Abstract
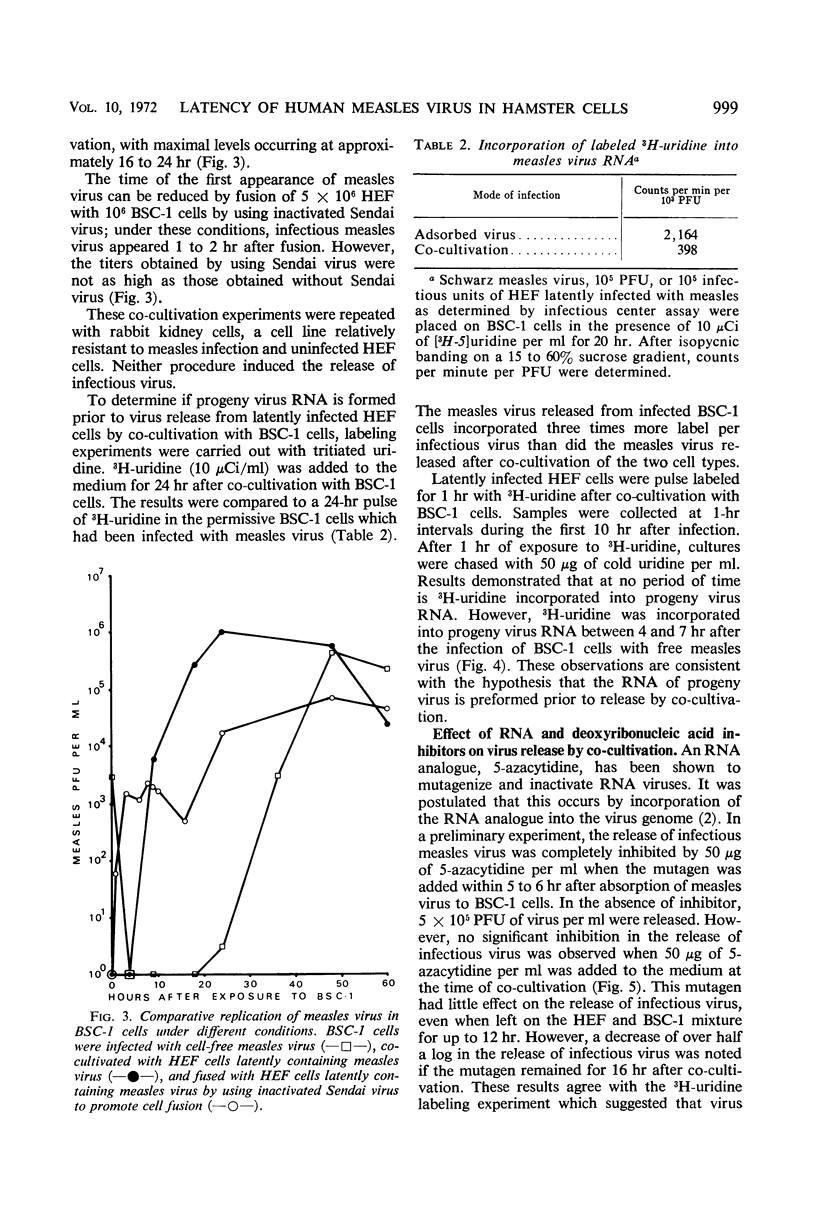
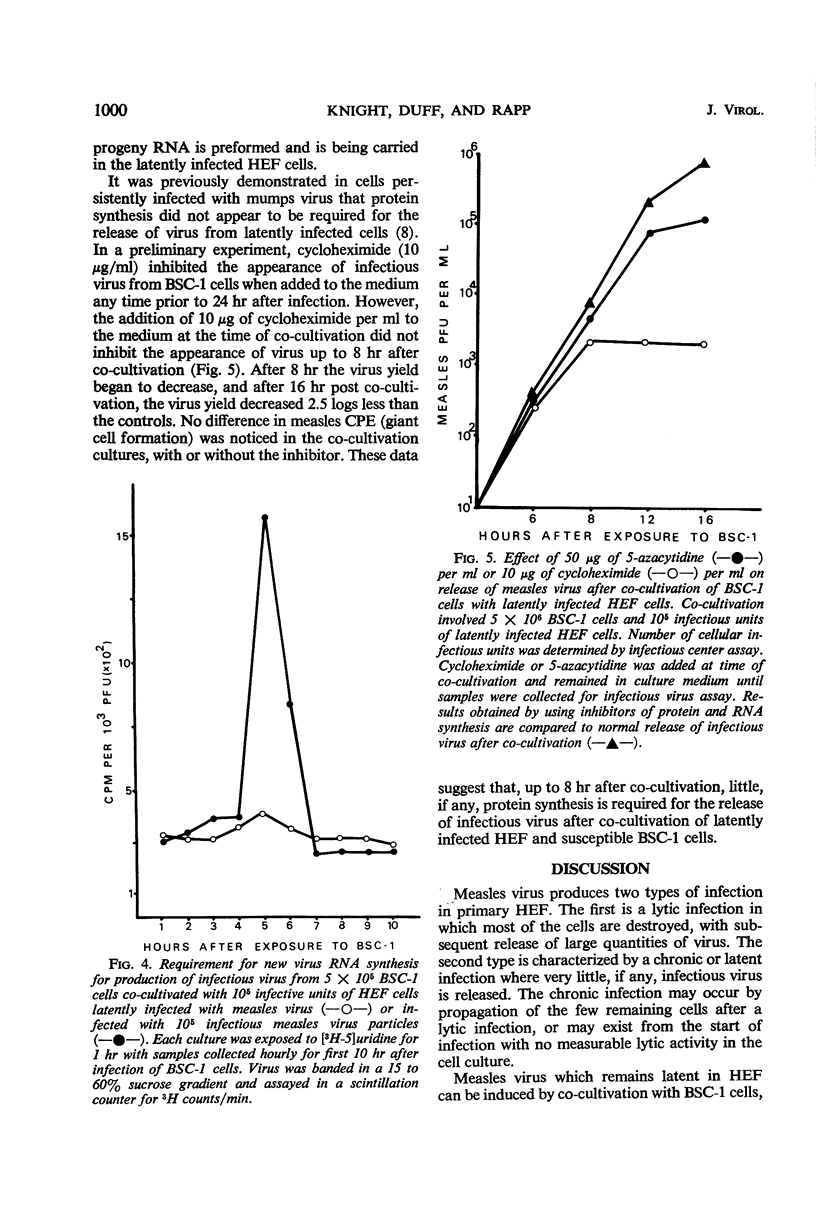
A latent system employing measles virus (Schwarz strain) was developed in hamster embryo fibroblasts (HEF). Measles virus-specific antigen was detected by immunofluorescence in 30 to 50% of HEF cells, and these cells released infectious virus when co-cultivated with a susceptible monkey cell line, BSC-1 cells. No infectious virus could be detected in the cells when measures were taken to exclude passage of viable latent cells onto the indicator BSC-1 cells. Infectious center assays demonstrated that about 1 in 10 of the latently infected cells in the population could release infectious virus. Infectious virus appeared within 6 hr after co-cultivation of the HEF cells with BSC-1 cells, as compared to 24 hr required for normal replication of measles virus in the BSC-1 cells. Furthermore, labeling of progeny virus ribonucleic acid (RNA) by using tritiated uridine, and inhibition of RNA or protein synthesis by 5-azacytidine or cycloheximide suggested that neither additional RNA nor protein synthesis is required after co-cultivation of the cells to effect early virus release. It can therefore be postulated that there is a block at a late step in virus replication in the latently infected hamster cells. The most obvious site would concern maturation of infectious virions at the cell membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. T., Watanabe I., Zeman W., Mealey J., Jr Subacute sclerosing panencephalitis: propagation of measles virus from brain biopsy in tissue culture. Science. 1969 Mar 14;163(3872):1193–1194. doi: 10.1126/science.163.3872.1193. [DOI] [PubMed] [Google Scholar]
- Halle S. 5-Azacytidine as a mutagen for arboviruses. J Virol. 1968 Oct;2(10):1228–1229. doi: 10.1128/jvi.2.10.1228-1229.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson T. E., Brody J. A., Sever J. L., Dyken M. L., Cannon J. Measles antibody titers in multiple sclerosis patients, siblings, and controls. JAMA. 1970 Mar 23;211(12):1985–1988. [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. T. Subacute sclerosing panencephalitis. J Infect Dis. 1970 Feb;121(2):227–230. doi: 10.1093/infdis/121.2.227. [DOI] [PubMed] [Google Scholar]
- Nakai T., Shand F. L., Howatson A. F. Development of measles virus in vitro. Virology. 1969 May;38(1):50–67. doi: 10.1016/0042-6822(69)90127-5. [DOI] [PubMed] [Google Scholar]
- Norrby E. A carrier cell line of measles virus in Lu 106 cells. Arch Gesamte Virusforsch. 1967;20(2):215–224. doi: 10.1007/BF01241275. [DOI] [PubMed] [Google Scholar]
- Northrop R. L. Effect of puromycin and actinomycin D on a persistent mumps virus infection in vitro. J Virol. 1969 Aug;4(2):133–140. doi: 10.1128/jvi.4.2.133-140.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyanagi S., ter Meulen V., Müller D., Katz M., Koprowski H. Electron microscopic observations in subacute sclerosing panencephalitis brain cell cultures: their correlation with cytochemical and immunocytological findings. J Virol. 1970 Sep;6(3):370–379. doi: 10.1128/jvi.6.3.370-379.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive mutants isolated from L cells persistently infected with Newcastle disease virus. J Virol. 1972 Feb;9(2):200–206. doi: 10.1128/jvi.9.2.200-206.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F. PLAQUE DIFFERENTIATION AND REPLICATION OF VIRULENT AND ATTENUATED STRAINS OF MEASLES VIRUS. J Bacteriol. 1964 Nov;88:1448–1458. doi: 10.1128/jb.88.5.1448-1458.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSTIGIAN R. A carrier state in HeLa cells with measles virus (Edmonston strain) apparently associated with non-infectious virus. Virology. 1962 Jan;16:101–104. doi: 10.1016/0042-6822(62)90212-x. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Bornstein M. B. Ultrastructural study of long-term measles infection in cultures of hamster dorsal-root ganglion. J Virol. 1971 Sep;8(3):318–329. doi: 10.1128/jvi.8.3.318-329.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. I. Development and characteristics of HeLa sublines persistently infected with complete virus. J Bacteriol. 1966 Dec;92(6):1792–1804. doi: 10.1128/jb.92.6.1792-1804.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Ward R., Strand M. The replication cycle of RNA bacteriophages. Adv Virus Res. 1969;15:1–59. doi: 10.1016/s0065-3527(08)60873-8. [DOI] [PubMed] [Google Scholar]
- Wear D. J., Rapp F. Encephalitis in newborn hamsters after intracerebral injection of attenuated human measles virus. Nature. 1970 Sep 26;227(5265):1347–1348. doi: 10.1038/2271347a0. [DOI] [PubMed] [Google Scholar]
- Wear D. J., Rapp F. Latent measles virus infection of the hamster central nervous system. J Immunol. 1971 Dec;107(6):1593–1598. [PubMed] [Google Scholar]