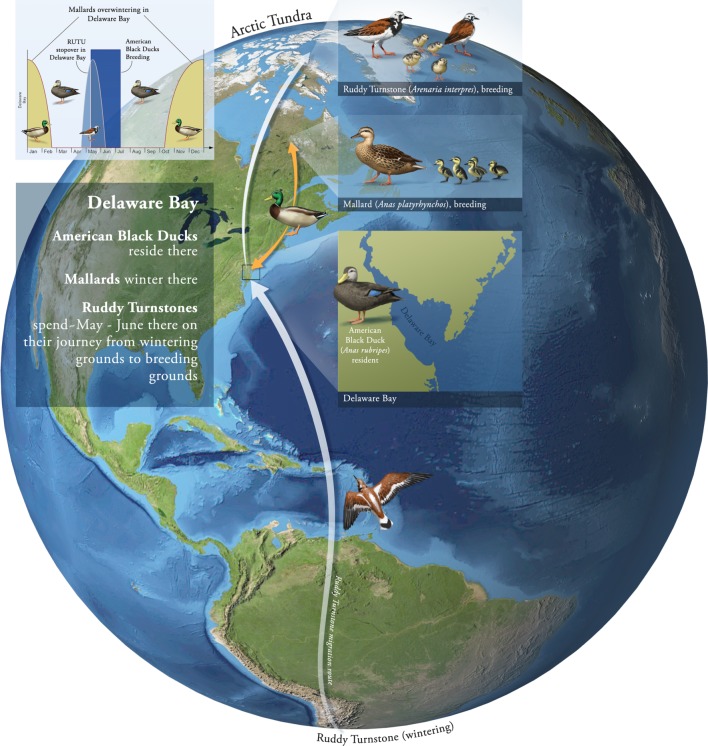
The initiation of the AIV prevalence peak observed in Delaware Bay in ruddy turnstones is not known. As studies suggest that AIV is not present year round in this species [26], it may therefore rely on the maintenance cycle of AIVs driven in part by resident and migratory ducks. To understand this system and examine its consequences for invasion of novel viruses, we develop a multi-host, multi-site AIV transmission model, with parameters estimated using existing prevalence data, that represents a simplified version of the interactions in Delaware Bay. We focus on three host species that we consider key to understanding transmission dynamics in Delaware Bay, with each interacting with the Delaware Bay environment for different periods of time during the year. All three species return a high average percentage of positive AIV isolations, either globally [9] or within Delaware Bay [26]. The three hosts, and their interaction with Delaware Bay, are: (i) ruddy turnstones (a short-term visitor to Delaware Bay)—of the shorebirds tested for AIV in Delaware Bay, this species most frequently returns positive results [26]; (ii) American black ducks (Anas rubripes; ‘resident ducks’ in our system)—a locally breeding species with resident and migratory birds present throughout the year; (iii) mallards (‘migrating ducks’ in our system), a long-term visitor to Delaware Bay—and a species with one of the highest reported percentages of AIV isolations [9]. The migration biology of this system, and the wintering/breeding sites included in the model for one or more of the migrating species, is illustrated in figure 1.
Figure 1.
Migratory ecology of the simplified three-host Delaware Bay system. The migration routes for ruddy turnstones and mallards are shown, with the inset showing the timing of their presence in Delaware Bay. Also marked in the inset (in dark blue) is the breeding season of resident ducks.
In addition to multiple host species, we consider mixed transmission dynamics and species-specific seasonality in breeding, hatching, mortality and migration. Our results show that the source and route of AIV infection varied throughout the year, depending on season-specific migration to and from Delaware Bay and which species were reproducing. Motivated by recent declines in horseshoe crab abundance [29], the model is studied to examine the consequences of continuing declines in resources (horseshoe crabs) for the ruddy turnstone population and the broader impact this has on AIV transmission in Delaware Bay. To quantify the chance that any future introduction of a novel strain to Delaware Bay will invade, and to determine the window of opportunity during which invasion is most probable, we calculated the local Lyapunov exponent (LLE; see S9 in the electronic supplementary material for a description). These results show that invasion is most likely when ruddy turnstones are in Delaware Bay or when hatching is occurring in any species.
2. The model
We address AIV transmission dynamics in Delaware Bay by constructing a deterministic, continuous time, three-host, susceptible–infectious–recovered–susceptible (SIRS) model. The key model ingredients are outlined below.
2.1. Seasonal migration
Two of the host species—ruddy turnstones and mallards—follow specific migration patterns. Ruddy turnstones are on their wintering grounds from September to May, in Delaware Bay for the majority of May and on their breeding grounds the rest of the year. Mallards winter in Delaware Bay from October to February and spend the rest of the year on their breeding grounds. The third host species—American black ducks—remains in Delaware Bay throughout the year. Details of the migration parameters are presented in the electronic supplementary material, §S3.
2.2. Seasonal hatching
The pulsed influx of susceptible juveniles is known to be important for transmission dynamics, both in the context of AIVs in bird populations [1] and more generally [30,31]. Therefore, we consider season-specific hatching rates in our model. Duck hatching rates are constant for a quarter of the year (during the hatching season) and zero otherwise [11,32]. The hatching season for ruddy turnstones is shorter, lasting for a tenth of a year [33]. These parameters are presented in the electronic supplementary material, §S4.
2.3. Seasonal mortality
In duck species, hunting is thought to be a significant contribution to annual mortality [34]. We include this element of duck life history by increasing the mortality rate in both duck species during the hunting season (October–January) [35] (see §S4 in the electronic supplementary material for parameter details).
2.4. Ruddy turnstone feeding ecology
While in Delaware Bay, ruddy turnstones feed on horseshoe crab eggs buried in high concentrations on coastal, sandy beaches [36]. Eggs are usually buried 15–20 cm beneath the surface, but are displaced by both other spawning crabs and tide movements [29,37]. Without a sufficient supply of horseshoe crab eggs, shorebirds are less likely to successfully complete their migration and breed [29]. To model this, we made the ruddy turnstone hatching rate dependent on the number of horseshoe crabs, E, as shown in the electronic supplementary material, equation (S1a). During our numerical analysis, we varied the number of horseshoe crabs, to assess how resource limitation affects AIV prevalence. We began with a large value of E and ran the numerical model for 500 years, retaining the peak prevalence values from the last 50 years. Using the final class sizes as our new initial conditions, we reset E to a smaller value and ran the numerical model. We repeated this for 100 values of E.
2.5. Direct transmission
Within each species, the direct transmission rate varies through the year. The contact rate in duck species is assumed to be lower immediately before and at the start of the hatching season, when birds form mating pairs and become aggressive towards conspecifics (thereby interacting less than at other times during the year) [11,32]. Transmission among ruddy turnstones is assumed to be low all year except for when they are in Delaware Bay, where contact rates are greatly increased (based on density estimates [26]). We use square wave functions to represent these variations. Between-species transmission rates are set to either zero or a non-zero constant, depending on the time of year. The transmission matrix and parameters are given in the electronic supplementary material, §S2. As supported by empirical evidence [38] and previous theoretical studies, we assume density-dependent transmission [39,40].
2.6. Environmental transmission
We include classes in our model for the environmental reservoir at each location, as in Breban et al. [39]. Virus is assumed to be shed at a constant rate into the environment by infected birds and to decay at a time-dependent rate, owing to temperature variation at the different locales (see the electronic supplementary material, §S5, for details). The virus concentration in the environment is represented by V in the model. The environmental transmission term represents the rate at which a susceptible bird consumes virions (ρSV), modified by a probability of infection term, ρV/(ρV + κ). Hence, ρV is the amount of virus consumed per unit time, kappa represents the ID50 (virus dose that has a 50% chance of generating an infection) and this expression determines infection probability per unit time. We estimate the value of ρ by fitting the model to existing prevalence data, as shown in the electronic supplementary material, §S7. Virus decay parameters are given in the electronic supplementary material, §S5.
2.7. Immunity
Our transmission model permits loss of immunity. Best-fit parameter estimates (see the electronic supplementary material, §S7) yielded a mean duration of immunity of approximately six months, consistent with experimental data suggesting that antibodies decline to undetectable levels within about eight months [18]. We assume the average duration of immunity in ruddy turnstones to be 1 year, based on empirical evidence that shows the majority of birds annually arrive in Delaware Bay seronegative and convert while there [41].
The system of equations describing a single-host model is given in (2.1). The full model and the seasonal parameters are given in the electronic supplementary material, §§S1–S5; parameter estimates for all hosts are given in table 1.
| 2.1a |
| 2.1b |
| 2.1c |
| 2.1d |
Here, N represents the total population size and is given by N = S + I + R (I is the infected class and R the immune class). We derive an expression for the effective basic reproductive value, R0e [47,48], assuming no seasonality (all parameters are constant) and the approximation ρV/(ρV + κ) = A (A const.). For comparison, we also present R0e when this assumption is not made. We can extend this to include the seasonally varying terms in our model by defining R0e(t) as the R0e value at time t when a single infected individual enters an otherwise susceptible population [49]. The expression for R0e(t) from equations (2.1) is
 |
2.2 |
The R0e(t) values that apply to each species are given in the electronic supplementary material, §S6. In §4, we use this time-varying R0e(t) to quantify the relative effect of interspecies mixing on AIV transmission dynamics in Delaware Bay.
Table 1.
Standard parameter values for each host species. The superscripts m,r,u stand for migrating ducks, resident ducks and ruddy turnstones, respectively.
| parameter | symbol | value/range | unit | source |
|---|---|---|---|---|
| mallards | ||||
| direct transmission (baseline) | 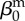 |
0.01 | year−1 | parametrization |
| amplitude of seasonality | 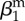 |
0.75 | parametrization | |
| birth rate | 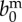 |
2 | year−1 | [42] |
| average death rate | 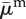 |
0.5 | year−1 | [42] |
| recovery rate | γm | 52 | year−1 | [1] |
| loss of immunity | εm | 2.004 | year−1 | parametrization |
| consumption rate | ρm | 1.3804 × 10−12 | year−1 | parametrization |
| infection shape parameter | κ | 100 | EID50 | [43] |
| shedding rate | ωm | 1012 | EID50 year−1 | [44] |
| persistence | η | 4.9–42.6 | year−1 | [19] |
| American black ducks | ||||
| direct transmission (baseline) | 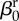 |
0.01 | year−1 | assumed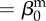
|
| amplitude of seasonality | 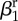 |
0.75 | assumed
|
|
| birth rate | 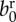 |
2 | year−1 | [34] |
| average death rate | 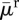 |
0.5 | year−1 | [34] |
| recovery rate | γr | 52 | year−1 | [1] |
| loss of immunity | εr | 2.004 | year−1 | assumed = εm |
| consumption rate | ρr | 1.3804 × 10−12 | year−1 | assumed = ρm |
| infection shape parameter | κ | 100 | EID50 | [43] |
| shedding rate | ωr | 1012 | EID50 year−1 | [44] |
| persistence | η | 13.9–42.6 | year−1 | [19] |
| ruddy turnstones | ||||
| direct transmission (baseline) | 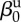 |
0.005 | year−1 | assumed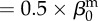
|
| amplitude of seasonality | 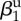 |
0.5 | estimated | |
| birth rate | 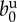 |
1.5 | year−1 | [45] |
| death rate | μu | 0.15 | year−1 | [45] |
| recovery rate | γu | 52 | year−1 | [46] |
| loss of immunity | εu | 1 | year−1 | [41] |
| consumption rate | ρu | 1.3804 × 10−12 | year−1 | assumed = ρm |
| infection shape parameter | κ | 100 | EID50 | — |
| shedding rate | ωu | 1010 | EID50 year−1 | D. Stallknecht, estimate based on unpublished data (2007–2008) |
| persistence | η | 1.6–167.9 | year−1 | [19] |
| predator shape parameter | θ | 10−4 | year−1 | — |
| number of horseshoe crabs | E | 1–105 | — |
3. The epizootiological data
The ideal data for fitting the model would be of high temporal resolution, with large numbers of samples at each time point, and would exist for multiple species across their migration ranges. Unfortunately, these data do not as yet exist; so we take a pragmatic approach and available data to guide model parametrization.
Two sources of surveillance data were used for model fitting. The first comprises published prevalence estimates from Stallknecht & Shane [50]. These data come from a variety of sources and studies, incorporating different bird-trapping methodologies and virus isolation techniques, but together represent the best source of information regarding prevalence cycles in dabbling ducks in North America. We apply least-squares estimation to these data to quantify four parameters for migrating ducks, and assume that the same values hold for resident ducks (the methodological detail is presented in the electronic supplementary material, §S7). Similarly, in the absence of independent information on consumption rate or infection shape parameter in ruddy turnstones, we take these values to be the same as those used for the duck species.
The second set of data are published here for the first time and come from the US Early Detection System for Highly Pathogenic Avian Influenza in Wild Birds (data collection described in Deliberto et al. [51]). These data were collected in Delaware during the winter months for three consecutive years (2007–2010) and are presented in the electronic supplementary material.
4. Results
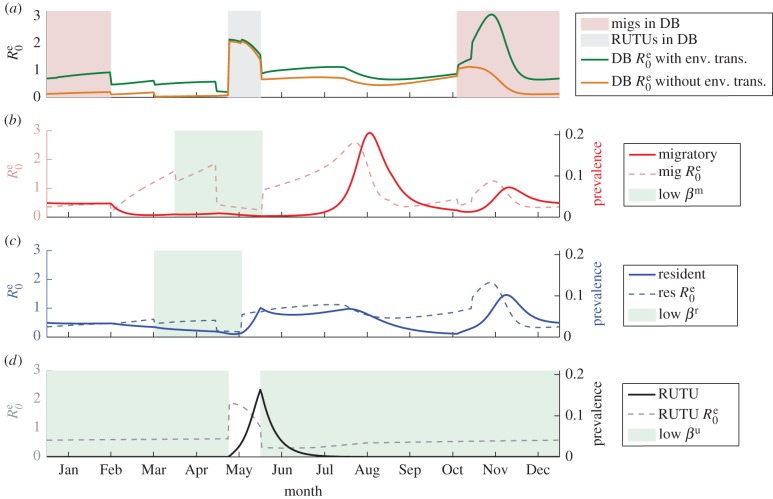
Our model explains that AIV dynamics in Delaware Bay are shaped by a combination of factors. The role of the environmental reservoir is apparent in a comparison of effective R0(t) values (R0e(t)) for species interacting in Delaware Bay in both the presence and absence of an environmental component (figure 2a), demonstrating that an environmental reservoir increases R0e. When migrating ducks and resident ducks are initially together in Delaware Bay, R0e(t) > 1 regardless of the environmental reservoir, although it is much higher when the environmental component is included. During the post-breeding period in resident ducks, inclusion of an environmental component produces R0e(t) > 1 (without it, R0e(t) < 1 during this period). Equally apparent is the role of interactions between host species—in particular, the interaction between ruddy turnstones and resident ducks. When considering R0e(t) for each species if modelled individually (i.e. as in (2.2)), R0e(t) for resident ducks when the ruddy turnstones are present in Delaware Bay is less than 1. However, the interaction between resident ducks and ruddy turnstones is such that R0e(t) for the two species is greater than 1, and a peak in prevalence in resident ducks is observed. This is seen in figure 2c, which shows both the prevalence curve and the individual R0e(t) for resident ducks against time. The impact of the interaction between both duck species is less obvious, as their individual R0e(t) > 1 during the timing of their interaction (figure 2b,c). However, the combined R0e(t) is a lot greater than the individual ones, contributing to the size of the prevalence peak observed.
Figure 2.
Prevalence curves from the multi-host model against effective R0(t) values [47]. (a) R0e(t) values for species in Delaware Bay both with and without environmental transmission; (b) the migratory duck (mig) prevalence alongside the effective R0(t) for migratory ducks individually; (c) the resident duck (res) prevalence and the effective R0(t) for resident ducks individually; and (d) the ruddy turnstone (RUTU) prevalence with the effective R0(t) for ruddy turnstones individually. In (b)–(d), the times of low transmission for the species shown are shaded and the R0e values are shown with environmental transmission. We assume the population size for each host to be 10 000 [33,39,52]. Initial conditions in the duck hosts are S(0) = 225, I(0) = 1, R(0) = 9774 (robustness to initial conditions is shown in the electronic supplementary material); for the ruddy turnstones they are S(0) = 9999, I(0) = 1, R(0) = 0. We assume that some virus is present initially at Delaware Bay and the duck breeding grounds, with V(0) = 100. We assume no virus is initially present at the ruddy turnstone wintering and breeding grounds [26,50].
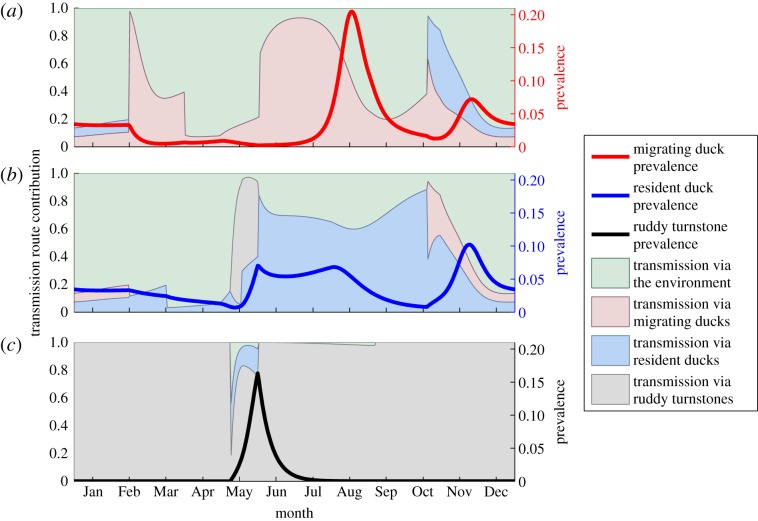
We can further use the model to determine the dominant transmission route throughout the year in each species. Each panel in figure 3 shows the prevalence for a particular host species, with a background that shows the proportion of cases generated via each transmission route throughout the year. Figure 3a shows the prevalence curve and contribution of each transmission route for migratory ducks. The main peak in prevalence in this host occurs prior to its arrival in Delaware Bay, after the influx of new susceptibles has occurred in the hatching season. Our model predicts that a second, smaller peak in prevalence is initiated by their arrival in Delaware Bay and mixing with resident ducks. The contributions from each of the transmission routes indicate that, outside Delaware Bay, the majority of infections in migrating ducks are caused by either within-species transmission or environmental transmission at different times of year. In Delaware Bay, our model suggests that within-species and between-species interactions contribute almost equally to new cases in both migrating and resident (figure 3b) ducks, although environmental transmission plays the largest role, accounting for approximately 80 per cent of cases during this time.
Figure 3.
Prevalence and incidence plots for the multi-host model showing the proportion of infections by their origin, alongside the prevalence curve, over time. (a) The migratory ducks, (b) the resident ducks and (c) the ruddy turnstones.
Figure 3b shows the equivalent curve for resident ducks. The numerical results suggest that three prevalence peaks occur every year. The model results demonstrate that the first peak results from the interaction between resident ducks and ruddy turnstones, and the final peak is due to the interaction between resident ducks and migrating ducks. The middle peak leads on from the first peak and is a response to the influx of susceptibles during the breeding season. These predictions suggest that the non-zero prevalence early in the year in both duck species is a consequence of the loss of immunity in ducks while migrating ducks are still in Delaware Bay. When the resident ducks are alone in Delaware Bay, within-species interactions account for between 60 and 80 per cent of transmission during the summer months, but environmental transmission is the dominant transmission route early in the year. The influence of ruddy turnstones is seen immediately before the first peak, when almost all transmission occurs via this species. Similarly, the role of migrating ducks is clear as they spark the peak in prevalence in residents, causing approximately 50 per cent of new cases as they arrive. The majority of transmission during this time period, however, is due to the environmental reservoir.
Figure 3c displays the cycle in ruddy turnstones, with peak prevalence occurring at the end of their stay in Delaware Bay. In assessing the contribution of each transmission route in this species, it can be seen that they show very little dependence on other species, with almost all transmission through within-species interactions. Notably, spikes in the proportion of infections transmitted via the environment and through interspecies interactions occur immediately before the prevalence peak, implying that these two factors are initiating their prevalence peaks. However, comparison of figure 3b and 3c indicates that the ruddy turnstones are a much greater influence on AIV prevalence among resident ducks than vice versa.
We can establish two results from the prevalence curves for each species and the contribution of the various transmission routes. First, it is clear that the presence of resident ducks in the model is a key factor in the persistence of AIV transmission in Delaware Bay. Further results presented in the electronic supplementary material (figure S13(b)) provide evidence of this, with outbreaks of AIV no longer occurring in ruddy turnstones when resident ducks are removed from the model. Moreover, these results (figure 3) show how important environmental transmission is, particularly in the case of the duck species. For much of the year in these species, transmission from the environmental reservoir is the dominant transmission route. We also find that interactions between the resident ducks and ruddy turnstones play a key role in the prevalence curves for each of these species, apparently providing the impetus for a peak in prevalence in both species.
We conducted a thorough sensitivity analysis on several of the model parameters, with the results presented in the electronic supplementary material, §S8. We found that the results are qualitatively very similar to the results presented here. The effect of changing the resident or ruddy turnstone population sizes is also presented in the electronic supplementary material, §S8, where we show that a small resident population has very little impact on the prevalence peaks in the migrating ducks but does change the height of the prevalence peak in ruddy turnstones. Similarly, altering the size of the ruddy turnstone population changes the peak prevalence in ruddy turnstones. Furthermore, we evaluated the role of each of the individual species in the system as a whole, by removing each in turn and considering the resulting prevalence curves and transmission routes (see the electronic supplementary material for sensitivity analyses).
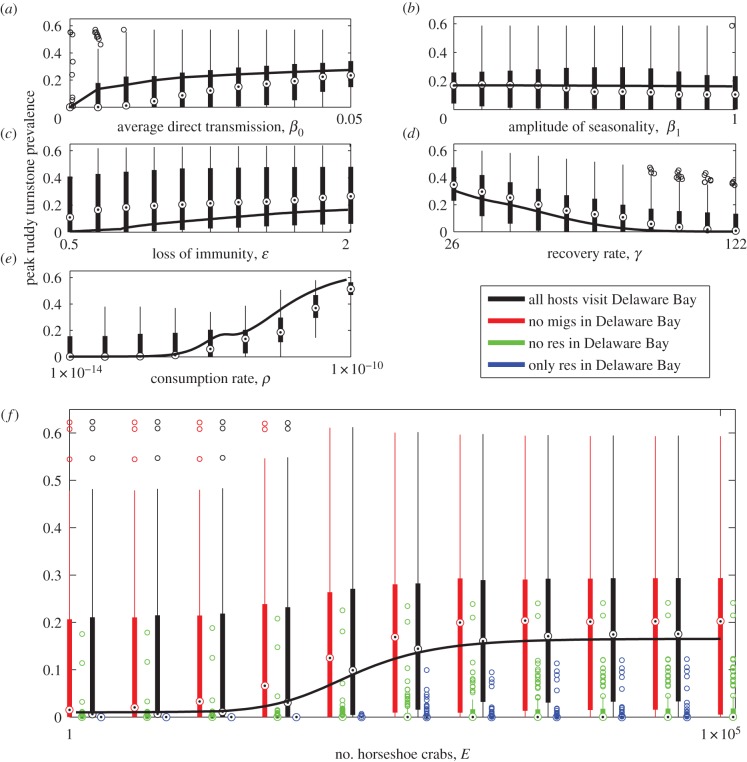
To systematically assess the contribution of key epizootiological parameters in our model output, we carried out a sensitivity analysis using Latin hypercube sampling (LHS). Specifically, we assigned a broad range of possible values to critical parameters—average direct transmission rate in the duck species; amplitude of seasonality in the aforementioned transmission rates; duration of immunity in the duck species; recovery rate in all species; consumption rate in all species; and number of horseshoe crabs—and generated 100 parameter sets using LHS (further detail is given in the electronic supplementary material, table S1). For each parameter set, peak prevalence in ruddy turnstones was noted to determine how it is influenced by changes in parametrization. We also compared these results with the peak prevalence found when only one of the parameters in question was allowed to vary and all others remained as given in table 1.
The results show (figure 4) that parameters that are more indirectly linked to peak prevalence in ruddy turnstones in Delaware Bay (such as amplitude of seasonality of direct transmission in the two duck species) have a smaller effect over their range than those that have a direct influence on AIV epizootiology in ruddy turnstones (e.g. recovery rate, consumption rate). In these two cases, mean peak prevalence in ruddy turnstones changes dramatically over the range of values tested (varying between 0.07 and 0.31 for the recovery rate and between 0.07 and 0.48 for the consumption rate).
Figure 4.
Effects of parameter variation on peak AIV prevalence in ruddy turnstones in Delaware Bay, using Latin hypercube sampling (LHS) across key epidemiological parameters. Each panel shows the results of varying one of these parameters and maintaining all other parameters as given in table 1 (some exceptions are required—see the electronic supplementary material for details) as a black line. The LHS for each parameter is then shown as a boxplot. (a) The results as the direct transmission rate in ducks varies; (b) the results as the amplitude of seasonality in the ducks' direct transmission rate varies; (c) the results from varying the duration of immunity of ducks; (d) the results as the recovery rate varies in all three species; (e) the results if the consumption rate varies in all species; and (f) how peak prevalence varies as the number of horseshoe crabs is varied. The range for each of the parameters used in the LHS is given in the electronic supplementary material, table S1.
Using LHS alongside different combinations of host species passing through Delaware Bay provides an insight into the role of multiple host species in the model system (figure 4f). Host species combinations are found to have a significant effect on peak prevalence in ruddy turnstones in Delaware Bay—specifically, interaction with resident ducks in the system is crucial for non-zero peak prevalence in ruddy turnstones. If they are either removed from the model or ruddy turnstones do not travel through Delaware Bay and so do not interact with them, mean peak prevalence in ruddy turnstones is zero (note that if ruddy turnstones do not travel through Delaware Bay, peak prevalence at the same time of year is shown instead).
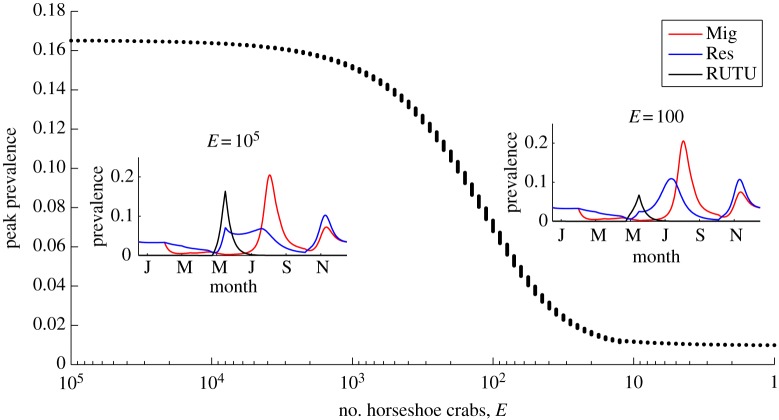
Results shown so far pertain to the known epizootiological situation. For a more prospective use of the model, we now turn to a key component in this system that is exhibiting a long-term trend—the number of horseshoe crabs. We find that the number of horseshoe crabs present can exert great influence on the prevalence curves for both the resident ducks and ruddy turnstones, with peak AIV prevalence in ruddy turnstones decreasing as horseshoe crab numbers, E, decline. The sharpest reduction occurs in the region 30< E<1000. Figure 5 shows this trend, with insets that show changing prevalence curves for all three hosts with (E = 10) and without (E = 105 ) resource limitation. Furthermore, we note that the decline in AIV prevalence in ruddy turnstones leads to an increase in the tallest prevalence peak in resident ducks, as their prevalence curve becomes more like that of the migrating ducks.
Figure 5.
Prevalence curves for reduced resources for the ruddy turnstones. The main figure shows the peak prevalence in ruddy turnstones as the number of horseshoe crabs (E) decreases for a ruddy turnstone population of 10 000, using updated initial conditions in the ruddy turnstone population. The inset figures show prevalence curves predicted by the model for two different values of E. Mig, migratory ducks; Res, resident ducks; RUTU, ruddy turnstones.
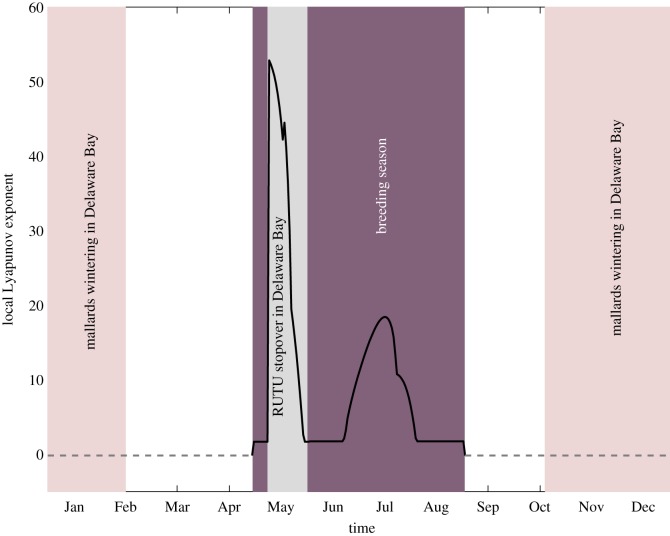
A potentially important dimension to the identification of Delaware Bay as an avian influenza hotspot concerns the likely role played by this site in the successful invasion of any novel AIV strain. That is, are there specific windows of vulnerability during which amplification of an introduced virus is predicted? We answer this question by calculating a dynamic and time-dependent measure of pathogen invasion potential, specifically the LLE (described in the electronic supplementary material, §S9). A negative LLE indicates that any perturbation (resulting from a virus introduction) will decay in the short term, while a positive exponent signals locally exponential growth [53]. From this metric, we find that seasonal hatching and the ruddy turnstones' stay in Delaware Bay are the two key determinants of the sign of the LLE, with the four-month period between May and August, covering these events, as the period during which Delaware Bay can act as a gateway. This is indicated by a positive LLE (figure 6), with especially large amplification potential during the ruddy turnstones' sojourn in Delaware Bay. This result helps establish the contribution of seasonal breeding and seasonal migration (in ruddy turnstones) to the definition of Delaware Bay as a ‘hotspot’.
Figure 6.
The local Lyapunov exponent (solid line, positive; dashed line, negative) against time over a year. The shaded areas show when the migrating ducks are in Delaware Bay, when the ruddy turnstones are in Delaware Bay and when hatching is occurring in the system (of any host). The x-axis ticks mark the midpoint of each month.
5. Discussion
Delaware Bay has long been recognized as an important and anomalous site in AIV epizootiology, although the reasons behind this discrepancy have not been fully understood. We have dissected this vital question, pinpointing some of the key mechanisms that are likely to contribute to the AIV dynamics observed at the site.
We parametrized our model using available data (as described in §3). Unfortunately, the prevalence curve for mallards does not come from a single data source but is the amalgamation of a variety of studies conducted in different months. These independent studies were carried out over different time periods and used different virus detection and isolation techniques, but together represent what is known about AIV prevalence in migrating mallards. We used these data together with prevalence estimates provided by the US Department of Agriculture (see §3 and the electronic supplementary material for greater detail), and the resulting model trajectory is therefore a compromise between these independent data sources. We minimized the sum of squared errors (see the electronic supplementary material for methodological details) to determine the best fit parameters—owing to the fragmented nature of the mallard data, it was not possible to adopt more elaborate statistical inference methods. We found that our parametrized model successfully captures key seasonal trends of the data, but systematically under-represents prevalence—particularly in the resident ducks. There are a number of possible reasons for this. Firstly, the fact that our parameter estimates are a compromise from fitting the model to two different data sources is likely to play a role. Secondly, the prevalence levels observed in PCR-based isolation data (for American black ducks in Delaware) are surprisingly high—understanding why presents an interesting topic for further work. Finally, it is possible that our model may not be capturing an element of the system that is driving the high prevalence levels observed. Uncovering whether this is the case, and what this element could be, is likely to be driven by long-term surveillance data from these birds—data that do not currently exist. However, given the ability of our model to successfully capture the seasonal trends present in both datasets, we are still able to draw useful inferences from our results.
The model analysis indicates that prevalence peaks occurring in Delaware Bay, in any of the species represented in our model, are a result of several integrated factors. The migrating ducks have an annual, pre-arrival peak in prevalence owing to both direct within-species transmission and transmission from the environmental reservoir. This peak in prevalence is succeeded by another through interactions with the resident ducks in Delaware Bay. Equally, the ruddy turnstones are capable of driving their own prevalence peaks, but these are initiated by both the environmental reservoir in Delaware Bay and interaction between ruddy turnstones and resident ducks. The model shows that prevalence peaks in resident ducks are sparked by the arrival of either the ruddy turnstones or the migrating ducks, and, in the summer months, maintained by the within-species transmission in resident ducks. In particular, this analysis suggests that both between-species interactions and transmission via the environment are important elements in the determination of Delaware Bay as a hotspot, as they are so influential in transmission. In particular, the importance of between-species interaction is highlighted when comparing peak prevalence in ruddy turnstones as different combinations of host species are included in the model. We find that, without the key interaction between ruddy turnstones and resident ducks, peak prevalence remains zero even when other epidemiological parameters are allowed to vary (figure 4f).
Our model predicts a multi-peaked prevalence curve in ducks, with the initiation of each peak through the year attributable to a different source. In our model, resident ducks display a peak in prevalence as migrating ducks arrive in Delaware Bay, followed by non-zero prevalence immediately prior to the departure of the migrating ducks. Notably, similar prevalence levels have been observed at the same time of year in data collected from Europe [54]. The summer peaks that occur in resident ducks cannot yet be verified as the necessary data are currently lacking, but our model suggests that they are a result of either interactions with the ruddy turnstones or the influx of new susceptibles in the post-breeding period.
The effective R0(t) values from the model offer an explanation for Delaware Bay as an AIV hotspot. A peak in AIV prevalence could occur in Delaware Bay while either of the migrating species is present or briefly during the summer months as a result of the influx of susceptible resident ducks. Outside Delaware Bay, the effective R0(t) for ruddy turnstones is too low for prevalence peaks to occur, but migrating ducks maintain a sufficiently large effective R0(t) to admit prevalence peaks, with annual peaks in prevalence prior to their arrival in Delaware Bay. The arrival of either of the other species in Delaware Bay increases the effective R0(t) value there and prompts a peak in prevalence in the resident ducks. The effective R0(t) values show the impact of heterospecific interactions, which greatly increase the effective R0(t) value.
The model predicts that peak prevalence in ruddy turnstones decreases as horseshoe crabs decrease in abundance. An annual prevalence cycle is apparent until the number of horseshoe crabs is so limited that ruddy turnstone populations can no longer be supported in Delaware Bay. The impact of this on the system is not straightforward. Although prevalence in ruddy turnstones declines, it leads to peak annual prevalence in the resident duck population increasing over time. This is a result of the resident duck prevalence curve losing its May–June peak and instead developing a prevalence curve similar to that in migrating ducks, with one main (post-hatching) peak in the year. Surveillance will need to be ongoing and long term to identify this consequence of decreasing ruddy turnstone prevalence.
We explored the question ‘when could a novel avian virus invade North America?’, by determining the time-dependent invasion potential in our system, as characterized by LLEs. Our model analysis established when Delaware Bay may serve as a potential amplification site for a new AIV subtype. Specifically, our results show that the two biggest predictors of this are when the ruddy turnstones are in Delaware Bay or during the hatching seasons, when there is an influx of susceptibles. Were it to occur while ruddy turnstones are in Delaware Bay, immediate transmission to the resident duck population would be likely. A successful invasion during the hatching season may have less wide-reaching consequences depending on the physiological effects of infection on its host, in particular whether migratory traits and, therefore, the spread to other host species and locations are affected. Identifying the potential origin of such a virus is beyond the scope of this work, but would contribute vital information to the role of Delaware Bay in the spread of AIVs. This result offers two more components of the system that promote Delaware Bay's status as a hotspot—seasonal migration and seasonal breeding.
Our work has provided insight into potentially important ecological parameters affecting AIV ecology in Delaware Bay. The combination of model analyses attests to the synergistic contributions of multiple host species, migration biology, virus kinetics in the environment and seasonal shifts in direct transmission in generating an AIV transmission hotspot. Along with Delaware Bay, four other sites in North America are key shorebird sites (Copper River Delta, Alaska; Gray's Harbor, Washington; Bay of Fundy, Canada; Cheyenne Bottoms, Kansas [55]) that may also prove to be AIV transmission hotspots. The work presented here provides key factors that contribute to the definition of Delaware Bay as a hotspot, providing vital information that may aid efforts to detect large-scale outbreaks of novel influenza virus in wild-bird populations in the USA.
Acknowledgements
This work was supported by the James S. McDonnell Foundation and the National Science Foundation (DEB-0917853). P.R. was also supported by the RAPIDD program of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health. D.S. and J.B. were also supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract HHSN266200700007C. Data were collected by numerous biologists from state and federal agencies participating in the US Early Detection System for HPAI in wild birds, and made available through the USDA-APHIS Wildlife Services National Wildlife Disease Program. The opinions expressed herein are those of the author(s) and do not necessarily reflect the views of any of the funding agencies.
References
- 1.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56, 152–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claas ECJ, Osterhaus ADME, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, Krauss S, Shortridge KF, Webster RG. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351, 472–477 10.1016/S0140-6736(97)11212-0 (doi:10.1016/S0140-6736(97)11212-0) [DOI] [PubMed] [Google Scholar]
- 3.Belshe RB. 2005. The origins of pandemic influenza: lessons from the 1918 virus. N. Engl. J. Med. 353, 2209–2211 10.1056/NEJMp058281 (doi:10.1056/NEJMp058281) [DOI] [PubMed] [Google Scholar]
- 4.Garten RJ, et al. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325, 197–201 10.1126/science.1176225 (doi:10.1126/science.1176225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li KS, et al. 2004. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430, 209–213 10.1038/nature02746 (doi:10.1038/nature02746) [DOI] [PubMed] [Google Scholar]
- 6.Garske T, Legrand J, Donnelly CA, Ward H, Cauchemez S, Fraser C, Ferguson NM, Ghani AC. 2009. Assessing the severity of the novel influenza A/H1N1 pandemic. Br. Med. J. 339, 220–224 10.1136/bmj.b2840 (doi:10.1136/bmj.b2840) [DOI] [PubMed] [Google Scholar]
- 7.Krauss S, Webster RG. 2010. Avian influenza virus surveillance and wild birds: past and present. Avian Dis. 54, 394–398 10.1637/8703-031609-Review.1 (doi:10.1637/8703-031609-Review.1) [DOI] [PubMed] [Google Scholar]
- 8.Swayne DE. (ed.) 2008. Avian influenza. New York, NY: John Wiley and Sons Inc [Google Scholar]
- 9.Olsen B, Munster VJ, Wallensten A, Waldenström J, Osterhaus ADME, Fouchier RAM. 2006. Global patterns of influenza A virus in wild birds. Science, 312, 384–388 10.1126/science.1122438 (doi:10.1126/science.1122438) [DOI] [PubMed] [Google Scholar]
- 10.Stallknecht DE, Brown JD. 2008. Ecology of avian influenza in wild birds. In Avian influenza (ed. Swayne DE.), pp. 43–58 New York, NY: John Wiley and Sons Inc. [Google Scholar]
- 11.Drilling N, Titman R, Mckinney F. 2002. Mallard (Anas platyrhynchos). In The birds of North America online (ed. Poole A.). Ithaca, NY: Cornell Lab of Ornithology; See http://bna.birds.cornell.edu/bna/species/658. [Google Scholar]
- 12.Alerstam T, Christie DA. 1993. Bird migration. Cambridge, UK: Cambridge Univeristy Press [Google Scholar]
- 13.Altizer S, Bartel R, Han BA. 2011. Animal migration and infectious disease risk. Science 331, 296–302 10.1126/science.1194694 (doi:10.1126/science.1194694) [DOI] [PubMed] [Google Scholar]
- 14.Kilpatrick AM, Chmura AA, Gibbons DW, Fleischer RC, Marra PP, Daszak P. 2006. Predicting the global spread of H5N1 avian influenza. Proc. Natl Acad. Sci. USA 103, 19 368–19 373 10.1073/pnas.0609227103 (doi:10.1073/pnas.0609227103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prosser DJ, et al. 2011. Wild bird migration across the Qinghai-Tibetan Plateau: a transmission route for highly pathogenic H5N1. PLoS ONE 6, e17622. 10.1371/journal.pone.0017622 (doi:10.1371/journal.pone.0017622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantin-Jackwood MJ, Swayne DE. 2009. Pathogenesis and pathobiology of avian influenza virus infection in birds. Rev. Sci. Tech. Off. Int. Epiz. 28, 113–136 [PubMed] [Google Scholar]
- 17.Kida H, Yanagawa R, Matsuoka Y. 1980. Duck influenza lacking evidence of disease signs and immune response. Infect. Immun. 30, 547–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fereidouni SR, Grund C, Hauslaigner R, Lange E, Wilking H, Harder TC, Beer M, Starick E. 2010. Dynamics of specific antibody responses induced in mallards after infection by or immunization with low pathogenicity avian influenza viruses. Avian Dis. 54, 79–85 [DOI] [PubMed] [Google Scholar]
- 19.Brown JD, Goekjian G, Poulson R, Valeika S, Stallknecht DE. 2009. Avian influenza virus in water: infectivity is dependent on pH and salinity and temperature. Vet. Microbiol. 136, 20–26 10.1016/j.vetmic.2008.10.027 (doi:10.1016/j.vetmic.2008.10.027) [DOI] [PubMed] [Google Scholar]
- 20.Stallknecht DE, Kearney MT, Shane SM, Zwank PJ. 1990. Effects of pH, temperature and salinity on persistence of avian influenza viruses in water. Avian Dis. 34, 412–418 [PubMed] [Google Scholar]
- 21.Brown JD, Swayne DE, Cooper RJ, Burns RE, Stallknecht DE. 2007. Persistence of H5 and H7 avian influenza viruses in water. Avian Dis. 51, 285–289 [DOI] [PubMed] [Google Scholar]
- 22.Hinshaw VS, Webster RG, Turner B. 1980. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can. J. Microbiol. 26, 622–629 [DOI] [PubMed] [Google Scholar]
- 23.Vong S, Ly S, Mardy S, Holl D, Buchy P. 2008. Environmental contamination during influenza A virus (H5N1) outbreaks, Cambodia. Emerg. Infect. Dis. 14, 1303–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markwell DD, Shortridge KF. 1982. Possible waterborne transmission and maintenance of influenza viruses in domestic ducks. Appl. Environ. Microbiol. 43, 110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers JP, Morrison RIG, Antas PZ, Harrington BA, Lovejoy TE, Sallaberry M, Senner SE, Tarak A. 1987. Conservation strategy for migratory species. Am. Sci. 75, 18–26 [Google Scholar]
- 26.Krauss S, Stallknecht DE, Negovetich NJ, Niles LJ, Webby RJ, Webster RG. 2010. Coincident ruddy turnstone migration and horseshoe crab spawning creates an ecological ‘hot spot’ for influenza viruses. Proc. R. Soc. B 277, 3373–3379 10.1098/rspb.2010.1090 (doi:10.1098/rspb.2010.1090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons KC. 2002. Integrated management of waterbird habitats at impounded wetlands in Delaware Bay and U.S.A. Waterbirds 25, (Special Publication 2), 25–41 [Google Scholar]
- 28.Shuster CN, Jr, Botton ML. 1985. A contribution to the population biology of horseshoe crabs. Limulus polyphemus (L), in Delaware Bay. Estuaries 8, 363–372 [Google Scholar]
- 29.Niles LJ, et al. 2009. Effects of horseshoe crab harvest in Delaware Bay on red knots: are harvest restrictions working? Bioscience 59, 153–164 10.I525/bio.2009.59.2.8 (doi:10.I525/bio.2009.59.2.8) [DOI] [Google Scholar]
- 30.He D, Earn DJD. 2007. Epidemiological effects of seasonal oscillations in birth rates. Theor. Popul. Biol. 72, 274–291 10.1016/j.tpb.2007.04.004 (doi:10.1016/j.tpb.2007.04.004) [DOI] [PubMed] [Google Scholar]
- 31.Keeling MJ, Rohani P. 2008. Modelling infectious diseases. Princeton, NJ: Princeton University Press [Google Scholar]
- 32.Longcore JR, Mcauley DG, Hepp GR, Rhymer JM. 2000. American black duck (Anas rubripes). In The birds of North America online (ed. Poole A.). Ithaca, NY: Cornell Lab of Ornithology; See http://bna.birds.cornell.edu/bna/species/481. [Google Scholar]
- 33.Nettleship DN. 2000. Ruddy turnstone (Arenaria interpres). In The birds of North America online (ed. Poole A.). Ithaca, NY: Cornell Lab of Ornithology; See http://bna.birds.cornell.edu/bna/species/537. [Google Scholar]
- 34.Francis CM, Sauer JR, Serie JR. 1998. Effect of restrictive harvest regulations on survival and recovery rates of American black ducks J. Wildl. Manage. 62, 1544–1557 [Google Scholar]
- 35.Delaware Department of Natural Resources and Environmental Control 2011. 2011–2012 Delaware migratory game bird season summary. See http://www.dnrec.delaware.gov/fw/Hunting/Documents/2011-2012. [Google Scholar]
- 36.Tsipoura N, Burger J. 1999. Shorebird diet during spring migration stopover on Delaware Bay. Condor 101, 635–644 [Google Scholar]
- 37.Loveland RE. 2001. The life history of Horseshoe crabs. In Limulus in the limelight; a species 350 million years in the making and in peril? (ed. Tanacredi JT.), pp. 93–102 Dordrecht, The Netherlands: Kluwer Academic [Google Scholar]
- 38.Roche B, Lebarbenchon C, Gauthier-Clerc M, Chang C-M, Thomas F, Renaud R, van der Werf S, Guégan J. 2009. Water-borne transmission drives avian influenza dynamics in wild birds: the case of the 2005–2006 epidemics in the Camargue area. Infect. Genet. Evol. 9, 800–805 10.1016/j.meegid.2009.04.009 (doi:10.1016/j.meegid.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 39.Breban R, Drake JM, Stallknecht DE, Rohani P. 2009. The role of environmental transmission in recurrent avian influenza epidemics. PLoS Comput. Biol. 5, e1000346. 10.1371/journal.pcbi.1000346 (doi:10.1371/journal.pcbi.1000346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohani P, Breban R, Stallknecht DE, Drake JM. 2009. Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proc. Natl Acad. Sci. USA 106, 10 365–10 369(doi:10.1073/pnas.0809026106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maxted AM, Luttrell MP, Goekjian VH, Brown JD, Niles LJ, Dey AD, Kalasz KS, Swayne DE, Stallknecht DE. 2012. Avian influenza virus infection dynamics in shorebird hosts. J. Wildl. Dis. 48, 322–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schekkerman H, Slaterus R. 2008. Population dynamics and prevalence of influenza A virus in mallard, mute swan and other wildfowl. Thetford, UK: British Trust for Ornithology [Google Scholar]
- 43.Swayne DE, Slemons RD. 2008. Using mean infectious dose of high- and low-pathogenicity avian influenza viruses originating from wild duck and poultry as one measure of infectivity and adaptation to poultry. Avian Dis. 52, 455–460 [DOI] [PubMed] [Google Scholar]
- 44.Webster RG, Yakhno M, Hinshaw VS, Bean WJ, Murti KC. 1978. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology 84, 268–278 10.1016/0042-6822(78)90247-7 (doi:10.1016/0042-6822(78)90247-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metcalfe NB, Furness RW. 1985. Survival, winter population stability and site fidelity in the turnstone Arenaria interpres. Bird Study 32, 207–214 10.1080/00063658509476881 (doi:10.1080/00063658509476881) [DOI] [Google Scholar]
- 46.Latorre-Margalef N, et al. 2009. Effects of influenza A virus infection on migrating mallard ducks. Proc. R. Soc. B 276, 1029–1036 10.1098/rspb.2008.1501 (doi:10.1098/rspb.2008.1501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson RM, May RM. 1991. Infectious diseases of humans. Oxford, UK: Oxford University Press [Google Scholar]
- 48.van den Driessche P, Watmough J 2002. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 180, 29–48 10.1016/S0025-5564(02)00108-6 (doi:10.1016/S0025-5564(02)00108-6) [DOI] [PubMed] [Google Scholar]
- 49.Grassly NC, Fraser C. 2006. Seasonal infectious disease epidemiology. Proc. R. Soc. B 273, 2541–2550 10.1098/rspb.2006.3604 (doi:10.1098/rspb.2006.3604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stallknecht DE, Shane SM. 1988. Host range of avian influenza virus in free-living birds. Vet. Res. Commun. 12, 125–141 [DOI] [PubMed] [Google Scholar]
- 51.Deliberto TJ, Swafford SR, Nolte DL, Pedersen K, Lutman MW, Schmit BB, Baroch JA, Kohler DJ, Franklin A. 2009. Surveillance for highly pathogenic avian influenza in wild birds in the USA. Integr. Zool. 4, 426–439 10.1111/j.1749-4877.2009.00180.x (doi:10.1111/j.1749-4877.2009.00180.x) [DOI] [PubMed] [Google Scholar]
- 52.Hess GK, West RL, Barnhill MV, III, Fleming LM. 2000. Birds of Delaware. Pittsburgh, PA: University of Pittsburgh Press. [Google Scholar]
- 53.Peitgen H-O, Jr, Gens H, Saupe D. 2004. Chaos and fractals: new frontiers of science. Berlin, Germany: Springer [Google Scholar]
- 54.Munster VJ, et al. 2007. Spatial, temporal and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog. 3, e61. 10.1371/journal.ppat.0030061 (doi:10.1371/journal.ppat.0030061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ehrlich PR, Dobkin DS, Wheye D. 1988. The birder's handbook: a field guide to the natural history of North American birds. New York, NY: Simon and Schuster [Google Scholar]